ABSTRACT
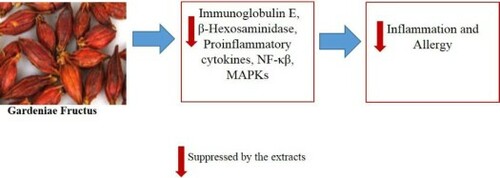
Atopic dermatitis (AD) is a common allergic, inflammatory skin disease. Here, we focused on the anti-allergic and anti-inflammatory effects of Gardenia jasminoides Ellis fructus extracts (GFE) in DNCB treated NC/Nga mice. In an in vivo study, GFE treatment significantly decreased immunoglobulin E (IgE) levels and cytokines expression in spleen and serum of treatment groups mice, compared with the negative controls (administered 0.2% DNCB only). Mice treated with GFE had fewer inflammatory cell infiltrates in the dermis and hypodermis compared to the negative controls. In addition, we also studied β-Hexosaminidase release assay and possible anti-inflammatory mechanism of GFE in RBL-2H3 cells. GFE suppressed the expression of COX-2 and TNF-α, the translocation of NF-κβ, and the phosphorylation of Syk, p38, JNK, and Erk1/2 in stimulated RBL-2H3 cells. These results strongly suggest that GFE inhibits the allergic response by reducing mast cell activation and may have therapeutic potential as an anti-atopic dermatitis agent.
GRAPHICAL ABSTRACT
Introduction
The occurrence of allergic disease has increased harmfully in the last few decades. Atopic dermatitis (AD) is an allergic inflammation disorder characterized by itchy, red, swollen, cracked skin (Hoare, Li Wan, & Williams, Citation2000; Leung, Boguniewicz, Howell, Nomura, & Hamid, Citation2004). Although the pathogenesis of AD is not fully understood, it is assumed that deregulation of T-helper 1 (Th1) and T-helper 2 (Th2) immune responses, a predominance of allergen-specific IgE, and interrupted epidermal barrier function are keys to the pathogenic mechanism. In addition, the allergic response is related to mast cells, which are generated from myeloid stem cells. RBL-2H3 is a mucosal mast cell line. The functions of these cells are similar to those of primary mast cells and normal basophils. Therefore, they are widely used to study IgE-mediated degranulation and are considered to be a good model for studying anti-allergic effects (Chung, Park, & Park, Citation2012; Han, Yim, An, Kim, & Kim, Citation1994; Marchand et al., Citation2003). They have been shown to promote the production of various inflammatory mediators and cytokines, including COX-2 and TNF-α (Gilfillan & Tkaczyk, Citation2006). Mast cell degranulation is induced in part through nuclear translocation of nuclear factor kappa-β (NF-κB) (Saito, Kato, & Matsumoto, Citation2009). Further, mitogen-activated protein kinases (MAPKs), such as extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), and p38, control various cellular functions, including cell proliferation, activation, and degranulation (Johnson & Lapadat, Citation2002). Activation of MAPKs is related to the allergic inflammatory response via translocation of NF-κB, which regulates the production of proinflammatory mediators and cytokines (Arthur & Ley, Citation2013; Hommes, Peppelenbosch, & van Deventer, Citation2003; May & Ghosh, Citation1998). Following degradation of nuclear factor of kappa light polypeptide gene enhancer in β-cells inhibitor, alpha (Iκβα) protein via phosphorylation of MAPKs, NF-κB is translocated into the nucleus, and it regulates the transcription of target genes. Therefore, inhibition of MAPKs and NF-κβ activation decreases the secretion of allergic mediators (Lian, Cheng, Zhong, & Wang, Citation2015). Further, AD can be understood through the study of animal models. A number of mouse models have been developed over the past two decades, particularly, to describe the Nc/Nga mouse as the first spontaneously occurring model of AD (Matsuda et al., Citation1997). These models display important features of human AD, resulting in a better understanding of the pathogenesis of this disease.
