ABSTRACT
Polysaccharides are one of many bioactive compounds found in edible mushrooms. Edible mushrooms have become attractive as “health foods” and as source materials for immunomodulators. The aim of this project was to study the immunoregulatory effects of a purified polysaccharide derived from wild Russula griseocarnosa (PRG1-1) on macrophages. Our data showed that in RAW264.7 macrophage cells, PRG1-1 increased expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Furthermore, PRG1-1 increased the production of nitric oxide (NO) and cytokines, including interleukin 6 (IL-6) and tumour necrosis factor alpha (TNF-α). Western blotting demonstrated that the regulation of NO and cytokines was mediated through the nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Therefore, PRG1-1 has the capacity to activate macrophages via the NF-κB and MAPK pathways. These findings helped to elucidate the immune-modulatory properties of the polysaccharide from R. griseocarnosa.
Introduction
Macrophages are an important type of immune cell that play a critical role in protecting the host against pathogens and phagocytosis through immune-inflammatory responses (Junt et al., Citation2007). The activation of macrophages initiates the innate immune response and induces antigen processing and presentation to promote adaptive immunity (Shi & Pamer, Citation2011).
Macrophage function is improved through the secretion of such factors as nitric oxide (NO) and pro-inflammatory cytokines (such as IL-1, IL-6, IL-10 and TNF-α) (Doyle & O’Neill, Citation2006; Kim, Cheon, Kim, Kim, & Kim, Citation1999; Wang & Mazza, Citation2002). It has been reported that many stimuli induce the activation of macrophages. NO endows host macrophages with cytotoxic activity against viruses, bacteria and tumour cells (Boscá, Zeini, Través, & Hortelano, Citation2005). Meanwhile, cytokines are key regulators mediating multiple processes, including the activation of T and B cells, anti-tumour processes, and anti-infection processes (Belardelli, Citation1995; Vitenberga & Pilmane, Citation2017). Hence, the activation of macrophages and the subsequent immune response is an effective strategy for promoting human health.
Recently, studies have demonstrated that polysaccharides have various bioactive properties and can potentially impede the function of macrophages (Deng et al., Citation2016). In particular, polysaccharides from edible mushrooms have attracted the interest of a wide range of researchers. These polysaccharides are considered to be a potential source of natural immunomodulatory agents that could be useful in protecting humans from many diseases, including cancers, diabetes mellitus and neurodegenerative diseases (Fu et al., Citation2015; Han et al., Citation2012; Sheng, Liu, Wang, Lv, & Du, Citation2017; Yamac et al., Citation2016). Crude polysaccharides from the medicinal mushroom Amauroderma rude were shown to increase macrophage activity as well as levels of spleen lymphocytes and natural killer cells (Pan et al., Citation2015). A medium-to-high molecular weight exopolysaccharide from Schizophyllum commune was shown to have intestinal anti-inflammatory activity (Du, Yang, Bian, & Xu, Citation2017). The polysaccharides from Dictyophora indusiata significantly affected macrophage immune function by promoting the secretion of NO and pro-inflammatory cytokines, including IL-1, -6, -12 and TNF-α (Deng et al., Citation2016).
R. griseocarnosa, a wild ectomycorrhizal mushroom commonly called Dahongjun, is found in southern China. This mushroom was first recorded as a new species in 2009 (Li et al., Citation2010). Previous studies of R. griseocarnosa-derived polysaccharides had mainly focused on their composition (X.-H. Chen, Xia, Zhou, & Qiu, Citation2010) and genetic diversity (Li et al., Citation2010), while reports of the biological activities of these polysaccharides are few. In our previous study, crude polysaccharides were extracted from R. griseocarnosa (PRG) using Response Surface Metholology (RSM) combined with a Box–Behnken Design (BBD) (Yuan et al., Citation2017). A 630 kDa polysaccharide component named as PRG1-1 was purified from PRG, and the monosaccharide composition of PRG1-1 was consisted of glucose, galactose, mannose, xylose and fructose in a molar ration of 66.5:29.2:3.17:0.663:0.447, and the presence of C=O, C–O-H, C–O-C and C–H bands were observed (Liu, Zhang, & Meng, Citation2017). In the present study, we evaluated the immune-enhancing effects and the potential mechanism of action of PRG1-1 in the mouse macrophage cell line RAW264.7.
