?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Europium (Eu3+) and horse radish peroxidase (HRP) are highly promising biosensors due to its excellent physical and chemical properties. The two biosensors are widely applied in time-resolved fluoroimmunoassay (TRFIA) and chemiluminescence enzyme-linked immunosorbent assay, respectively. In this work, the two technologies were integrated with parallel input and parallel output (PI–PO) characters. Eu3+ was adopted as probe I in TRFIA for deoxynivalenol and de-epoxyldexoylnivalenol with a limit of detection (LOD) of 4.74 and 4.56 ng/mL, while HRP was performed as probe II in CL-ELISA for FB1 with an LOD of 0.088 ng/mL. Compared to the traditional individual assay, this method exhibited fast and broad detection characteristics by integrating two immunoassays into one.
1. Introduction
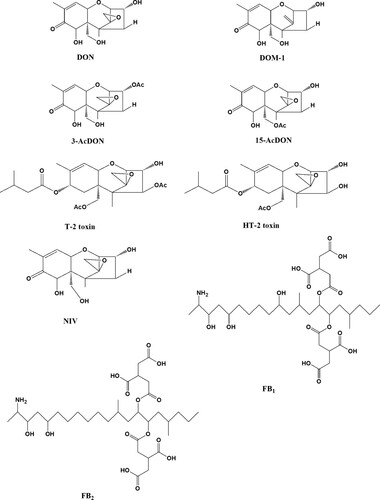
Mycotoxins, such as Fumonisins B1 (FB1), deoxynivalenol (DON), and its major metabolite de-epoxyldexoylnivalenol (DOM-1), are mainly produced by fungi when storage of cereals (structure shown in ) is inadequate (He et al., Citation2017; Kong, Xie, Liu, Song, & Kuang, Citation2017). These mycotoxins can induce various hazardous effects on animals and human beings even at a very low level (Bottalico & Perrone, Citation2002; Juan, Raiola, Mañes, & Ritieni, Citation2014). Due to the worldwide observed rise in food-borne diseases, mycotoxin poisoning has drawn increasing concerns of consumers. In order to control the contaminant of mycotoxins, an end-of-line product inspection is in urgent need to assess the critical points in both food production and storage (Craig, Franca, & Irudayaraj, Citation2013). Therefore, a broad instrument and immunoassay method has been developed for detection of mycotoxins. A number of methodologies have been reported for mycotoxins’ detection, such as gas chromatography coupled to mass spectrometry (Tacke & Casper, Citation1996) and liquid chromatography tandem mass spectrometry (LC-MS/MS) (Li, Hu, Huo, & Xu, Citation2007). However, these chromatography methods require costly instruments and time-consuming steps for sample preparation prior to the instrumental analysis step. On the other hand, demands for rapid screening techniques cannot be fulfilled by these instrumental analytical methods.
Figure 1. Chemical structures of DON, DOM-1, FB1, and analogues (3-AcDON, 15-AcDON, NIV, T-2, HT-2, and FB2).
Particularly, immunoassays are indispensable screening tools to detect the presence of mycotoxins in cereal samples and products. Several systems for multi-toxin determination have been described via different immunoassay methods. Multi-component detection applying microarray-based assays is a very common pattern. The target analytes are separated and recognised by antibodies or other proteins on the microarray (Delehanty & Ligler, Citation2002; Shlyapnikov et al., Citation2012).
For multi-analysis in immunoassays, the first important step is to produce the antibody that can recognise a group of analogues. Recently, there were several reports on development of broad-specificity antibodies for veterinary drugs which are structure-related analogues (Adrian, Font, Diserens, Sanchez-Baeza, & Marco, Citation2009; Li et al., Citation2008; Zhu et al., Citation2011). In another way, with the help of the phage display technique, recombinant antibodies have been developed quickly which can be modified artificially to bind to more analogues (Korpimaki, Hagren, Brockmann, & Tuomola, Citation2004; Korpimaki et al., Citation2002). Receptors, another new protein, are also utilised as the broad-specificity protein in immunoassays (Lamar & Petz, Citation2007). However, these novel technologies require complicated preparation of recognition factors, which limited the application for drugs’ detection.