Gardeniae Fructus is the fruit of Gardenia jasminoides Ellis, which is a flowering plant belonging to the genus Gardenia in the family Rubiaceae. This fruit has been traditionally used as a folk medicine in many Asian countries, including Korea, China, and Japan, for the treatment of inflammation, jaundice, headache, edema, fever, hepatic disorders, and hypertension (Debnath et al., Citation2011). Gardenia jasminoides Ellis fructus extracts (GFE) has been shown to inhibit the expression of matrix metalloproteinases in a human fibrosarcoma cell line, have anti-inflammatory effects on carrageenan-induced edema in mice, and effectively treat ecchymoses in rats (Hwang, Choi, Kim, & Hwang, Citation2009). In addition, previous study has demonstrated the antioxidant effects of GFEs (Debnath et al., Citation2011). However, there is no data on the efficacy of GFE for treating AD.
In this study, we assessed the anti-allergic effects of GFE using the RBL-2H3 cell line and a model of AD in NC/Nga mice. In the in vivo study, mice were treated with 2% DNCB to induce AD-like skin lesions, and then treated with 2% and 1% of GFE for eight weeks. GFE application significantly suppressed IgE levels and inflammatory cytokines expressions in spleen and serum as well as inhibited the expression of COX-2 and TNF-α, NF-κβ translocation and the phosphorylation of Syk, p38, JNK, and Erk1/2 in stimulated RBL-2H3 cells. Therefore, these observations suggest that GFE may be an effective agent for treating AD-like allergic skin inflammation.
Materials and methods
Reagents
Cell culture materials and antibodies were purchased from Thermo Fisher Scientific, Korea and Cell Signaling Technology Inc. (Beverly, MA, USA). IgE was purchased from Bethyl (Montgomery, TX, USA). Desonide was supplied by Konkuk University Hospital (Chungju, Korea). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Wako Chemical Co. (Tokyo, Japan). DNP-specific-IgE, DNP-BSA (Albumin from Bovine Serum (BSA), 2,4-Dinitrophenylated), 4-methylumbelliferyl-N-acetyl-β-D-glucosaminide, and 1-chloro-2,4-dinitro-benzene (DNCB) were obtained from (Sigma-Aldrich, South Korea). TNF-α (#3707), NF-κβ (#8242), Sky (#2712), p-Syk (#2711), p38 (#9212), p-p38 ((#9211), p-JNK (#9251), JNK (#9252) and p-Erk1/2 (#4370), Erk1/2 (#9102) were purchased from Cell Signaling Technology (Danvers, MA).
Sample preparation
Dried fruits were obtained from Wando, Korea and ground to a powder. 100 g powder (equivalent to 1000 g of fresh weight) was extracted with 700 ml de-ionized water (DW) at 85°C for 3 h (Debnath et al., Citation2011). The extraction was performed at three times. After filtration, the extracts were evaporated under reduced pressure at 40°C in a vacuum rotary evaporator. Afterwards, the concentrated mass was dried and weighed to determine total extractable compounds. Finally, the extracts were stored in a refrigerator at 4°C until use. The stock samples were prepared by dissolving the extracted samples into DW to perform different assays.
MTT assay
RBL-2H3 cells were seeded onto a 96-well plate (1 × 104 cells/well) in DMEM with 10% FBS at 37∘C overnight. The cells were washed and incubated with DNP-IgE (10 μg/mL) for 24 h. The IgE sensitized cells were incubated with GFE (10–100 μg/mL) for 1 h and stimulated with DNP-BSA (100 ng/mL) for 4 h. Cell viability was determined by measuring the difference in absorbance at a wavelength of 450 nm.
