ABSTRACT
Ara h 1 is a key peanut allergen and its activity is significantly affected by protein intrinsic structure which is found to be regulated by heat treatment, although the molecular basis for this regulation has remained largely unknown. Here, we explored the effect of boiling on the structure and allergenicity of recombinant peanut protein Ara h 1 (rAra h 1). rAra h 1 was purified from E. Coli BL21(DE3) plysS cells and structurally studied. According to the results, rAra h 1 undergoes degradation during the heating process, and the aggregation of fragments happened after 20 min of heating. An increased surface hydrophobic index and a decreased content of α-helixes were found in rAra h 1, indicating a looser protein structure of rAra h 1 caused by heat treatment. Destroyed epitopes during protein degradation and aggregation could be a mechanism of reducing the allergenic nature of rAra h 1 by heat treatment.
Introduction
Allergy to peanut is a common, severe and generally persistent medical disorder, affecting more than 1% of children all over the world (Sicherer & Sampson, Citation2014; Teodorowicz, Fiedorowicz, Kostyra, Wichers, & Kostyra, Citation2013). There are currently no effective therapies for peanut allergy; peanut-free diet is the only option for patients (Burks et al., Citation2015; Hurlburt, McBride, Nesbit, Ruan, & Maleki, Citation2014). There are growing numbers of patients in North America and several European countries (about threefold increase noted from 1997 to 2008) (Bunyavanich et al., Citation2014; Du Toit et al., Citation2008), while the prevalence in China is not as high as it is in the western countries (Beyer et al., Citation2001; Lee, Thalayasingam, & Lee, Citation2013). It has been reported that heat treatment like boiling and roasting can modify the structure of allergens existing in the peanut and therefore change the sensitivity of the peanut (Niess et al., Citation2011; Shen et al., Citation2015; Vissers et al., Citation2011).
Peanut allergens surrounded by other food matrixes, like sugar and fat, could be easily modified structurally and thus produce advanced glycation end products (AGEs) during the thermal process (Mattison et al., Citation2015; Moghaddam et al., Citation2014).Choosing one specific allergen as subject can help us understand how the thermal process, instead of the other components existing inside of the peanut kernel, affects the structure and allergenicity of the allergen. Ara h 1 is one of the dominant allergens in peanut. It was reported that 55-95% of peanut allergic patients have serum-specific IgE against this protein (Blanc et al., Citation2011), and there are approximately 90% of subjects with peanut allergy having antibodies against Ara h 1 (Ramesh et al., Citation2016). Ara h 1 is a vicilin, member of the 7S globulin family and makes up roughly 15% of the peanut kernel (Hurlburt et al., Citation2014).
Lots of experiments have been conducted to explore effective methods which can be used to eliminate or lower the sensitivity of Ara h 1. It has been reported that peanut roasted over 130°C for 20 min can significantly reduce the solubility and sensitivity of Ara h 1 (Rao et al., Citation2016). However, more extreme thermal processing such as roasting at 140°C appeared to enhance IgE-binding capacity of Ara h 1 (Rao et al., Citation2016). According to Blanc et al. (Citation2011), Ara h 1 boiled in the absence or presence of glucose produced aggregates and thus maintained a relatively lower sensitivity. Researchers utilized various thermal methods to treat peanut kernel or a certain allergen and explored the influence of heat treatment on the immunoreactivity, and the results were not consistent with each other. That is possibly because of the different experimental conditions and the complex food matrix existing inside the peanut kernel.
Besides, it has been reported that recombinant allergens have a similar sensitivity like the natural ones and can be utilized as vaccine in the allergy treatment (Perez-Riverol, Justo-Jacomini, Zollner, & Brochetto-Braga, Citation2015; Valenta, Campana, Focke-Tejkl, & Niederberger, Citation2016). According to the results of animal experiments, hypoallergenic derivatives converted from recombinant allergens, which exhibit decreased IgE-binding capacity and retain T cell reactivity, could be used in allergen-specific immunotherapy. For instance, recombinant allergen Ara h 2 was reported to have a steadily decreased immunogenicity over thermal processing, and thus could be considered for immunotherapy (Shen et al., Citation2015). Advancement in the field of recombinant DNA technology has paved the way for improved diagnosis and research on food allergy (Satitsuksanoa, Głobińska, Jansen, van de Veen, & Akdis, Citation2018).
Clarifying the mechanism of how the sensitivity of recombinant Ara h 1 is influenced by heat treatment is essential for the vaccine development. In sum, we expect to analyse the impacts of boiling on the structure and immunoreactivity of rAra h 1, and reveal the relationship between molecular features and sensitivity change of rAra h 1. Results from this research would bring new understanding to the sensitization mechanism of peanut allergen and the guidance of hypoallergenic food processing.