Materials and methods
Chemicals and materials
Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS) and biomyc-3 antibiotic solution were obtained from Biological Industries. Primary antibodies against COX-2, iNOS, phospho-SAPK/JNK, phospho-p38, phospho-c-Jun, phospho-NF-κB p65, IKKβ, IκBα and phospho-IκBα were purchased from Cell Signalling (Boston, MA, USA). NE-PER Nuclear and Cytoplasmic extraction reagents were purchased from Pierce Biotechnology (Rockford, IL, USA). The Thermo Scientific Pierce BCA protein assay kit was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Mouse IL-6 and TNF-α ELISA kits were purchased from eBioscience, Inc. (San Diego, CA, USA). LPS (Escherichia coli 055:B5) was obtained from Sigma-Aldrich (St. Louis, MO, USA). The TransDetect Cell Counting Kit (CCK) was obtained from TransGen Biotech (Beijing, China) and the NO assay kit was purchased from Beyotime Biotechnology (Jiangsu, China). The murine macrophage cell line RAW264.7 was obtained from the China Cell Line Bank (Beijing, China).
Purification of PRG1-1
PRG was extracted as our previous report using RSM [18], and the PRG1-1 was purified from PRG with multiple column chromatography including DEAE-52 and Sephadex G-100 (Liu et al., Citation2017).
Cell culture and cell viability assays
The murine macrophage cell line RAW264.7 was cultured in DMEM medium supplemented with 10% FBS and 1% biomyc-3. Cells were grown at 37°C in a humidified 5% CO2 atmosphere.
Cell viability was determined using the Cell Counting Kit according to the manufacturer’s instructions. 1 × 104 RAW264.7 cells were plated into 96-well plates, incubated overnight, and then treated with different concentrations (0, 50, 100, 150, 200, 300, 400 μg/mL) of PRG1-1 for 24 h. The supernatants were removed and 100 μL CCK solution (10 μL CCK: 100 μL medium) was added to each well. The plate was incubated at 37°C for 2 h in the dark. After 2 h, absorbance was detected at 450 nm using the mirco-plate reader.
Measurement of nitric oxide (NO)
Griess reagent was used to determine NO levels in the cell culture medium. 100 μL of RAW264.7 cells (1 × 105 cells/mL) were plated into 96-well plates overnight and stimulated with different concentrations of PRG1-1 (0, 50, 100, 150, 200, 300, 400 μg/mL) or LPS (1 μg/mL) for 24 h. The supernatant was collected for NO detection. 100 μL of supernatant was mixed with an equal volume of Griess reagent (0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulfanilamide, and 2.5% H3PO4). After 15 min, the optical density of each well was read at 450 nm. The concentration of nitrite in the supernatant was determined by comparison to a sodium nitrite standard curve.
Determination of TNF-α and IL-6 production
Macrophage RAW264.7 cells were plated in 96-well plates and stimulated with PRG1-1 (50, 100, 150 and 200 μg/mL) or LPS (1 μg/mL) for 24 h. The supernatant was collected by centrifugation and the TNF-α and IL-6 levels were determined by ELISA kits according to the manufacturer’s protocol. All experiments were performed in triplicate.
Extraction of nuclear and cytosolic protein fractions
Cytoplasmic and nuclear proteins were isolated using the NE-PER Nuclear and Cytoplasmic extraction reagents. The cells were digested with trypsin, collected by centrifugation, and subsequently washed with PBS. Protein was isolated at 4°C or on ice following the manufacturer’s instructions.