β-Hexosaminidase release assay
After synthesis, β-hexosaminidase is stored in secretory granules and released into the extracellular medium during degranulation. RBL-2H3 cells were grown in 24-well plates (2.5 × 106 cells per well) and were sensitized overnight with 10 μg/mLof DNP-IgE (Tang et al., Citation2015). Next, the growth medium was replaced with modified Siraganian buffer (119 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L CaCl2, 0.4 mmol/L MgCl2, 25 mmol/L PIPES, 5.6 mmol/L glucose, 40 mmol/L NaOH, and 0.1% [w/v] BSA, pH 7.2). The IgE-sensitized RBL-2H3 cells were preincubated for 15 min. After incubation, the cells were treated with different concentrations (10–100 μg/mL) of GFE (20 μL) and incubated for 1 h, followed by 4 h incubation with DNP-BSA (100 ng/mL). To determine the amount of β-hexosaminidase released into the culture medium, culture supernatant (30 μL) and β-hexosaminidase substrate (1 mM, 30 μL) were incubated in 96-well plates for 60 min at 37°C. At the end of the experiment, the supernatant was incubated with an equal volume of substrate solution (0.1 mol/L citrate-sodium citrate buffer, pH 4.5). The pH value of solution was then adjusted to 11.0 by the addition of aqueous Na2CO3/NaHCO3 solution (0.1 mol/L) to terminate the enzymatic reaction. The fluorescence of the enzymatic reaction product (4-methylumbelliferone) was measured by spectrofluorimetry at 405 nm using an ELISA plate reader (TECAN, Männedorf, Switzerland).
Animals and treatments
Four-week-old, male NC/Nga mice (n = 25) were provided by Orientbio (Seongnam, Korea). The animals were acclimatized under controlled conditions for 1 week before beginning the experiment, and animals were housed in individual cages under a 12-h light/dark cycle at 20–24°C. All animals were provided commercial mouse chow and water ad libitum according to a protocol approved by the Institutional Animal Care and Use Committee of the Konkuk University. AD was induced in NC/Nga mice by chemical sensitization with DNCB. Briefly, the hair on the backs of the mice was cut with electric clippers, and residual hair was removed with a depilatory cream. Dermatitis was induced by topical application of 1% DNCB dissolved in acetone and olive oil (3:1; 200 µL per animal). Then, the animals were repeatedly sensitized with 0.2% DNCB for three days. The control and GFE groups were treated with 0.2% DNCB three times each week for eight weeks. GFE was prepared daily at doses of 0.2% or 1%.
AD mice were randomly allocated into five experimental groups of five animals each, which were treated for eight weeks as follows: normal group (administered 0.2 mL of an acetone:olive oil mixture, 3:1), negative control group (administered 0.2% DNCB), positive control group (administered 0.2% DNCB and 0.5 μg/kg desonide), 0.2% GFE group (administered 0.2% DNCB and 0.2% GFE), and 0.1% GFE group (administered 0.2% DNCB and 0.1% GFE). At the end of the experimental period, mice were fasted overnight, blood was collected from the retro-orbital venous plexus under mild anesthesia, and the mice were then euthanized.
Measurement of IgE
The serum was isolated after centrifugation at 3000 rpm for 15 min and 100 mg of spleen tissue was collected and homogenized in 1 mL PBS in an ice bath. After centrifugation at 3000 rpm for 15 min at 4°C, the supernatant of splenocyte was collected. Serum and splenocyte IgE levels were determined using a commercially available ELISA kit (Bethyl, TX, USA) according to the manufacturer’s instructions.
Western blotting
The protein concentrations in RBL-2H3 cell lysates or NC/NGA mice splenocyte lysates were determined using Bio-Rad DC Protein Assay Reagents (Hercules, CA, USA), and equal proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were transferred to PVDF membranes (Amersham Pharmacia Biotech, Buckinghamshire, UK). The immunoblot was incubated overnight with a blocking solution, followed by incubation with dilution of polyclonal antibodies against TNF-α, COX-2, interleukin (IL)-4, interferon (IFN)-γ. The blots were washed twice with Tween 20/ Tris-buffered saline and incubated with diluted solutions of the respective secondary antibodies (1:1500) for 1 h at RT. After applying the secondary antibody, the blots were incubated with Enhanced Chemiluminescence Western Blotting Detection System reagent (Thermo Fisher) and exposed to photographic film. Band intensity was measured using quantification software (Bio-Rad). Band intensities were quantified using Scion-Image software for Windows.