Methods
Protein preparations
The coding DNA of full-length Ara h 1 adding 6 × his-tag was cloned into a pET-32a expression vector by Shenggong Biology Company (Shanghai, China). The expression of rAra h 1 was purified as previously described (Wu et al., Citation2015) with some modifications. Briefly, the recombinant plasmids pET-32a-Ara h 1 was transformed into Escherichia coli BL21(DE3) pLysS cells. Single clones selected on the ampicillin plate were grown at 37°C until the OD600 reached 0.6. 300 mM IPTG (isopropyl-β-D-thiogalactopyranoside) was added and cells were cultured at 24°C overnight (Alves et al., Citation2015). Cell pellets were then resuspended in lysis buffer (50 mM Tris-Cl, 0.1 mM EDTA, 200 mM NaCl, 62 mM Triton, 15 mM SLS, 1 mM PMSF, 1 mM DTT, 1 mg/mL lysome, 20 mM imidazole, pH 8.4) and sonicated. His-tagged rAra h 1 was affinity-purified using Ni-NTA (Qiagen, USA) and collected in elution buffer (50 mM Tris-Cl, 0.1 mM EDTA, 600 mM NaCl, 1 mM DTT, 300 mM imidazole, pH 8.4).
Natural Ara h 1 was purified from raw peanuts (obtained from a local market) as described by Wu (Wu et al., Citation2015) with slight modification. Briefly, peanut kernels were ground with liquid nitrogen, and defatted by acetone and ethyl. Peanut protein was extracted by the extraction buffer (50 mM phosphate, 0.15 M sodium chloride, pH 7.4). DEAE-fast flow column (GE Healthcare Company) was then utilized to purify Ara h 1. By continuously increasing the concentration of sodium chloride to 0.4 mol/L at a flow rate of 1.0 mL/min, Ara h 1 was eluted in the 73rd minute of elution process and collected in the receptor machine. Then, Ara h 1 was separated by Superdex 200 pg gel filtration chromatography (GE Healthcare, USA), eluted with Tris-HCl buffer (50 mM, pH 8.0) at 0.3 mL/min and collected in the 45th minute of elution process.
Heat treatment
Purified rAra h 1 in elution buffer was ultra-filtrated and concentrated to 1.0 mg/mL. Then, the protein buffer was boiled at 100°C for 2–40 min.
SDS-PAGE
Methods from Rao et al. (Citation2016) were modified as follows: protein samples were identified by using sodium dodecyl sulphonate polyacrylamide gel electrophoresis (SDS-PAGE) on a mini-protean II gel apparatus (Bio-Rad, USA). Samples in SDS-loading buffer were boiled and loaded (10 μg/lane) on a 12% polyacrylamide gel and afterwards dyed using Coomassie brilliant blue R250 for 40 min, bleached in destaining solution (1.31 M HAc, 1.23 M methanol) for 12 h. BIO-RAD GelDoc 2000 gel imaging system was used to photograph and analyse the pictures of electrophoresis results.
Western blotting
According to methods reported by Hurlburt et al. (Citation2014), western blots were performed as follows: peanut protein from the SDS-PAGE gel was transferred to a nitrocellulose membrane and blocked for 1 h at room temperature. Then, the membrane was incubated overnight with the rabbit anti-Ara h 1 antibody at 4°C. After washing 5 times, the membrane was incubated with goat anti-rabbit IgE-HRP for 2 h at 37°C. The results were observed using an enhanced chemiluminescense detection system (Kangweishiji, Beijing, China).
ELISA assay
According to Liu et al. (Citation2012), peanut proteins diluted to 10 μg/mL were added into the 96-well plates and incubated at 4°C for 12 h. After washing, Tris Buffered Saline (TBS) (10 mM Tris-HCl, pH 7.5) containing 1% bovine serum albumin (w/v) was utilized to block the plates at 37°C for 1 h and the rabbit anti-Ara h 1 (1:5000, v:v) was added to each microwell. The horseradish peroxidase-labelled goat anti-rabbit IgE (1:4000, v:v) was added after washing the plates. After incubating for 1 h in 37°C, 100 μL TMB reagent (3,3,5,5-tetramethyl benzidine) was added to each well and reacted for 15 min. H2SO4 (2 mol/L) was used to terminate the reaction. The absorbance was measured at 450 nm.
Matrix-Assisted Laser Desorption/lonization Time of Flight Mass Spectrometry (MALDI-TOF MS) analysis
SDS-PAGE-purified rAra h 1 was cut according to the bands that appeared in the gel, and sealed in sterile distilled water before identification. Proteins digested by trypsin were analysed on a Voyager-DE PRO matrix-assisted laser desorption/ionization time of flight (4700 MALDI TOF/TOF) mass spectrometer (Applied Biosystems, USA). Sequence analysis was performed by using the MASCOT (Matrix Sciences, England) system, and the data acquired were compared with data from National the Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/).
Dynamic light scattering
According to the methods reported by Vissers et al. (Citation2011), protein size of rAra h 1 was measured by dynamic light scattering before and after heating. The experiment was performed on a Dynapro NanoStar DLS machine (WYATT, USA). Each sample was measured in triplicate and data were presented as intensity by size distribution.
O-phthaldialdehyde assay
Methods from Małgorzata, Konrad, and Zieliński (Citation2016) were modified as follows: after being mixed with the O-phthaldialdehyde assay (OPA) reagent in a ratio of 1:10 (v/v), the protein solution (250 mg/mL) was then incubated for 20 min at 25°C. The absorbance was measured at 340 nm and samples were measured in triplicate. Unreacted amino groups were estimated from a calibration curve established with L-leucine.