Western blot analysis
Western blotting was performed according to previously described methods (X. Chen et al., Citation2017). The BCA assay kit was used to assess concentrations of protein extracts according to the manufacturer’s protocol. 40 μg of protein was separated by SDS-PAGE gel electrophoresis and then transferred to a polyvinylidene fluoride (PVDF) membrane. The membranes were incubated with the optimal primary antibody dilution at 4°C for 12 h. The membrane was washed with PBST and then incubated with the optimal horseradish peroxidase (HRP)-conjugated secondary antibody dilution for 1 h. The bands were visualized using a chemiluminescence (ECL) plus reagent (Amersham Pharmacia Biotech, Piscataway, NJ) and detected using the ChemiDoc XRS imaging system (Bio-Rad, Richmond, CA). Band intensities were quantified using Image Lab software. All experiments were performed in triplicate.
Statistical analysis
All values are presented as the mean ± standard deviation (SD). Data were analyzed with one-way analysis of variance (ANOVA) followed by Student’s t-test to compare various experimental groups. GraphPad Prism (version 5.0) was used to carry out the analyses. All experiments were repeated at least three times (n ≥ 3), and *<0.05 or **<0.01 was considered to be statistically very significant and significant.
Results
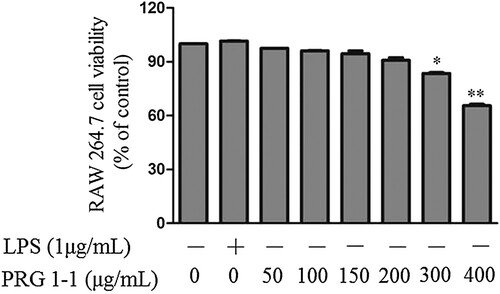
Effects of PRG1-1 on viability of RAW264.7 macrophage cells
The effects of PRG1-1 on macrophage viability were determined using the CCK kit. RAW264.7 cells were incubated with various concentrations (0, 50, 100, 150, 200, 300, 400 μg/mL) of PRG1-1. As shown in , concentrations below 150 μg/mL had no significant effect on macrophage viability. Macrophage viability began to decrease at 200 μg/mL, and the decrease reached significance at 300 μg/mL of PRG1-1. When the PRG1-1 concentration was increased to 400 μg/mL, cell viability was dramatically reduced (65.5%). These results indicated that PRG1-1 was not toxic to RAW264.7 cells below 200 μg/mL but was significantly cytotoxic at concentrations higher than 200 μg/mL. Thus, concentrations below 200 μg/mL were selected for further investigation.
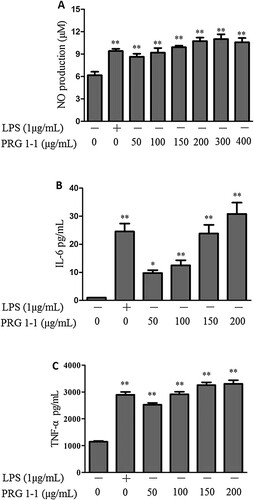
Effects of PRG1-1 on the production of NO and pro-inflammatory cytokines
Our results demonstrated that PRG1-1 significantly induced the production of NO compared with the untreated group (A). Additionally, to evaluate the effect of PRG1-1 on immune stimulation, cytokine levels were measured using ELISA kits. As shown in B-C, stimulation with PRG1-1 for 24 h significantly increased the secretion of cytokines, including IL-6 and TNF-α, compared with untreated cells.
Figure 2. Effects of PRG1-1 on NO (A), IL-6 (B) and TNF-α (C) levels in RAW264.7 cells. RAW264.7 cells were stimulated with different concentrations of PRG1-1 or with a standard concentration of LPS (1 μg/mL). LPS was used as the positive control. Values are presented as the means ± SD (n = 3). Analyses were performed using a one-way ANOVA. *p < 0.05, **p < 0.01 compared to the untreated control.