In order to avoid this technological problem, several couples’ coating antigens and antibodies are applied for a multi-compounds’ analysis with a labelled second antibody for monitoring so as to provide a simple “YES/NO” answer with parallel input and single output (Zhu, Dietrich, Didier, Acar, & Martlbauer, Citation2013) (Scheme 1(B)). However, it could not give a further “How many” result. In order to obtain a quantitative and semi-quantitative result, a parallel input and parallel output detection model would be sufficient by providing not only “YES/NO” answer but also “How many” in a “YES” result (Scheme 1(C)).
Scheme 1. Main types of assays for the detection of mycotoxins in microtitre plates. (A) ELISA procedure for a single input and single output; (B) detection of multiple analytes with parallel input and single output; (C) parallel input for the detection of multiple analytes with parallel output.
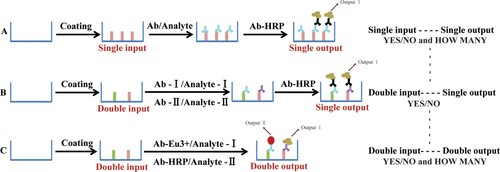
During the past years, antibody-based techniques applying different biosensors with a single-input and single-output result are the key elements in many modern immunoassay applications, such as lanthanide chelates, quantum dots, and horse radish peroxidase (HRP) (Scheme 1(A)). Lanthanide chelates have been used as luminescent inorganic fluorophores in time-resolved fluoroimmunoassay (TRFIA) since the 1980s with non-isotopic labels (Hemmilä & Laitala, Citation2005; Li et al., Citation2007). Chemiluminescence ELISA (CL-ELISA) with HRP performed as a biosensor is also widely used in the veterinary fields because of its simple, rapid, sensitive characters, and wide linear working range (Li, Zhang, Cao, et al., Citation2012; Li, Zhang, Shi, et al., Citation2012). These single-input and single-output assays can be considered as a first step towards a simple immunoassay design, which could be adopted as a basis of parallel analytical purposes. In this investigation, we integrate two monoclonal antibodies (mAbs) (anti-DON mAb and anti-FB1 mAb) to demonstrate a simultaneous multiplex assay for different mycotoxins present in the same sample in a single well with a binary “YES/NO” answer and “How many” in the “YES” answer (Scheme 1(C)).
2. Materials and methods
2.1. Reagents and materials
The anti-DON mAb (No. 4A4) (Li, Shi, Shen, et al., Citation2012) and anti-FB1 mAb (No. 2D7) (Sheng et al., Citation2012) were obtained from the College of Veterinary Medicine, China Agricultural University (Beijing, China). DOM-1 was purchased from Sigma Chemical Co. (St. Louis, MO, USA). DON, 3-acetyl-deoxynivalenol (3-AcDON), 15-acetyl-deoxynivalenol (15-AcDON), nivalenol (NIV), T-2 toxin, HT-2 toxin, Fumonisin B1 (FB1), and Fumonisin B2 (FB2) standard were purchased from Fermentek Biotechnology (Jerusalem, Israel) (structure shown in ). HRP was obtained from Boehringer Mannheim Biochemicals (Indianapolis, IN). SuperSignal CL substrate solution was purchased from Thermo Fisher (USA). A Eu-labelled kit was obtained from PE-Life-Science-Wallac (Turku, Finland). A HRP-labelled kit was obtained from Weideweikang (Beijing, China). Other chemicals and solvents were of analytical grade and were obtained from Beijing Chemical Reagent Co. (Beijing, China).
ELISA microtitre plates were purchased from Costar (Costar Inc., Milpitas, CA, USA). A spectra Max M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) was employed for this study. Ultrafiltration tubes (Millipore, Billerica, MA, USA) and a Mikro 22R centrifuge from Hettich Laborapparate (Tuttlingen, Germany) were employed for these studies. Deionised water was prepared using a Milli-Q water purification system (Millipore, Bedford, MA, USA).