Circular dichroism spectra
A Chirascan spectroscope (Applied Photophysics Ltd, England) was used for Circular dichroism (CD) measurements. The scanning interval ranged from 190 to 250 nm, the scanning speed is 500 nm/min and the accumulation frequency is 3. All the data were analysed by CDNN software package (Applied Photophysics Ltd, England).
Protein surface hydrophobic measurement (H0)
According to Mondoulet et al. (Citation2005), a fluorescent probe method was used to measure the surface hydrophobic index of protein. Protein samples were diluted to 0.2, 0.1, 0.05, 0.025 and 0.0125 mg/mL. 20 μL of 8 mmol/L 8-benzene amino-1-naphthalene sulfonic acid was added to 4 mL protein samples. A Hitachi F2500 fluorescence spectrometer (Tokyo, Japan) was used to measure the fluorescence intensity of mixture, with 390 nm as the excitation wavelength and 470 nm as emission wavelength. The initial slope of fluorescence intensity versus protein concentration plot was used as an index of H0.
Mediator release assay
RBL-2H3 cell (Kebai Biology Company, Nanjing, China) was utilized to analyse the sensitivity of rAra h 1 (Vissers et al., Citation2011). Cells were cultured in minimum Eagle's medium (MEM) medium supplemented with 15% fetal calf serum at 37°C in a humidified atmosphere with 5% CO2. Cells in the stationary growth phase were harvested and plated in plates at 1.5 × 106 cells/mL. Balb/c mice serum was added and incubated overnight. After washing, the cells were stimulated for 1 h with the allergens diluted in Tyrode’s buffer containing 50% deuterium oxide (Sayers et al., Citation2016). The sensitivity of allergen was quantified by measuring β-hexosaminidase activity and presented as percentage of the total β-hexosaminidase content which will be measured by lysing the cells with 1% Triton X-100.
Statistical analysis
The statistical analysis was performed using SPSS Software (v15.0, SPSS Inc., USA). The difference was considered statistically significant when P-values were less than .05.
Results
Expression and purification of (r)Ara h 1
As seen in (A), rAra h 1 expression was successfully induced in strain BL21(DE3) pLysS. Following growth to mid-log phase, virtually no rAra h 1 was observed on the SDS-PAGE gel of whole cells (lane 1). However, reasonable induction was achieved in the same clones treated with IPTG for 24 h (lane 2). The arrow indicates the position of inducibly expressed Ara h 1. Solubility of the protein was examined following lysis and centrifugation. The results are shown in (A) (lanes 3 and 4). It appears that more than half of the expressed protein is in the soluble fraction ((A), lane 3), and there is roughly 45% of rAra h 1 contained in the sediment ((A), lane 4). There appear to be a few proteolytic cleavage products in both the soluble and insoluble fractions. Ni-beads binding with the supernate of lysis buffer have been also identified in (A) lane 5; it appears that the rAra h 1 could attach to the Ni-NTA beads effectively and thus could be purified in the elution process ((A), lanes 6–8). Besides, natural Ara h 1 (63.5 kDa) was purified from peanuts and identified by SDS-PAGE ((C), lanes 9–12).
Figure 1. The purification and identification of (r)Ara h 1. (A) SDS-PAGE analysis of recombinant protein, M: protein maker, 1: protein expression without IPTG induction, 2: protein expression after IPTG induction, 3: protein expression in the supernatant of lysis buffer, 4: protein expression in the sediment after lysis process, 5: Ni-beads after binding with the supernatant of lysis buffer, 6–8: eluted protein by using Ni-affinity chromatography. (B) The identification of rAra h 1 by using MALDI-TOF analysis. Nine characteristic peptides are matched to the Ara h 1, and the match rate is calculated to be 426. (C) SDS-PAGE analysis of purified natural Ara h 1, M: protein maker, 9–10: eluted protein by using anion change chromatography, 11–12: eluted protein by using gel chromatography.
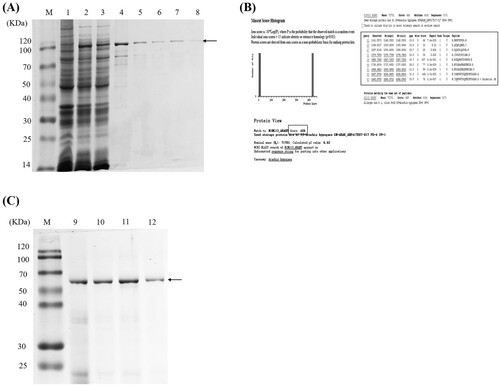
Identification of purified rAra h 1 by MALDI-TOF MS
To verify the purified protein from BL21(DE3) pLysS cells was exactly Ara h 1, affinity-purified protein was separated by SDS-PAGE. The indicated regions enriched with bands at 110 kDa were cut and identified via MALDI-TOF MS analysis ((B)). There are nine typical peptides identified and matched well with natural Ara h 1, indicating that the purified rAra h1 shares a similar amino acids sequence (score: 426) with natural Ara h 1. Furthermore, another band (75 kDa), which appeared in the supernate and sediment of lysis buffer, was analysed by MALDI-TOF MS and the results showed that this band also matches with natural Ara h1 by score 248 (supporting material 2).