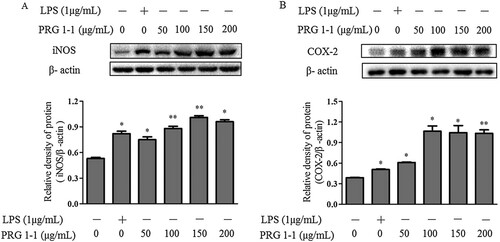
PRG1-1 induces iNOS and COX-2 production in RAW264.7 macrophages
Stimulation of RAW264.7 cells using various concentrations of PRG1-1 (0, 50, 100, 150, 200 μg/mL) significantly increased iNOS and COX-2 production compared to the negative control (A-B). As shown in A, LPS (1μg/mL) clearly enhanced the production of iNOS and COX-2. PRG1-1 concentrations between 0 and 150 μg/mL significantly increased the production of iNOS in RAW264.7 cells in a concentration-dependent manner. However, stimulation with 200 μg/mL PRG1-1 led to a lower iNOS level than that produced with 150 μg/mL PRG1-1. This might be due to decreased cell viability at 200 μg/mL. COX-2 production trends in PRG1-1-induced RAW264.7 cells were consistent with the iNOS trends (B).
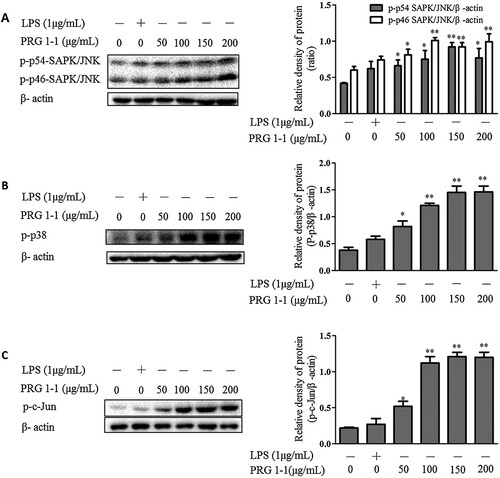
Effects of PRG1-1 on the mitogen-activated protein kinase (MAPK) pathway in RAW264.7 macrophages
MAPK is widely recognized as a typical inflammatory pathway. It plays a crucial role in the regulation of cell growth, differentiation and cellular response to cytokines (Chardin, Schenk, Hirt, Colcombet, & Krapp, Citation2017). We examined whether PRG1-1 induced the secretion of NO and cytokines, including TNF-α and IL-6, through the MAPK pathway. Western blot data showed an increase in the phosphorylation of SAPK/JNK (A), p38 (B) and c-Jun (C) in RAW264.7 cells exposed to PRG1-1 compared with the negative control.
Figure 4. PRG1-1 activates the MAPK pathway in RAW264.7 macrophage cells. Cells were stimulated with PRG1-1 (0, 50, 100, 150 and 200 μg/mL μg/mL) or with LPS (1 μg/mL). Phospho-SAPK/JNK (A), phospho-p38 (B) and phospho-c-Jun (C) protein levels were determined by Western blot analysis. LPS was used as the positive control. Values are presented as the means ± SEM (n = 3). Analyses were performed using a one-way ANOVA. *p < 0.05, **p < 0.01 compared to untreated control.
Effects of PRG1-1 on the NF-κB pathway in RAW264.7 macrophages
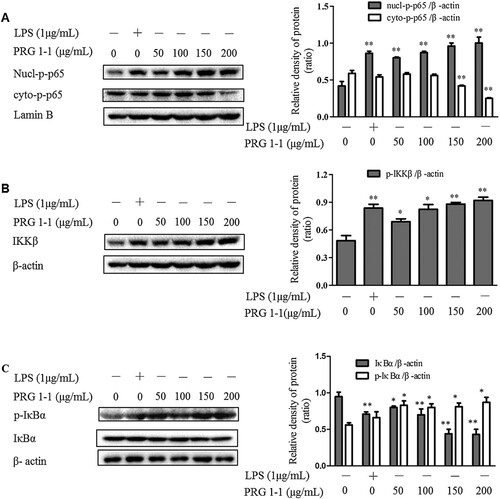
Our results suggested that stimulation with PRG1-1 significantly increased nuclear levels of NF-κB (p65) and reduced cytoplasmic levels of NF-κB (p65) compared with the untreated group (A-B). Furthermore, we found that PRG1-1 increased IKKβ and phospho-IκB-α levels in RAW264.7 cells. Additionally, degradation of IκB-α was observed after treatment with various concentrations of PRG1-1. These results suggested that PRG1-1 treatment activates the NF-κB pathway in RAW264.7 macrophages.