2.2. Preparation of biosensor I and biosensor II
Biosensor I is a lanthanide chelate (Eu3+) which is labelled to anti-DON mAb according to a previous report (Wang et al., Citation2009), which is applied for one of the PI–PO TRFIA detection protocols for DON and DOM-1. Brief conjugation steps were as follows. Two milligrams of anti-DON mAb (dissolved in Na2CO3–NaHCO3 containing 0.155 mol/L NaCl, pH 8.5) was mixed with 0.5 mg Eu3+–DTTA, and the mixture was stirred at room temperature for 20 h. The resulting mixture was fractionated by an ultra-filtration device to get rid of unbound Eu3+–DTTA. For storage, bovine serum albumin (1 g/L) and 0.04% NaN3 were added. The final tracer solution was stored at 4°C.
HRP is performed as biosensor II, and is labelled to anti-FB1 mAb for the CL-ELISA detection of FB1. Briefly, 2 mg of activated HRP was dissolved in 0.2 mL of PBS (0.1 M, pH 7.4), and then 0.2 mL of reaction buffer was added. After that, 2 mg of anti-FB1 mAb (dissolved in PBS, 0.1 M, pH 7.4) was added and mixed for 1 h for conjugation. After the reaction, 2 mL of HRP conjugates’ dilution buffer was added and 50 μL of stop solution was used to terminate the reaction. The final HRP-mAb solution was stored at 4°C.
2.3. Sample preparation
Cereal samples obtained from the local market were homogenised and 2 g of each was transferred to a 50 mL polypropylene centrifuge tube. Ten millilitres of 20% methanol was added and vortexed for the extraction. The samples were centrifuged at 5000×g for 15 min at 4°C, and then the supernatant was transferred to another centrifuge tube. The samples were diluted with assay buffer and 50 μL of the extract was used for the following immunoassay.
Due to the hydroxyl group in structure, the target analytes could be dissolved easily in the aqueous phase. Therefore, a high percentage of the aqueous phase (20% methanol in water) was applied as the extract solution, which is kept in accordance with the previous studies (Li, Shi, Shen, et al., Citation2012). Additionally, the dilutions resulted in a lower percentage of the organic phase of the samples.
2.4. Parallel input and parallel output immunoassay (PI-POIA)
This developed PI-POIA assay integrated the two separated single-input and single-output assay (Scheme 1(C)). The details were as follows: both DON and FB1 coating antigen were coated on the microtitre plates. Mixture of DON and FB1 competitors were added into each well for competitive reaction as well as the two mAbs. Then, each well was washed with PBS containing 2% tween-20. Enhancement solution was added to the output signal of TRFIA for DON and DOM-1 detection, and chemiluminescent substrate solution was added to the other output signal of CL-ELISA for FB1 detection.
3. Results and discussion
3.1. Confirmation of labelled biosensors
3.1.1. Biosensor I
The concentration of Eu3+ was determined by fluorescence measurement. The concentration of the Eu-labelled protein (C) is spectra photometrically determined at 280 nm and calculated using the following equation:
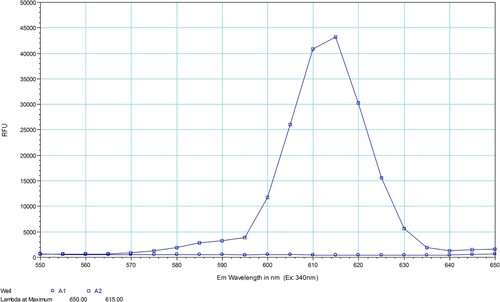
The number of Eu3+ ions incorporated per immunoglobulin molecule was calculated based on the concentration of Eu3+ and mAb. On average, two Eu3+ ions were incorporated per IgG molecule. The fluorescence spectra of Eu-mAb were obtained in PBS and the resulting emission spectra with narrow intense emission bands and maximum wavelength at approximately 615 nm are shown in .