Preparation of cytoplasmic and nuclear extracts and immunoblotting detection
Cells were preincubated for 2 h with or without GFE at indicated concentrations for NF-κβ, Iκβα, p-Iκβα (Cell signallingand MAPKs Western blot analysis and then, stimulated for 30 min with LPS (10 μg/mL). Cytosolic extract (CE) and nuclear extract (NE) were prepared according to the instruction provided in kit manuals (NE-PER nuclear and cytoplasmic extraction reagents, Thermo scientific) and the protein content of the cell lysates was determined using the Bradford reagent (Bio-Rad, Hercules, CA). The levels of phosphorylated IκBα (p-IκBα) and IκBα protein in CE were determined by western blot.
Histology
The dorsal skin of each mouse was removed, fixed in 10% formalin, embedded in paraffin, and then cut into 10-μm thick sections. Each deparaffinized section was placed on a glass slide, dried, stained with hematoxylin and eosin (H&E), and analyzed by light microscopy (Olympus CK-2; Tokyo, Japan).
Statistical analysis
Experimental results are expressed as means ± SD. A one-way analysis of variance was used for multiple comparisons (GraphPad Prism, version 5.00 for Windows; San Diego, CA, USA).
Results and discussion
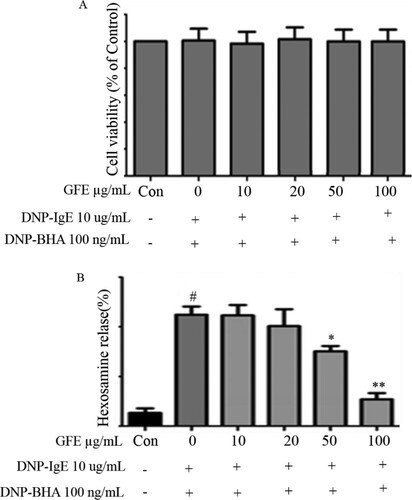
The yield was found to be 5.9% for the water-based extracts. Mucosal mast cells secrete chemical mediators, such as histamine, β-hexosaminidase, pro-inflammatory cytokines, and MAPKs, during specific immune responses (Barnes, Citation2008). Herein, we evaluated the cytotoxicity of GFE and antigen (DNP-BSA) co-treatment to find the optimal concentrations of GFE for this study. We treated the RBL-2H3 mast cells with different GFE concentrations ranging from 10–100 μg/mL in the experiments. Cell viability testing showed that GFE was not cytotoxic at the concentrations ((A)). The stimulated release of β-hexosaminidase is widely accepted marker for mast cell degranulation. Here, the effect of GFE on the degranulation of RBL-2H3 cells was examined. β-hexosaminidase enzyme is stored in mast cell secretory granules together with allergic mediators such as histamine. β-hexosaminidase is released while the mast cells are immunologically stimulated to degranulate, As shown in (B), degranulation was suppressed by the addition of GFE in a dose-dependent manner. The β-hexosaminidase release rate at the maximum concentration of GFE was nearly equal to that of the control, suggesting that GFE has degranulation-suppressing activity in RBL-2H3 cells stimulated by antigen.