Figure 5. PRG1-1 activates the NF-κB pathway in RAW264.7 macrophage cells. Cells were stimulated with PRG1-1 (0, 50, 100, 150 and 200 μg/mL) or with LPS (1 μg/mL). Protein levels of nucl-phospho-p65, cyto-phospho-p65 (A), IKKβ (B), phospho-IκBα, and IκBα (C) were determined by Western blot analysis. LPS was used as the positive control. Values are presented as means ± SEM (n = 3). Analyses were performed using a one-way ANOVA. *p < 0.05, **p < 0.01 compared to untreated control.
Discussion
Polysaccharides derived from edible mushrooms have been widely investigated. Many mushroom-derived polysaccharides have potential biological activities, including antitumor, antiviral, antioxidant, anti-inflammatory and immunoregulatory activities (Minato, Laan, Ohara, & van Die, Citation2016; Siu, Xu, Chen, & Wu, Citation2016; Tian, Zhao, Zeng, Zhang, & Zheng, Citation2016). In this study, we investigated the immunostimulatory effects of a purified polysaccharide from R. griseocarnosa on macrophages and the mechanism by which PRG1-1 enhances the expression of NO and pro-inflammatory cytokines.
Macrophages play a critical role in the host defensive immune response. These cells eliminate pathogens and recruit other cells to sites of ongoing inflammation by secreting regulatory cytokines [24]. The activation of macrophages promotes the production of NO and pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α. Our results demonstrated that PRG1-1 significantly increased the production of NO in RAW264.7 macrophages compared to the untreated group. It is acknowledged that iNOS and COX-2 are crucial in the regulation of NO and are the key mediators in many inflammatory conditions (Lee, Lee, Shin, Jang, & Lee, Citation2017). PRG1-1 treatment increased the expression levels of iNOS and COX-2, both of which are associated with NO production. The release of NO is involved in many physiological processes, such as vasodilatation (Khan et al., Citation2017), neurotransmission (Kusek et al., Citation2017), immune responses (Cinzia, Maria Pia, Angela, & Marco, Citation2016), and platelet aggregation (Smyth et al., Citation2017). Furthermore, activated macrophages produce cytokines, including IL-6 and TNF-α, that play important roles in the cellular immune process by aiding in the elimination of abnormal cells(Ying et al., Citation2009). In our study, we found that PRG1-1 significantly induced the expression of immunostimulatory cytokines, including TNF-α and IL-6, in macrophage cells. NO, TNF-α, and IL-6 all play critical roles in the development of innate immunity during septic shock and inflammation (Zhao et al., Citation2007). Based on the results above, we concluded that PRG1-1 is involved in immune enhancement in RAW264.7 cells.
To elucidate the possible mechanism by which PRG1-1 activates the MAPK pathway in RAW264.7 macrophage cells, we examined the effects of PRG1-1 on p-SAPK/JNK, p-p38 and p–c-Jun. SAPK/JNK and p38 are members of the MAPK family. We found that the MAPK pathway was activated by PRG1-1 through enhancement of SAPK/JNK and p38 phosphorylation. MAPKs are Ser/Thr protein kinases that convert extracellular stimuli into a wide range of cellular responses, including the production of macrophage-related cytokines and chemokines. When SAPK/JNK is activated as a dimer, it can translocate into the nucleus and regulate transcription through c-Jun. Our results showed that c-Jun phosphorylation levels were increased upon PRG1-1 treatment in a concentration dependent manner. In addition, we found that PRG1-1 activated the NF-κB pathway. NF-κB is an essential transcription factor that regulates genes involved in both the innate and adaptive immune responses. Our results showed that PRG1-1 induced phosphorylation and degradation of IκBα, up-regulated the phosphorylation of p65 in the nucleus, and down-regulated the phosphorylation of p65 in the cytoplasm. These data suggested that PRG1-1 enables the nuclear translocation of NF-κB. Therefore, PRG1-1 exerts its immunostimulatory effects through the activation of the MAPK and NF-κB pathways in RAW264.7 macrophages.