Heat treatment altered the constitution and allergenicity of rAra h 1
According to (A), the bands of the protein extracts became shallower after being treated with heat, indicating that the soluble protein contents in samples were decreased. And there appeared some bands with low molecular weight, which are assumed to be the degradation products of rAra h 1. On the contrary, rAra h 1 without heat treatment remains a relatively high intensity.
Figure 2. The effect of heat treatment on the conformation and allergenicity of rAra h 1. (A) SDS-PAGE analysis of heat-treated Ara h 1. M: protein maker, C: raw rAra h 1 without heating, 2–40 refer to rAra h 1 heated for 2, 6, 10, 14, 20, 30, 40 min, respectively. (B) Detection of allergen contents and IgE-binding efficiency by using western blot. C: raw rAra h 1 without heating, 2–40 refer to rAra h 1 heated for 2, 6, 10, 14, 20, 30, 40 min, respectively. (C) ELISA assay with natural Ara h 1, raw and heat-treated rAra h 1s. Values were means of three determinations. Statistical analyses were performed using Student’s t-test at 95% confidence.
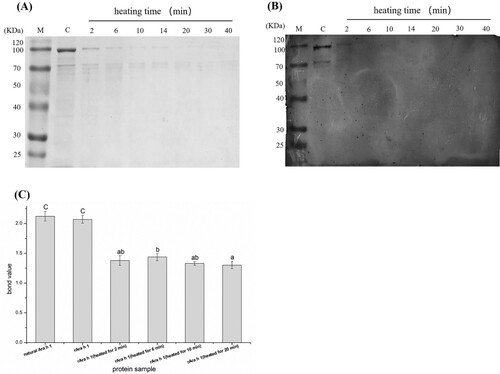
The binding efficiency between rAra h 1 and antibody was evaluated by western blot. As seen in (B), bands can be seen in the first two tracks and approximately no bands in the other lanes, demonstrating that the immunogenicity decreased significantly in heat-treated rAra h 1s. ELISA was used to quantify the bound values between allergen and antibody. According to (C), the sensitivity of rAra h 1 was similar with natural Ara h 1. Recombinant Ara h 1 without heat treatment maintained the highest sensitivity, while rAra h 1 heated for 2 min and 10 min have a similar allergenicity, which is also significantly lower than that of raw rAra h 1. When heated for 20 min, the sensitivity of rAra h 1 reaches the bottom among the five kinds of rAra h 1s. Heating for a longer time cannot assure a relatively low level of allergenicity of rAra h 1, because the binding efficiency between rAra h 1 and its antibody does not decrease gradually with the extension of heating time.
Mediator release of RBL-2H3 cell induced by rAra h 1 treated with heat
The IgE cross-linking capacities of raw and heated rAra h 1 were determined using degranulation tests that were performed using passively sensitized rat stripped basophils. As seen in (A), after being heated for 10 min, the allergenicity of rAra h 1 decreases gradually along with the increasing concentration, showing a degranulation rate of 47% at a protein concentration of 1 ng/mL compared with 33% at 1 mg/mL. When heated for 2, 6 and 20 min, the trend of sensitivity change presents an initial increase and then decreases along with the increased concentration of rAra h 1.
Figure 3. β-hexosaminidase release of RBL-2H3 cell stimulated by heat-treated rAra h 1. (A) β-hexosaminidase released by an RBL-2H3 cell, which was stimulated by different concentrations of heat-treated rAra h 1. (B) β-hexosaminidase released by RBL-2H3 cell stimulated by rAra h 1, which was heat treated for a different time.
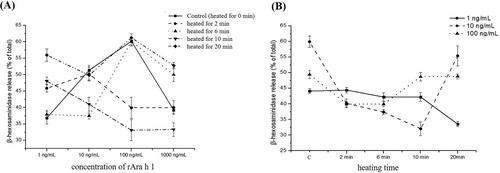
According to (B), non-heated rAra h 1 demonstrated a profoundly higher biological activity than heated rAra h 1. When the concentration of rAra h 1 is 1 ng/mL and 100 ng/mL, the β-hexosaminidase release rate of raw allergen is higher than that of heat-treated ones. When the concentration of rAra h 1 reached 10 ng/mL, the β-hexosaminidase release rate showed an obvious drop in the first 10 min, and then a significant increase in the next 10 min. The sensitivity of rAra h 1 decreased after heat treatment, and the degree of reduction is not in accordance with the length of heating time. This observation is consistent with the result of ELISA assay.
Heat treatment caused degradation and aggregation of rAra h 1
According to (A), the content of free amino groups of the heated rAra h 1 samples showed a consistent increase during the heating process, starting from 100% (raw) to about 140% (heated for 20 min). The growing number of free amino acids indicates the existence of protein degradation. However, the surface hydrophobicity index showed a gradual increase during the first half period of the heating process, indicating the hydrophobic groups of the rAra h 1 were more exposed to the outside and the protein structure was looser. Then, the index showed a drop when the heating time extended to 20 min, only slightly higher than the controlled one.