3.1.2. Biosensor II
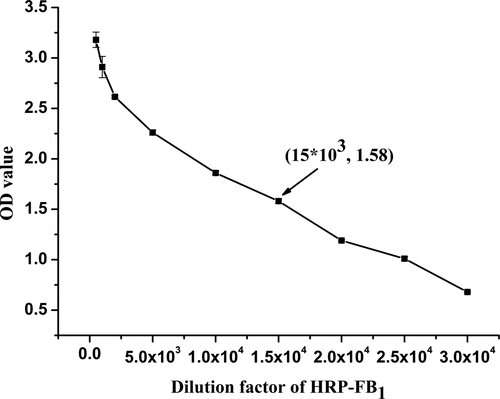
This synthesised HRP-mAb was characterised using a single-input and single-output ELISA procedure. The OD (absorbance values) values of HRP-FB1 at a series of concentrations in this procedure are shown in . From the figure, it can be concluded that the conjugation exhibited high sensitivity at a dilution range from 1:500 to 1:20,000. Considering that an OD value of about 1.5 exhibited the optimum conditions, a dilution of HRP-mAb at 1:15,000 was adopted.
3.2. Specificity and sensitivity
The specificity (evaluated by cross-reactivity, CR) of each mAb was determined via this proposed PI-POIA protocol. A 50% inhibition of control (IC50) values of each analyte was determined. The ability of each mAb to recognise other analogues was investigated by evaluating the percent of CR:
The regression equations were plotted using a four-parameter equation from the Origin, version 8.0 (OriginLab, Northampton, MA, USA). The sensitivity was evaluated using the values of IC50 and limit of detection (LOD, determined by IC10) obtained from the standard curve (). CR values of anti-DON mAb and anti-FB1 mAb were determined with the other eight mycotoxins applied to the developed procedure (). When the CR value was <2%, the binding affinity of the mAb was neglected. Therefore, the anti-DON mAb bound to DON, DOM-1, and 3-AcDON, while the anti-FB1 mAb specifically bound to FB1. The aforementioned results demonstrate that both the mAbs corresponding to the target mycotoxins were good candidates for use in developing an assay with high sensitivity and specificity to those targets. 3-AcDON is the metabolite of DON and it is not a very common contaminant in cereals. Therefore, in this study, biosensor I was adopted for DON and DOM-1 detection for one output, while biosensor II was applied for FB1 detection for the other one. LOD, evaluated by IC10, for the three mycotoxins were 4.74, 4.56, and 0.088 ng/mL.
Figure 4. Standard curves of PI-POIA for the two classes of mycotoxins TRFIA standard curve for DON and DOM-1 (right) () and CL-ELISA standard curve for FB1 (left) (
) (n = 3).
![Figure 4. Standard curves of PI-POIA for the two classes of mycotoxins TRFIA standard curve for DON and DOM-1 (right) (Y={(2095−742)/[1+(X/65)]0.8379}+742) and CL-ELISA standard curve for FB1 (left) (Y={(103,847−162)/[1+(X/0.56)]1.1887}+162) (n = 3).](/cms/asset/54671e75-8a42-4053-bec1-ba132d675640/cfai_a_1424121_f0004_oc.jpg)
Table 1. IC50, cross-reactivity (%CR) and LOD (measured by IC10) of the DON/FB1 analogues (DON, DOM-1, 3-AcDON, 15-AcDON, NIV, T-2, HT-2, FB1, and FB2).
3.3. Accuracy and precision
To ensure the system for the target analytes’ detection, rice samples were applied to this newly developed procedure. Accuracy and precision were evaluated by determining recoveries of DON, DOM-1, and FB1 in fortified samples with six replicates each on three different days. The fortified levels were 0.5, 1.0, and 1.5 mg/kg. The mean recovery ranged from 68.9% to 112.4% for DON, from 64.3% to 114.3% for DOM-1, and from 62.8% to 112.4% for FB1 with the coefficient of variation (CV) less than 17.5%, 17.9%, and 18.5%, respectively ().