The immunoregulatory activity of polysaccharides has raised the interest of many researchers. Minato reported that Pleurotus citrinopileatus-derived polysaccharides could induce the activation of human dendritic cells through multiple pathways(Minato et al., Citation2016). Wu found that polysaccharides derived from Boletus edulis had anti-inflammatory effects in the pathology of asthma (Wu, Wang, Yang, & Cui, Citation2016). Many studies suggest that polysaccharides from herbal medicines, plants and mushrooms function as immunomodulators by recognizing cell surface receptors (such as TLRs) to trigger an immune response (J. Chen & Seviour, Citation2007; Zhang, Liu, Peng, Han, & Yang, Citation2014). Additionally, it has been reported that the MAPK pathway plays a critical role in TLR4 signalling and the production of pro-inflammatory mediators (Kawai & Akira, Citation2010). In this study, we robustly demonstrated that PRG1-1 enhances cytokine production in RAW264.7 cells through the MAPK and NF-κB pathways. The RAW264.7 receptors targeted by PRG1-1 still need to be identified.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Zhaoli Meng http://orcid.org/0000-0002-4908-7253
Additional information
Funding
References
- Belardelli, F. (1995). Role of interferons and other cytokines in the regulation of the immune response. APMIS, 103(1-6), 161–179. doi: https://doi.org/10.1111/j.1699-0463.1995.tb01092.x
- Boscá, L., Zeini, M., Través, P. G., & Hortelano, S. (2005). Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology, 208(2), 249–258. doi: https://doi.org/10.1016/j.tox.2004.11.035
- Chardin, C., Schenk, S. T., Hirt, H., Colcombet, J., & Krapp, A. (2017). Review: Mitogen-activated protein kinases in nutritional signaling in arabidopsis. Plant Science, 260, 101–108. doi: https://doi.org/10.1016/j.plantsci.2017.04.006
- Chen, J., & Seviour, R. (2007). Medicinal importance of fungal β-(1→3), (1→6)-glucans. Mycological Research, 111(6), 635–652. doi: https://doi.org/10.1016/j.mycres.2007.02.011
- Chen, X.-H., Xia, L.-X., Zhou, H.-B., & Qiu, G.-Z. (2010). Chemical composition and antioxidant activities of Russula griseocarnosa sp. nov. Journal of Agricultural and Food Chemistry, 58(11), 6966–6971. doi: https://doi.org/10.1021/jf1011775
- Chen, X., Zhang, S., Xuan, Z., Ge, D., Chen, X., Zhang, J., Liu, B. (2017). The phenolic fraction of mentha haplocalyx and its constituent linarin ameliorate inflammatory response through inactivation of NF-κB and MAPKs in lipopolysaccharide-induced RAW264.7 cells. Molecules, 22(5). doi: https://doi.org/10.3390/molecules22050811
- Cinzia, F., Maria Pia, A., Angela, S., & Marco, C. (2016). Immunoregulatory and effector activities of nitric oxide and reactive nitrogen species in cancer. Current Medicinal Chemistry, 23(24), 2618–2636. doi: https://doi.org/10.2174/0929867323666160727105101
- Deng, C., Shang, J., Fu, H., Chen, J., Liu, H., & Chen, J. (2016). Mechanism of the immunostimulatory activity by a polysaccharide from Dictyophora indusiata. International Journal of Biological Macromolecules, 91, 752–759. doi: https://doi.org/10.1016/j.ijbiomac.2016.06.024
- Doyle, S. L., & O’Neill, L. A. J. (2006). Toll-like receptors: From the discovery of NF-κB to new insights into transcriptional regulations in innate immunity. Biochemical Pharmacology, 72(9), 1102–1113. doi: https://doi.org/10.1016/j.bcp.2006.07.010
- Du, B., Yang, Y., Bian, Z., & Xu, B. (2017). Molecular weight and helix conformation determine intestinal anti-inflammatory effects of exopolysaccharide from Schizophyllum commune. Carbohydrate Polymers, 172, 68–77. doi: https://doi.org/10.1016/j.carbpol.2017.05.032