Figure 4. The aggregation and degradation of rAra h 1. (A) The content of amino acids and surface hydrophobic index of raw and heat-treated rAra h 1s. (B) The molecular size of raw and 6-, 10-, 20-min heat-treated rAra h 1.
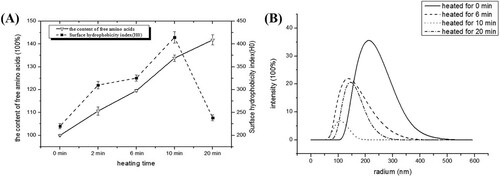
The molecular sizes were measured by dynamic light scattering. According to (B), most particles that existed in the raw rAra h 1 sample were at a radium range of 220–230 nm. The common sizes of molecules that existed in the 6- and 10-min heated samples were 145 and 100 nm, respectively. However, after being heated for 20 min, most molecules were at a range of 140–150 nm, which is bigger than the common size of particles that existed in the 10 min-heated sample. It can also be deducted that the parts of these degraded polypeptides that were produced in the earlier stage of heating process were assembled into much larger aggregated structures when the heating time reached 20 min. This result is consistent with (A), in which the surface hydrophobicity index showed a drop when heated for 20 min, demonstrating a cross linking happened between small fractions. It is clear that in the first stage of boiling (up to 10 min), the particles tend to degrade and more free amino acids were released to the buffer. However, when heated for 20 min, the interaction between molecules became stronger and thus polymers were produced.
The effect of heat treatment on the secondary structure of rAra h 1
Circular dichroism was utilized to explore the secondary structural changes in rAra h 1. According to (A), the curves of natural and recombinant Ara h 1 were coinciding with each other, indicating the secondary structure of those two proteins were similar. There were typical α-helixes that existed in raw and heat-treated rAra h 1 (peaks in 192 nm and negative spikes in 208 and 222 nm). Heating caused a partial loss of secondary structure, as indicated by the lowered positive absorption at 192–195 nm in heat-treated samples. Furthermore, rAra h 1 heated for 20 min appeared to be highly denatured having only a slight positive absorption at 192 nm. The positive spectrum band in 185 nm indicated that β-sheet existed in the raw and heat-treated rAra h 1. Compared with the raw rAra h 1, there was a relatively lowered peak that appeared in 185 nm of 20-min heat-treated rAra h 1, which indicated that the content of β-sheet is the least among five kinds of Ara h 1.
Figure 5. The effect of heat treatment on the secondary structure of rAra h 1. (A) CD spectra of natural Ara h 1, raw and heat-treated rAra h 1s. (B) The content of different kinds of secondary structures of natural Ara h 1, raw and heat-treated rAra h 1s.
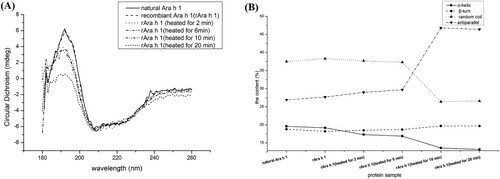
The proportion of α-helixes, β-turns, random coils and antiparallel were analysed via CDNN software ((B)). Compared with natural Ara h 1 and rAra h 1, the heat-treated protein had a gradual reducing α-helical content among the heating process, from 18.2% in raw rAra h 1 to 13.2% in 20-min heat-treated rAra h 1. However, the content of β-turns in the samples presented a slight increase during the heating process, rising from 18.2% in raw rAra h 1 to 19.7% in 20-min heat-treated rAra h 1. In the meantime, the amount of antiparallel was increased significantly among the whole heating process, which demonstrated that re-folding, re-curling and re-swing of proteins appeared in the heating process.
Discussion
Peanuts that experienced heat treatment commonly have an attractive aroma and tasteful quality. However, several studies have testified that thermal processing can enhance or reduce the immunoreactivity of peanuts (Rao et al., Citation2016; Shen et al., Citation2015; Teodorowicz et al., Citation2013). Boiling has been proved by researches that can reduce the allergenicity of peanut allergens mainly because the low-molecular-weight allergens that existed inside of the peanut kernel would transfer into the cooking water (Schmitt, Nesbit, Hurlburt, Cheng, & Maleki, Citation2010; Starkl, Citation2011), and thus produce a relatively low sensitivity peanut maintaining decreased content of allergen. Besides, the water molecules would attack the allergens and thus cause alterations to their structures. Epitopes mapping on the quaternary structure of allergens could be destroyed, the secondary structures like α-helixes and β-sheets would collapse (Rahaman, Vasiljevic, & Ramchandran, Citation2016) and therefore cannot protect the epitopes anymore (Blanc et al., Citation2011). In this research, we studied the effects of boiling on the structure and immunoreactivity of rAra h 1, and tried to reveal the relationship between conformational alterations and sensitivity change.