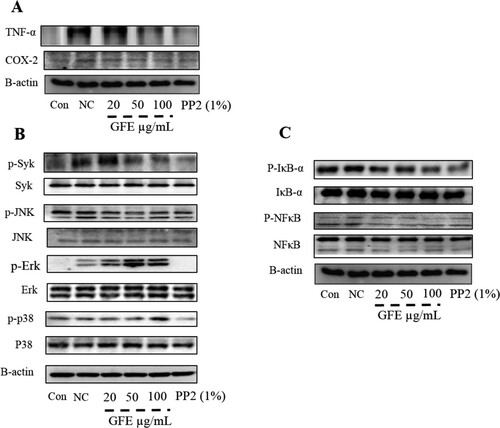
The release of inflammatory mediators and cytokines from immune cells has been implicated in Type-I allergy. Further, it is reported that TNF-α and COX-2 play an important role in AD like allergic responses (Fogh, Herlin, & Kragballe, Citation1989; Tang et al., Citation2015). Here, we found that GFE inhibited the production of COX-2 and TNF-α in stimulated RBL-2H3 cells ((A)). Therefore, the inhibitory effect of GFE on TNF-α and Cox-2 formation may point to its additional advantage as an anti-allergy agent. Further, IgE-induced degranulation in mast cells is associated with the release of COX-2 and TNF-α through the phosphorylation of spleen tyrosine kinase (Syk) (Roth, Chen, & Lin, Citation2008). In sequence, the activation of Syk associates with the activation of the MAPKs (Roth et al., Citation2008). Then, we examined the effects of GFE on the phosphorylation of Syk, Erk 1/2, p38, and JNK (a marker of PI3-kinase activation) in antigen-stimulated RBL-2H3 cells. IgE-antigen complex induced the phosphorylation of Syk and MAPKs, including Erk 1/2, p38, and JNK, in RBL-2H3 cells ((B)). Treatment with GFE attenuated the phosphorylation of Syk and MAPKs ((B)). In addition, GFE reduced the phosphorylation of IκB-α and NF-κB in a dose-dependent manner ((C)). These results suggest that GFE suppress proinflammatory cytokines via IκBα degradation and NF-κB activation as well as suppression of Syk and MAPKs (p38, ERK1/2, and JNK) in stimulated RBL-2H3 cells, which offers a potential mechanism for the anti-allergic activity of GFE.
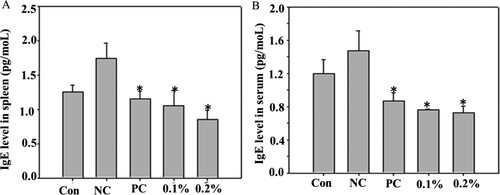
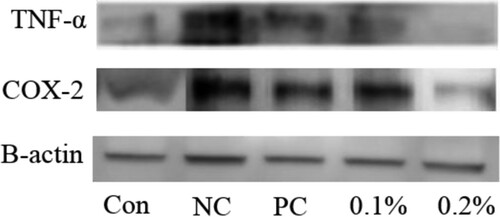
AD is an allergic skin conditions. In the present study, we determined the effects of GFE on AD-like lesions in NC/Nga mice induced by 0.2% DNCB. We observed the characteristics of human AD-like skin lesions, including dryness, itching, and hemorrhage in NC/Nga mice treated with DNCB for eight weeks. We also showed that GFE application reduced the AD lesions when compared with those with the negative control. Further, patients with AD have elevated levels of IgE in their blood which was correlated with disease severity in some studies (Chen et al., Citation2010; Kim, Lee, Park, & Choue, Citation2010; Novak, Bieber, & Leung, Citation2003). Subsequent cross-linking of IgE with allergens triggers the release of histamine and other inflammatory mediators such as COX-2 and TNF-α. Therefore, the regulation of IgE synthesis has been a focus of intensive research. Here, we found that the expression of IgE increased in negative control (only DNCB group) mice compared with other’s. Topical application of GFE reduced the levels of IgE in both splenocytes and serum ((A) and (B)). Then, we checked the effects GFE on cytokine productions in mice splenocytes (). We found that the expression of COX-2 and TNF-α were elevated in DNCB-sensitized mice. However, the treatment with GFE lowered the production levels of COX-2 and TNF-α in splenocytes compared with the levels in the negative controls. Any significant diferences were not found on the reduction of COX-2 and TNF-α expression among the GFE treated and positive control groups. Overall, the results of the present study suggest that the anti-allergic effects of GFE in AD mice are closely related to regulation of the activation of mast cells and IgE as well as cytokines production in inflamed tissues.