Table 2. Accuracy and precision of DON, DOM-1, and FB1 in spiked rice and wheat samples at concentrations of 0.5, 1.0, and 1.5 mg/kg.
3.4. Apply to real samples
Twenty commercial cereal samples obtained from the local supermarket were analysed using this developed assay. Eight samples were determined as positive for DON and DOM-1 biosensor I and six of them were positive for FB1 biosensor II. It can be concluded that this newly developed PI-POIA method is indeed reliable and could be applied for DON, DOM-1, and FB1 detection in cereal samples.
4. Conclusion
In this research, a novel integrated immunoassay with PI-POIA character was described for the detection of DON, DOM-1 (biosensor I), and FB1 (biosensor II). This developed PI-POIA method combined europium (Eu3+) and HRP as labelling biosensors, and exhibited the advantages of high throughput, short analysis time, and reduced overall cost per assay. The PI-POIA described in this work showed promise as a multiplex immunoassay for the qualitative and quantitative screening of two classes of chemical residues in cereals.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adrian, J., Font, H., Diserens, J. M., Sanchez-Baeza, F., & Marco, M. P. (2009). Generation of broad specificity antibodies for sulfonamide antibiotics and development of an enzyme-linked immunosorbent assay (ELISA) for the analysis of milk samples. Journal of Agricultural and Food Chemistry, 57(2), 385–394. doi: https://doi.org/10.1021/jf8027655
- Bottalico, A., & Perrone, G. (2002). Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. European Journal of Plant Pathology, 108(7), 611–624. doi: https://doi.org/10.1023/A:1020635214971
- Craig, A. P., Franca, A. S., & Irudayaraj, J. (2013). Surface-enhanced Raman spectroscopy applied to food safety. Annual Review of Food Science and Technology, 4(4), 369–380. doi: https://doi.org/10.1146/annurev-food-022811-101227
- Delehanty, J. B., & Ligler, F. S. (2002). A microarray immunoassay for simultaneous detection of proteins and bacteria. Analytical Chemistry, 74(21), 5681–5687. doi: https://doi.org/10.1021/ac025631l
- He, Q., Peng, H., Yang, J., Xu, Z., Fan, C., & Sun, Y. (2017). QuEChERS extraction followed by enzyme-linked immunosorbent assay for determination of deoxynivalenol and zearalenone in cereals. Food and Agricultural Immunology, 28(6), 1477–1495. doi: https://doi.org/10.1080/09540105.2017.1348491
- Hemmilä, I., & Laitala, V. (2005). Progress in lanthanides as luminescent probes. Journal of Fluorescence, 15(4), 529–542. doi: https://doi.org/10.1007/s10895-005-2826-6
- Juan, C., Raiola, A., Mañes, J., & Ritieni, A. (2014). Presence of mycotoxin in commercial infant formulas and baby foods from Italian market. Food Control, 39(1), 227–236. doi: https://doi.org/10.1016/j.foodcont.2013.10.036
- Kong, D., Xie, Z., Liu, L., Song, S., & Kuang, H. (2017). Development of IC-ELISA and lateral-flow immunochromatographic assay strip for the detection of citrinin in cereals. Food and Agricultural Immunology, 28(5), 754–766. doi: https://doi.org/10.1080/09540105.2017.1312293
- Korpimaki, T., Hagren, V., Brockmann, E. C., & Tuomola, M. (2004). Generic lanthanide fluoroimmunoassay for the simultaneous screening of 18 sulfonamides using an engineered antibody. Analytical Chemistry, 76(11), 3091–3098. doi: https://doi.org/10.1021/ac049823n
- Korpimaki, T., Rosenberg, J., Virtanen, P., Karskela, T., Lamminmaki, U., Tuomola, M., … Saviranta, P. (2002). Improving broad specificity hapten recognition with protein engineering. Journal of Agricultural and Food Chemistry, 50(15), 4194–4201. doi: https://doi.org/10.1021/jf0200624