Immune response of allergens is significantly affected by protein molecular structure which is found to be regulated by heat treatment. Previous studies show that proteins tend to unfold on heating at temperatures over 90°C, and then adopt a random coil conformation and form dimers and higher-order oligomers (Peng, Ren, & Guo, Citation2016; Wolz & Kulozik, Citation2015), accompanied by hydrolysis on the peptide backbone (Long et al., Citation2016; Niess et al., Citation2011). The compound formed by Ara h 1 and Ara h 6 has a strong immunoreactivity when compared with the native allergen (Guillon, Bernard, Drumare, Hazebrouck, & Adel-Patient, Citation2016). It has also been reported by Blanc et al. (Citation2011) that complex branched aggregates of natural Ara h 1 were produced through boiling, and thus caused a relatively decreased sensitivity. Similar to this result, our data showed that rAra h 1 tends to aggregate after 20 min of heating ((A,B)). Such structural features account for the decreased IgE-binding capacity of rAra h 1 and are consistent with previous observations that boiled natural Ara h 1 had a reduced capacity to elicit histamine release (Blanc et al., Citation2011). Along with the wet-thermal process, the allergenicity presented a dynamic change and reached the bottom value when the heating time extended to 20 min ((C) and (B)). This may be because conformational epitopes get more easily destroyed the thermal process than linear epitopes (Mills, Sancho, Rigby, Jenkins, & Mackie, Citation2009), oligomers produced during the heating process caused the formation of new intro-intermolecular interactions, which could help to decrease the binding efficiency between allergens and antibodies.
As seen in (A), the surface hydrophobicity index of 20-min heat-treated rAra h 1 was the minimum among the five kinds of rAra h 1. It is estimated that epitopes will find it much harder to bind antibodies when the hydrophobic interaction becomes weaker, due to the blocking of lysine and/or arginine residues (Chen et al., Citation2016). After heated for 20 minutes, hydrophobic side chains of amino acids are estimated to be driven into the inner part of protein. Reversible unfolding of the protein structure and the rearrangements of disulfide bonds would lead to the formation of aggregates, which could lock the epitopes to the inner part of molecues and thus lower the sensitivity of the allergen. On the contrary, allergens extracted from baked peanuts were reported to have a higher surface hydrophobic index and proved to have a stronger IgE-binding efficiency (Rao et al., Citation2016), indicating the IgE-binding ability of peanut was positively correlated with the surface hydrophobic index.
It is hard to say that longer the boiling time can consequently reduce the immunoreactivity of rAra h 1, but we can see that the sensitivity of rAra h 1 decreased after heating ( and ). Similar to natural Ara h 1, boiling for 20 min did not completely abrogate the IgE reactivity of rAra h 1 (Blanc et al., Citation2011). The mechanism of how construction alteration modulates the sensitivity of rAra h 1 could be explained from two aspects. On the one hand, degradation can damage the complex structure of allergens and release the epitopes. On the other hand, the conformational epitopes mapping on the surface of protein molecules will be easily destroyed during the thermal process. The dynamic changes of protein structure can alter the sensitivity of rAra h 1, and there is no linear relationship between these two, parameters like heating time and temperature could have a strong influence on the immunoreactivity of allergens (Rao et al., Citation2016; Sayers et al., Citation2016). According to our results, physical processes like boiling can induce the generation of allergen polymers, which has a relatively lower sensitivity and can be provided as a novel form of antigen in immunotherapy.
Conclusion
To clarify the connections between heat treatment and allergenicity alteration of rAra h 1, conformational changes were monitored during the whole thermal process. According to the results, boiling can lower the sensitivity of rAra h 1, but there is no linear relationship between the sensitivity decrease and the length of heating time. During the boiling process, rAra h 1 tends to degrade first, and then the small fractions produced tend to aggregate again when heated for 20 min. Conserved secondary structures like α-helixes showed a decreased content along with the extension of heating time, indicating the destruction impact of the thermal process on the allergen’s construction. Destruction of conformational epitopes could be the reason why immunoreactivity showed a decline when rAra h 1 was treated with heat.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alves, R. C., Pimentel, F. B., Nouws, H. P. A., Marques, R. C. B., González-García, M. B., Oliveira, M. B. P. P., & Delerue-Matos, C. (2015). Detection of Ara h 1 (a major peanut allergen) in food using an electrochemical gold nanoparticle-coated screen-printed immunosensor. Biosensors and Bioelectronics, 64, 19–24. doi: https://doi.org/10.1016/j.bios.2014.08.026