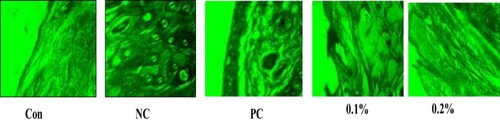
Histological assessment revealed that mice treated with GFE had a fewer inflammatory cells and less mast cell infiltration in the dermis and hypodermis compared to the negative controls (). This finding is consistent with a previous study by (Hyung, Min, & Kim, Citation2010). They reported that GFE successfully preserved lipid lamellae in the epidermis and inhibited the infiltration of mast cells into the dermis of nude mice exposed to irradiation with UVB light and in mice administered squalene monohydroperoxide (Hyung et al., Citation2010).
Conclusion
In this study, we evaluated the anti-allergic and anti-inflammatory effects of GFE using the RBL-2H3 cell line and NC/Nga mice. We studied the anti-inflammatory mechanism of GFE in vitro, and showed that GFE suppressed COX-2 and TNF-α expression, NF-κB activation, and the phosphorylation of Syk, p38, JNK, and Erk1/2 in stimulated RBL-2H3. Then, GFE application effectively suppressed the IgE level and pro-inflammatory cytokines expression in spleen and serum of AD mice. Histological examination showed that the GFE-treated mice had less inflammatory cell infiltration than negative control AD mice. Therefore, GFE may be an active agent for the treatment of AD like skin allergy related inflammation.
Acknowledgement
This study was supported by Konkuk University in 2017.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arthur, J. S., & Ley, S. C. (2013). Mitogen-activated protein kinases in innate immunity. Nature Reviews Immunology, 13(9), 679–692. doi: https://doi.org/10.1038/nri3495
- Barnes, P. J. (2008). The cytokine network in asthma and chronic obstructive pulmonary disease. Journal of Clinical Investigation, 118(11), 3546–3556. doi: https://doi.org/10.1172/JCI36130
- Chen, Q. C., Zhang, W. Y., Kim, H., Lee, I. S., Ding, Y., Youn, U. J., … Bae, K. (2010). Effects of Gardeniae Fructus extract and geniposide on promoting ligament cell proliferation and collagen synthesis. Phytotherapy Research, 24(Suppl 1), S1–S5. doi: https://doi.org/10.1002/ptr.2839
- Chung, M. J., Park, J. K., & Park, Y. I. (2012). Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. International Immunopharmacology, 12(2), 453–459. doi: https://doi.org/10.1016/j.intimp.2011.12.027
- Debnath, T., Park, P. J., Deb Nath, N. C., Samad, N. B., Park, H. W., & Lim, B. O. (2011). Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chemistry, 128, 697–703. doi: https://doi.org/10.1016/j.foodchem.2011.03.090
- Fogh, K., Herlin, T., & Kragballe, K. (1989). Eicosanoids in skin of patients with atopic dermatitis: Prostaglandin E and leukotriene B are present in biologically active concentrations. Journal of Allergy and Clinical Immunology, 83(1989), 450–455. doi: https://doi.org/10.1016/0091-6749(89)90132-2
- Gilfillan, A. M., & Tkaczyk, C. (2006). Integrated signalling pathways for mast-cell activation. Nature Reviews Immunology, 6(3), 218–230. doi: https://doi.org/10.1038/nri1782
- Han, M. K., Yim, C. Y., An, N. H., Kim, H. R., & Kim, U. H. (1994). Glycosylphosphatidylinositol-anchored NAD glycohydrolase is released from peritoneal macrophages activated by interferon-gamma and lipopolysaccharide. Journal of Leukocyte Biology, 56(6), 792–796. doi: https://doi.org/10.1002/jlb.56.6.792
- Hoare, C., Li Wan, P. A., & Williams, H. (2000). Systematic review of treatments for atopic eczema. Health Technology Assessment, 4(37), 1–191.
