?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The matrix effect is a serious problem for the immunoassay of antibiotic residues, which greatly restricts its real application in fishery products as well as other foodstuffs. Till now the related information on the colloidal gold immunochromatographic assay (GICA) is still limited. Therefore, five species of fish as samples, and the interference of main matrix components on the analysis of ciprofloxacin was investigated. Some water-soluble proteins were demonstrated to have significant affluence on GICA. Moreover, magnesium is also an important source of the matrix interference was revealed for the first time, and its interaction with some immunoglobin (Ig)G was assumed to be a possible reason for this. Based on these results, corresponding approaches for elimination of the interference were also explored. The matrix interference was fully eliminated and the sensitivity of GICA was increased about 8 times for the quantitative analysis of ciprofloxacin via acid pre-treatment followed by pH adjustment.
Introduction
Recently, GICA is gaining great attention because of its significant advantages such as simplicity, low cost, and suitability for an on-site analysis (Boris, Nadezhda, Alexandr, & Anatoly, Citation2014; Jiachi, Arthur, Hang, & Wing-tak, Citation2015; Mohammad et al., Citation2016). Numerous studies have reported the application of GICA for various food hazards detection including drug residues, toxins, and pathogens (Jiachi et al., Citation2015; Mohammad et al., Citation2016; Wu et al., Citation2017; Xie, Yang, Kong, Yang, & Yang, Citation2015).
In general, GICA performance is divided into two stages: sample pre-treatment and analyte detection. In comparison to the excellent rapidity in the detection stage (usually less than 10 min) (Huang, Zoraida, Xu, Lai, & Xiong, Citation2016), most of the sample pre-treatments process still require time-consuming procedures such as full homogenization, complex extraction, multiple cleaning-up etc, which gives great limitation to the real “rapidity” and “simplicity” of the analysis. In addition, the real application of GICA is also limited by its relatively poor accuracy and even “false positive” results (Du, Lin, Sui, Wang, & Cao, Citation2014; Selby, Citation1999).
All these tasks are considered to be closely related with the interference from the complex food components (Tiwari et al., Citation2010), which is usually called “matrix effect” and has been widely indicated in GICA and enzyme-linked immune sorbent assay (ELISA) of fishery products (Christiane & Christin, Citation2008; Tiwari et al., Citation2010; Wang, Lin, Sui, & Cao, Citation2013). Recently understanding of the matrix effect on ELISA has made significant progress, for example, some proteins have been verified as the main interference-inducing components in fishery products, and their chemical, physical and biological characteristics, as well as the action mechanism, have been clarified (Wang et al., Citation2016). Based on these results, new and more effective approaches have been developed for the elimination of the matrix interference by the exploitation of 5-sulfosalicylic acid dehydrate during pre-treatments (Cui, Lin, Wang, Cao, & Sui, Citation2015; Qu et al., Citation2016). For the matrix interference to GICA, though the effect of proteins and Na+ was proposed in food or urine samples (Li et al., Citation2013; Xu, Citation2013), overall the detailed information is still very limited, especially for fishery products and other food samples, which usually have complex components. Till now, the exact source as well as the mechanism involved has not been clarified, which makes it very difficult for the reasonable development of pre-treatments strategies to further significantly modify GICA performance.
With the purpose of in-depth understanding about the matrix effects on GICA and improving the efficiency of the detection technology, here we used ciprofloxacin as a target, and the effect of some main components (such as proteins and ions) in fishery samples are investigated. Based on the results, the source and mechanism of the matrix interference are preliminarily clarified, and strategies to solve the problem are also proposed and validated.
Materials and methods
Materials and reagents
Frozen Scophthalmus maximus, Cyprinus carpio, Siniperca chuatsi, Aristichthys nobilis, and Ctenopharyngodon idella were obtained from The Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (Qingdao, China), and have been verified of containing no ciprofloxacin. The edible parts of these samples were mashed fully by Waring Blender and stored at 4°C.