- Beyer, K., Morrowa, E., Li, X.-M., Bardina, L., Bannon, G. A., Burks, A. W., & Sampson, H. A. (2001). Effects of cooking methods on peanut allergenicity. Journal of Allergy and Clinical Immunology, 107(6), 1077–1081. doi: https://doi.org/10.1067/mai.2001.115480
- Blanc, F., Vissers, Y. M., Adel-Patient, K., Rigby, N. M., Mackie, A. R., Gunning, A. P., … Mills, E. N. C. (2011). Boiling peanut Ara h 1 results in the formation of aggregates with reduced allergenicity. Molecular Nutrition & Food Research, 55(12), 1887–1894. doi: https://doi.org/10.1002/mnfr.201100251
- Bunyavanich, S., Rifas-Shiman, S. L., Platts-Mills, T. A. E., Workman, L., Sordillo, J. E., Gillman, M. W., … Litonjua, A. A. (2014). Peanut allergy prevalence among school-age children in a US cohort not selected for any disease. Journal of Allergy and Clinical Immunology, 134(3), 753–755. doi: https://doi.org/10.1016/j.jaci.2014.05.050
- Burks, A. W., Wood, R. A., Jones, S. M., Sicherer, S. H., Fleischer, D. M., Scurlock, A. M., … Sampson, H. A. (2015). Sublingual immunotherapy for peanut allergy: Long-term follow-up of a randomized multicenter trial. Journal of Allergy and Clinical Immunology, 135(5), 1240–1248.e3. doi: https://doi.org/10.1016/j.jaci.2014.12.1917
- Chen, Y., Tu, Z., Wang, H., Zhang, L., Sha, X., Pang, J., … Yang, W. (2016). Glycation of β-lactoglobulin under dynamic high pressure microfluidization treatment: Effects on IgE-binding capacity and conformation. Food Research International, 89, 882–888. doi: https://doi.org/10.1016/j.foodres.2016.10.020
- Du Toit, G., Katz, Y., Sasieni, P., Mesher, D., Maleki, S. J., Fisher, H. R., … Lack, G. (2008). Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. Journal of Allergy and Clinical Immunology, 122(5), 984–991. doi: https://doi.org/10.1016/j.jaci.2008.08.039
- Guillon, B., Bernard, H., Drumare, M.-F., Hazebrouck, S., & Adel-Patient, K. (2016). Heat processing of peanut seed enhances the sensitization potential of the major peanut allergen Ara h 6. Molecular Nutrition & Food Research, 60(12), 2722–2735. doi: https://doi.org/10.1002/mnfr.201500923
- Hurlburt, B., McBride, J., Nesbit, J., Ruan, S., & Maleki, S. (2014). Purification of recombinant peanut allergen Ara h 1 and comparison of IgE binding to the natural protein. Foods (basel, Switzerland), 3(4), 642–657. doi: https://doi.org/10.3390/foods3040642
- Lee, A. J., Thalayasingam, M., & Lee, B. W. (2013). Food allergy in Asia: How does it compare? Asia Pacific Allergy, 3(1), 3. doi: https://doi.org/10.5415/apallergy.2013.3.1.3
- Liu, C.-Y., Tao, S., Xue, J.-Y., Zhang, H., Xue, W.-T., & Chen, F.-S. (2012). Identification and purification of a novel fish allergen from largemouth bass (Micropterus salmoides). Food and Agricultural Immunology, 25(1), 70–81. doi: https://doi.org/10.1080/09540105.2012.745122
- Long, F., Yang, X., Sun, J., Zhong, Q., Wei, J., Qu, P., & Yue, T. (2016). Effects of combined high pressure and thermal treatment on the allergenic potential of peanut in a mouse model of allergy. Innovative Food Science & Emerging Technologies, 35, 133–138. doi: https://doi.org/10.1016/j.ifset.2016.04.003
- Małgorzata, W., Konrad, P. M., & Zieliński, H. (2016). Effect of roasting time of buckwheat groats on the formation of Maillard reaction products and antioxidant capacity. Food Chemistry, 196, 355–358. doi: https://doi.org/10.1016/j.foodchem.2015.09.064
- Mattison, C. P., Dinter, J., Berberich, M. J., Chung, S.-Y., Reed, S. S., Le Gall, S., & Grimm, C. C. (2015). In vitro evaluation of digestive and endolysosomal enzymes to cleave CML-modified Ara h 1 peptides. Food Science & Nutrition, 3(4), 273–283. doi: https://doi.org/10.1002/fsn3.215
- Mills, E. N. C., Sancho, A. I., Rigby, N. M., Jenkins, J. A., & Mackie, A. R. (2009). Impact of food processing on the structural and allergenic properties of food allergens. Molecular Nutrition & Food Research, 53(8), 963–969. doi: https://doi.org/10.1002/mnfr.200800236
- Moghaddam, A. E., Hillson, W. R., Noti, M., Gartlan, K. H., Johnson, S., Thomas, B., … Sattentau, Q. J. (2014). Dry roasting enhances peanut-induced allergic sensitization across mucosal and cutaneous routes in mice. Journal of Allergy and Clinical Immunology, 134(6), 1453–1456. doi: https://doi.org/10.1016/j.jaci.2014.07.032