- Hommes, D. W., Peppelenbosch, M. P., & van Deventer, S. J. (2003). Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut, 52(1), 144–151. doi: https://doi.org/10.1136/gut.52.1.144
- Hwang, K., Choi, H. G., Kim, D. J., & Hwang, S. H. (2009). The effect of hydrolyzed gardeniae fructus extract hydrogel on the treatment of ecchymoses in a rat model. Dermatologic Surgery, 35(10), 1525–1531. doi: https://doi.org/10.1111/j.1524-4725.2009.01268.x
- Hyung, S. H., Min, K. J., & Kim, Y. C. (2010). Alleviating effect of Gardeniae fructus water extract on inflammation and skin barrier damage. Journal of Cosmetic and Laser Therapy, 6, 63–72.
- Johnson, G. L., & Lapadat, R. (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science, 298(5600), 1911–1912. doi: https://doi.org/10.1126/science.1072682
- Kim, J., Lee, I., Park, S., & Choue, R. (2010). Effects of Scutellariae radix and Aloe vera gel extracts on immunoglobulin E and cytokine levels in atopic dermatitis NC/Nga mice. Journal of Ethnopharmacology, 132(2), 529–532. doi: https://doi.org/10.1016/j.jep.2010.08.049
- Leung, D. Y., Boguniewicz, M., Howell, M. D., Nomura, I., & Hamid, Q. A. (2004). New insights into atopic dermatitis. Journal of Clinical Investigation, 113(5), 651–657. doi: https://doi.org/10.1172/JCI21060
- Lian, Q., Cheng, Y., Zhong, C., & Wang, F. (2015). Inhibition of the IgE-mediated activation of RBL-2H3 cells by TIPP, a novel thymic immunosuppressive pentapeptide. International Journal of Molecular Sciences, 16(1), 2252–2268. doi: https://doi.org/10.3390/ijms16012252
- Marchand, F., Mecheri, S., Guilloux, L., Iannascoli, B., Weyer, A., & Blank, U. (2003). Human serum IgE-mediated mast cell degranulation shows poor correlation to allergen-specific IgE content. Allergy, 58(10), 1037–1043. doi: https://doi.org/10.1034/j.1398-9995.2003.00251.x
- Matsuda, H., Watanabe, N., Geba, G. P., Sperl, J., Tsudzuki, M., Hiroi, J., … Ra, C. (1997). Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. International Immunology, 9(3), 461–466. doi: https://doi.org/10.1093/intimm/9.3.461
- May, M. J., & Ghosh, S. (1998). Signal transduction through NF-kappa B. Immunology Today, 19(2), 80–88. doi: https://doi.org/10.1016/S0167-5699(97)01197-3
- Novak, N., Bieber, T., & Leung, D. Y. (2003). Immune mechanisms leading to atopic dermatitis. Journal of Allergy and Clinical Immunology, 112, S128–S139. doi: https://doi.org/10.1016/j.jaci.2003.09.032
- Roth, K., Chen, K. M., & Lin, T. J. (2008). Positive and negative regulatory mechanisms in high-affinity IgE receptor-mediated mast cell activation. Archivum Immunologiae et Therapiae Experimentalis, 56, 385–399. doi: https://doi.org/10.1007/s00005-008-0041-2
- Saito, H., Kato, A., & Matsumoto, K. (2009). Role of mast cells in allergy. Nihon Rinsho, 67(11), 2083–2087.
- Tang, F., Chen, F., Ling, X., Huang, Y., Zheng X., Tang, Q., & Tan, X. (2015). Inhibitory effect of methyleugenol on IgE-mediated allergic inflammation in RBL-2H3 cells. Mediators of Inflammation, 2015, 9, Article ID 463530.