Ciprofloxacin hydrochloride standard was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). 5-sulfosalicylic acid dihydrate was purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). N, N, N′, N′-Tetramethylethylenediamine (TEMED) and Coomassie brilliant blue G-250 were obtained from SIGMA-ALDRICH (Shanghai, China). Ciprofloxacin colloidal gold immunochromatography test kits were purchased from Suzhou Airuide Biological Technology Co. Ltd (Suzhou, China). Protein marker, sodium dodecyl sulfate (SDS), and bovine serum albumin (BSA) were purchased from Solarbio Co. Ltd (Beijing, China).
Vortex mixer was offered by Guangzhou Instrument Laboratory Technology Co. Ltd (Guangzhou, China). All the centrifugation was accomplished with the refrigerated centrifuge from SIGMA-ALDRICH (Shanghai, China). The experimental results were collected by HP Scanjet G4050 scanner from Seiko Epson Nagano (HP, Japan). DYY-7C electrophoresis was obtained from Beijing June First Factory (Beijing, China). The nitrogen blowing instrument was made from Tianjin Automatic Science Instrument Co. Ltd (Tianjin, China).
Preparation of the sample extracts
For analysis of ciprofloxacin, crude fish extracts were prepared according to the procedure of Chen and Huang (Citation2002). 5 g of fish muscles was mixed with 5 ml phosphate-buffered saline (PBS, 0.01 M, pH 7.4) in a centrifugal tube and homogenized fully. Then the mixture was centrifuged at 4132 × g for 15 min at 4°C. The precipitate was collected and extracted again with the same procedure. The supernatants were combined and preserved at 4°C, PBS was used for the blank control.
The crude extracts were further mixed by 4% 5-sulfosalicylic acid dehydrate to a final volume of approximately 2:1 (v/v) of the original volume in order to further remove the protein (Cui et al., Citation2015). The solution was then centrifuged at 4132 × g for 15 min at 4°C and the supernatants were collected again. Following that the supernatants were combined and adjusted the pH to neutral by mixing with 0.1 M NaOH for further testing. Blank controls were prepared in the same way as described above without fish muscle samples.
Approaches to eliminate the matrix interference
The fish muscle samples were homogenized with 2% 5-sulfosalicylic acid at a ratio of 1:2 (w/v) and centrifuged at 4132 × g for 15 min at 4°C. The pH of supernatant was adjusted to 12.0 with 2 M NaOH, KOH, Ca(OH)2, respectively. Then the supernatants were collected and adjusted to neutral pH with 2 M 5-sulfosalicylic acid for further detection. As a comparison, the same fish samples were homogenized with 2% 5-sulfosalicylic acid dehydrate and directly adjusted the pH to 12.0 with three different alkalis. The mixture was centrifuged and the supernatants were adjusted to neutral pH for further detection. Blank controls were prepared in the same way as described above without fish muscles.
Determination of components
The protein content was measured by the Coomassie brilliant blue method (Konstantinos, Christos, & Yves-Jacques, Citation2015) and the protein composition was analyzed by polyacrylamide gel electrophoresis (Wang, Li, Zheng, Liu, & Lin, Citation2011). The lipid content was measured according to the chloroform/methanol extraction (Carla, Davide, & Carola, Citation2009), and removed by diatomite filtration (Wang et al., Citation2016). The total sugar content was measured by the vitriolic acid – phenol method with reference to the specific operation described by Tatsuya et al. (Citation2005) and the sugar composition of the concentrated crude extracts was analyzed by polyacrylamide gel electrophoresis (Theodore et al., Citation2005). The polysaccharide simulate solution was prepared according to the method described by Zhang et al. (Citation2011). The concentration of metal ions was measured by inductively coupled plasma atomic emission spectrometry (ICP-AES, Optima 5300 DV, PerkinElmer, Shelton, WA, USA). The simulated iron solution was prepared with PBS according to the concentration of the crude extracts.