- Fu, H., Deng, C., Teng, L., Yu, L., Su, T., Xu, X., … Yang, C. (2015). Immunomodulatory activities on RAW 264.7 macrophages of a polysaccharide from veiled lady mushroom. Dictyophora indusiata (Higher Basidiomycetes), 17(2), 151–160. doi: https://doi.org/10.1615/IntJMedMushrooms.v17.i2.60
- Han, X.-Q., Chung Lap Chan, B., Dong, C.-X., Yang, Y.-H., Ko, C.-H., Gar-Lee Yue, G., … Han, Q.-B. (2012). Isolation, structure characterization, and immunomodulating activity of a hyperbranched polysaccharide from the fruiting bodies of Ganoderma sinense. Journal of Agricultural and Food Chemistry, 60(17), 4276–4281. doi: https://doi.org/10.1021/jf205056u
- Junt, T., Moseman, E. A., Iannacone, M., Massberg, S., Lang, P. A., Boes, M., von Andrian, U. H. (2007). Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature, 450(7166), 110–114. Retrieved from http://www.nature.com/nature/journal/v450/n7166/suppinfo/nature06287_S1.html doi: https://doi.org/10.1038/nature06287
- Kawai, T., & Akira, S. (2010). The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nature Immunology, 11(5), 373–384. doi: https://doi.org/10.1038/ni.1863
- Khan, S. G., Melikian, N., Shabeeh, H., Cabaco, A. R., Martin, K., Khan, F., … Shah, A. M. (2017). The human coronary vasodilatory response to acute mental stress is mediated by neuronal nitric oxide synthase. American Journal of Physiology - Heart and Circulatory Physiology, 313(3), H578–H583. doi: https://doi.org/10.1152/ajpheart.00745.2016
- Kim, H. K., Cheon, B. S., Kim, Y. H., Kim, S. Y., & Kim, H. P. (1999). Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochemical Pharmacology, 58(5), 759–765. doi: https://doi.org/10.1016/S0006-2952(99)00160-4
- Kusek, M., Tokarska, A., Siwiec, M., Gadek-Michalska, A., Szewczyk, B., Hess, G., & Tokarski, K. (2017). Nitric oxide synthase inhibitor attenuates the effects of repeated restraint stress on synaptic transmission in the paraventricular nucleus of the rat hypothalamus. Frontiers in Cellular Neuroscience, 11, 127. doi: https://doi.org/10.3389/fncel.2017.00127
- Lee, S.-B., Lee, W. S., Shin, J.-S., Jang, D. S., & Lee, K. T. (2017). Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW 264.7 macrophages. International Immunopharmacology, 49, 21–29. doi: https://doi.org/10.1016/j.intimp.2017.05.021
- Li, M., Liang, J., Li, Y., Feng, B., Yang, Z.-L., James, T. Y., & Xu, J. (2010). Genetic diversity of dahongjun, the commercially important “big red mushroom” from southern China. PLOS ONE, 5(5), e10684. doi: https://doi.org/10.1371/journal.pone.0010684
- Liu, Y., Zhang, J., & Meng, Z. (2017). Purification, characterization and anti-tumor activities of polysaccharides extracted from wild Russula griseocarnosa. International Journal of Biological Macromolecules. doi: https://doi.org/10.1016/j.ijbiomac.2017.11.093
- Minato, K.-I., Laan, L. C., Ohara, A., & van Die, I. (2016). Pleurotus citrinopileatus polysaccharide induces activation of human dendritic cells through multiple pathways. International Immunopharmacology, 40, 156–163. doi: https://doi.org/10.1016/j.intimp.2016.08.034
- Pan, H., Han, Y., Huang, J., Yu, X., Jiao, C., Yang, X., … Yang, B. B. (2015). Purification and identification of a polysaccharide from medicinal mushroom Amauroderma rude with immunomodulatory activity and inhibitory effect on tumor growth. Oncotarget, 6(19), 17777–17791. doi: https://doi.org/10.18632/oncotarget.4397