- Lamar, J., & Petz, M. (2007). Development of a receptor-based microplate assay for the detection of beta-lactam antibiotics in different food matrices. Analytica Chimica Acta, 586(1–2), 296–303. doi: https://doi.org/10.1016/j.aca.2006.09.032
- Li, X., Hu, Y., Huo, T., & Xu, C. (2007). Comparison of the determination of chloramphenicol residues in aquaculture tissues by time-resolved fluoroimmunoassay and with liquid chromatography and tandem mass spectrometry. Food and Agricultural Immunology, 17(3–4), 191–199. doi: https://doi.org/10.1080/09540100601090349
- Li, Y., Shi, W., Shen, J., Zhang, S., Cheng, L., & Wang, Z. (2012). Development of a rapid competitive indirect ELISA procedure for the determination of deoxynivalenol in cereals. Food and Agricultural Immunology, 23(1), 41–49. doi: https://doi.org/10.1080/09540105.2011.589046
- Li, C., Wang, Z., Cao, X., Beier, R. C., Zhang, S., Ding, S., … Shen, J. (2008). Development of an immunoaffinity column method using broad-specificity monoclonal antibodies for simultaneous extraction and cleanup of quinolone and sulfonamide antibiotics in animal muscle tissues. Journal of Chromatography A, 1209(1–2), 1–9. doi: https://doi.org/10.1016/j.chroma.2008.08.116
- Li, Y., Zhang, Y., Cao, X. Y., Wang, Z. H., Shen, J. Z., & Zhang, S. X. (2012). Development of a chemiluminescent competitive indirect ELISA method procedure for the determination of gentamicin in milk. Analytical Methods, 4(7), 2151–2155. doi: https://doi.org/10.1039/c2ay25141h
- Li, Y., Zhang, Y., Shi, W., Wang, Z., Shen, J., & Zhang, S. (2012). Determination of T-2 toxin and HT-2 toxin in milk: A comparison of three formats of immunoassays. Analytical Letters, 45(16), 2425–2435. doi: https://doi.org/10.1080/00032719.2012.686134
- Sheng, Y., Jiang, W., De Saeger, S., Shen, J., Zhang, S., & Wang, Z. (2012). Development of a sensitive enzyme-linked immunosorbent assay for the detection of fumonisin B1 in maize. Toxicon, 60(7), 1245–1250. doi: https://doi.org/10.1016/j.toxicon.2012.08.011
- Shlyapnikov, Y. M., Shlyapnikova, E. A., Simonova, M. A., Shepelyakovskaya, A. O., Brovko, F. A., Komaleva, R. L., … Morozov, V. N. (2012). Rapid simultaneous ultrasensitive immunodetection of five bacterial toxins. Analytical Chemistry, 84(13), 5596–5603. doi: https://doi.org/10.1021/ac300567f
- Tacke, B. K., & Casper, H. H. (1996). Determination of deoxynivalenol in wheat, barley, and malt by column cleanup and gas chromatography with electron capture detection. Journal of AOAC International, 79(2), 472–475.
- Wang, K., Huang, B., Zhang, J., Zhou, B., Gao, L., Zhu, L., & Jin, J. (2009). A novel and sensitive method for the detection of deoxynivalenol in food by time-resolved fluoroimmunoassay. Toxicology Mechanisms and Methods, 19(9), 559–564. doi: https://doi.org/10.3109/15376510903380720
- Zhu, K., Dietrich, R., Didier, A., Acar, G., & Martlbauer, E. (2013). Versatile antibody-sensing Boolean logic for the simultaneous detection of multiple bacterial toxins. Chemical Communications, 49(81), 9314–9316. doi: https://doi.org/10.1039/c3cc45370g
- Zhu, K., Li, J., Wang, Z., Jiang, H., Beier, R., Xu, F., … Ding, S. (2011). Simultaneous detection of multiple chemical residues in milk using broad-specificity antibodies in a hybrid immunosorbent assay. Biosensors and Bioelectronics, 26(5), 2716–2719. doi: https://doi.org/10.1016/j.bios.2010.09.011