- Mondoulet, L., Paty, E., Drumare, M. F., AH-Leung, S., Scheinmann, P., Willemot, R. M., … Bernard, H. (2005). Influence of thermal processing on the allergenicity of peanut proteins. Journal of Agricultural and Food Chemistry, 53, 4547–4553 doi: https://doi.org/10.1021/jf050091p
- Niess, J.-H., Vissers, Y. M., Blanc, F., Skov, P. S., Johnson, P. E., Rigby, N. M., … Adel-Patient, K. (2011). Effect of heating and glycation on the allergenicity of 2S albumins (Ara h 2/6) from peanut. PLoS ONE, 6(8), e23998. doi: https://doi.org/10.1371/journal.pone.0023998
- Peng, X., Ren, C., & Guo, S. (2016). Particle formation and gelation of soymilk: Effect of heat. Trends in Food Science & Technology, 54, 138–147. doi: https://doi.org/10.1016/j.tifs.2016.06.005
- Perez-Riverol, A., Justo-Jacomini, D., Zollner, R., & Brochetto-Braga, M. (2015). Facing hymenoptera venom allergy: From natural to recombinant allergens. Toxins, 7(7), 2551–2570. doi: https://doi.org/10.3390/toxins7072551
- Rahaman, T., Vasiljevic, T., & Ramchandran, L. (2016). Effect of processing on conformational changes of food proteins related to allergenicity. Trends in Food Science & Technology, 49, 24–34. doi: https://doi.org/10.1016/j.tifs.2016.01.001
- Ramesh, M., Yuenyongviwat, A., Konstantinou, G. N., Lieberman, J., Pascal, M., Masilamani, M., & Sampson, H. A. (2016). Peanut T-cell epitope discovery: Ara h 1. Journal of Allergy and Clinical Immunology, 137(6), 1764–1771.e4. doi: https://doi.org/10.1016/j.jaci.2015.12.1327
- Rao, H., Tian, Y., Tao, S., Tang, J., Li, X., & Xue, W.-T. (2016). Key factors affecting the immunoreactivity of roasted and boiled peanuts: Temperature and water. LWT – Food Science and Technology, 72, 492–500. doi: https://doi.org/10.1016/j.lwt.2016.05.014
- Satitsuksanoa, P., Głobińska, A., Jansen, K., van de Veen, W., & Akdis, M. (2018). Modified allergens for immunotherapy. Current Allergy and Asthma Reports, 18(2), 3, doi: https://doi.org/10.1007/s11882-018-0766-x
- Sayers, R. L., Johnson, P. E., Marsh, J. T., Barran, P., Brown, H., & Mills, E. N. C. (2016). The effect of thermal processing on the behaviour of peanut allergen peptide targets used in multiple reaction monitoring mass spectrometry experiments. The Analyst, 141(13), 4130–4141. doi: https://doi.org/10.1039/c6an00359a
- Schmitt, D. A., Nesbit, J. B., Hurlburt, B. K., Cheng, H., & Maleki, S. J. (2010). Processing can alter the properties of peanut extract preparations. Journal of Agricultural and Food Chemistry, 58(2), 1138–1143. doi: https://doi.org/10.1021/jf902694j
- Shen, L.-L., Zhu, Q.-Q., Huang, F.-W., Xu, H., Wu, X.-L., Xiao, H.-F., … Liu, Z.-G. (2015). Effect of heat treatment on structure and immunogenicity of recombinant peanut protein Ara h 2.01. LWT – Food Science and Technology, 60(2), 964–969. doi: https://doi.org/10.1016/j.lwt.2014.10.044
- Sicherer, S. H., & Sampson, H. A. (2014). Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. Journal of Allergy and Clinical Immunology, 133(2), 291–307.e5. doi:10.1016/j.jaci.2013.11.020.
- Starkl, P. (2011). Heating affects structure, enterocyte adsorption and signalling, as well as immunogenicity of the peanut allergen Ara h 2. The Open Allergy Journal, 4(1), 24–34. doi: https://doi.org/10.2174/1874838401104010024
- Teodorowicz, M., Fiedorowicz, E., Kostyra, H., Wichers, H., & Kostyra, E. (2013). Effect of Maillard reaction on biochemical properties of peanut 7S globulin (Ara h 1) and its interaction with a human colon cancer cell line (Caco-2). European Journal of Nutrition, 52(8), 1927–1938. doi: https://doi.org/10.1007/s00394-013-0494-x
- Valenta, R., Campana, R., Focke-Tejkl, M., & Niederberger, V. (2016). Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: Lessons from the past and novel mechanisms of action for the future. Journal of Allergy and Clinical Immunology, 137(2), 351–357. doi: https://doi.org/10.1016/j.jaci.2015.12.1299
- Vissers, Y. M., Iwan, M., Adel-Patient, K., Stahl Skov, P., Rigby, N. M., Johnson, P. E., … Wichers, H. J. (2011). Effect of roasting on the allergenicity of major peanut allergens Ara h 1 and Ara h 2/6: the necessity of degranulation assays. Clinical & Experimental Allergy, 41(11), 1631–1642. doi: https://doi.org/10.1111/j.1365-2222.2011.03830.x
- Wolz, M., & Kulozik, U. (2015). Thermal denaturation kinetics of whey proteins at high protein concentrations. International Dairy Journal, 49, 95–101. doi: https://doi.org/10.1016/j.idairyj.2015.05.008
- Wu, Z., Yan, F., Wei, X., Li, X., Tong, P., Yang, A., … Chen, H. (2015). Purification and recombinant expression of major peanut allergen Ara h 1. Preparative Biochemistry and Biotechnology, 45(5), 438–446. doi: https://doi.org/10.1080/10826068.2014.940972