- Sheng, Y., Liu, G., Wang, M., Lv, Z., & Du, P. (2017). A selenium polysaccharide from Platycodon grandiflorum rescues PC12 cell death caused by H2O2 via inhibiting oxidative stress. International Journal of Biological Macromolecules, 104(Part A), 393–399. doi: https://doi.org/10.1016/j.ijbiomac.2017.06.052
- Shi, C., & Pamer, E. G. (2011). Monocyte recruitment during infection and inflammation. Nature Reviews. Immunology, 11(11), 762–774. doi: https://doi.org/10.1038/nri3070
- Siu, K.-C., Xu, L., Chen, X., & Wu, J.-Y. (2016). Molecular properties and antioxidant activities of polysaccharides isolated from alkaline extract of wild Armillaria ostoyae mushrooms. Carbohydrate Polymers, 137, 739–746. doi: https://doi.org/10.1016/j.carbpol.2015.05.061
- Smyth, E., Solomon, A., Birrell, M. A., Smallwood, M. J., Winyard, P. G., Tetley, T. D., & Emerson, M. (2017). Influence of inflammation and nitric oxide upon platelet aggregation following deposition of diesel exhaust particles in the airways. British Journal of Pharmacology, 174(13), 2130–2139. doi: https://doi.org/10.1111/bph.13831
- Tian, Y., Zhao, Y., Zeng, H., Zhang, Y., & Zheng, B. (2016). Structural characterization of a novel neutral polysaccharide from Lentinus giganteus and its antitumor activity through inducing apoptosis. Carbohydrate Polymers, 154, 231–240. doi: https://doi.org/10.1016/j.carbpol.2016.08.059
- Vitenberga, Z., & Pilmane, M. (2017). Inflammatory, anti-inflammatory and regulatory cytokines in relatively healthy lung tissue as an essential part of the local immune system. Biomedical Papers, 161(2), 164–173. doi: https://doi.org/10.5507/bp.2017.029
- Wang, J., & Mazza, G. (2002). Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor α in LPS/IFN-γ-activated RAW 264.7 macrophages. Journal of Agricultural and Food Chemistry, 50(15), 4183–4189. doi: https://doi.org/10.1021/jf011613d
- Wu, S., Wang, G., Yang, R., & Cui, Y. (2016). Anti-inflammatory effects of Boletus edulis polysaccharide on asthma pathology. American Journal of Translational Research, 8(10), 4478–4489.
- Yamac, M., Zeytinoglu, M., Senturk, H., Kartkaya, K., Kanbak, G., Bayramoglu, G., Van Griensven, L. J. L. D. (2016). Effects of black hoof medicinal mushroom, Phellinus linteus (agaricomycetes). Polysaccharide Extract in Streptozotocin-Induced Diabetic Rats, 18(4), 301–311. doi: https://doi.org/10.1615/IntJMedMushrooms.v18.i4.30
- Ying, L., Fang, J., Yang, Q., Wei, L., Ying, Q., Chixia, T., … Chunying, C. (2009). Immunostimulatory properties and enhanced TNF- α mediated cellular immunity for tumor therapy by C60 (OH)20 nanoparticles. Nanotechnology, 20(41), 415102. doi: https://doi.org/10.1088/0957-4484/20/41/415102
- Yuan, Y., Liu, Y., Liu, M., Chen, Q., Jiao, Y., Liu, Y., & Meng, Z. (2017). Optimization extraction and bioactivities of polysaccharide from wild Russula griseocarnosa. Saudi Pharmaceutical Journal, 25(4), 523–530. doi: https://doi.org/10.1016/j.jsps.2017.04.018
- Zhang, P., Liu, W., Peng, Y., Han, B., & Yang, Y. (2014). Toll like receptor 4 (TLR4) mediates the stimulating activities of chitosan oligosaccharide on macrophages. International Immunopharmacology, 23(1), 254–261. doi: https://doi.org/10.1016/j.intimp.2014.09.007
- Zhao, X., Zhang, Y., Strong, R., Zhang, J., Grotta, J. C., & Aronowski, J. (2007). Distinct patterns of intracerebral hemorrhage-induced alterations in NF-κB subunit, iNOS, and COX-2 expression. Journal of Neurochemistry, 101(3), 652–663. doi: https://doi.org/10.1111/j.1471-4159.2006.04414.x