The concentration of protein content or magnesium ions of crude extraction and other samples from different pre-treatment methods was recorded as C0 and C, respectively. The removal ratio of protein and magnesium was calculated as follows:
(1)
(1)
GICA procedures
The GICA exploits the indirect competitive principle (Wang, Liu, Zhang, & Wang, Citation2007) and the procedures were according to the operating instruction of the test kit. Standard solutions or 100 μL of sample extracts were added in the sample wells and allowed to react at room temperature for 5 min, then the results were distinguished by naked eyes. Considering the significant difference (P < 0.05) between the colour darkness on the test line and the control line (a), the results were distinguished according to the following:
Negative results (−), the sample extracts should make the colour darkness on test line the same as that on the test line of blank controls, indicating an antibiotic concentration below the detection limit.
Positive results (+), the colour darkness on test line was significantly lighter than that on the test line of blank controls, indicating an antibiotic concentration higher than the detection limit.
The control line was supposed to be always visible, clear and uniform, otherwise, the result was invalid.
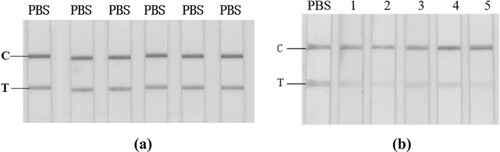
Figure 1. GICA results for PBS (a) and crude extracts of different negative fish samples (b). From left: PBS; 1, Scophthalmus maximus; 2, Cyprinus carpio; 3, Siniperca chuatsi; 4, Aristichthys nobilis; 5, Ctenopharyngodon idella.
For quantitative analysis (Zeng et al., Citation2017), the image of the result was obtained by a flatbed scanner, and the relative brightness value of test line and control line was calculated by subtracting background brightness value from the test zone signal. B01 and B1 were used to designate the relative brightness of the test line at PBS (B01) and the crude extract (B1), respectively. The relative brightness values of extracts after different pre-treatments and the corresponding blank controls were recorded as B and B02, respectively. The calibration curve was prepared by the relative brightness on the test line and the corresponding ciprofloxacin standard concentration (ranging from 0 to 20 ng/ml) in solvents of blank controls, and the data were fit to a quadratic function. The lowest limit of detection (LOD) for GICA was defined as the average concentration corresponding to the relative brightness of test line of negative samples plus three times the standard deviation (SD, n = 3).
The removal ratio of matrix interference was calculated as follows:
(2)
(2)
Statistical analysis
All experiments were performed at least in duplicate, and the data were expressed as Mean ± SEM, unless indicated otherwise. The Student’s t test was carried out to determine the significant difference (P = 0.05) between different groups.
Results and discussion
Overall effect of the fish matrix on the GICA
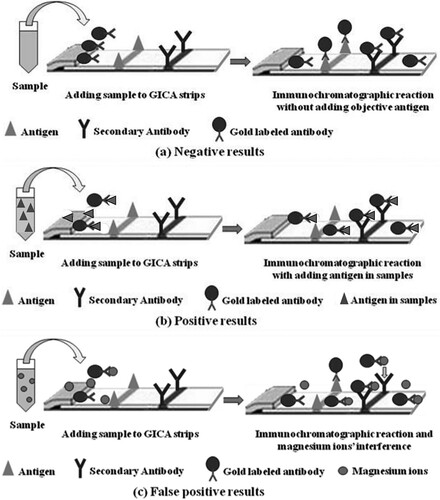
In the presence of aqueous fish extracts, the reddish colour of the test line on the GICA kit was significantly faded in comparison to the control in PBS (b). Such a result verified the interference of some water-soluble matrix to the GICA. No significant difference was observed among the five fish species, indicating that such a matrix effect may be of universal meaning for fishery products. The interference may result in false positive results, which seriously reduced the accuracy, precision, and sensitivity of GICA, and therefore greatly limited its real application, especially for the detection of trace analyte.
Effect of protein components on the GICA
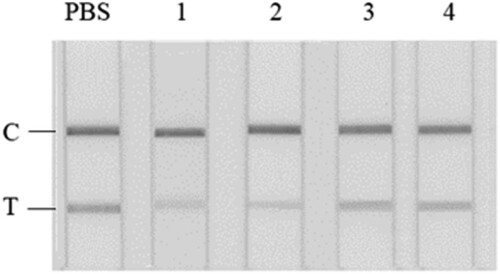
Proteins contribute much of the water-soluble components in the fish crude extracts (). In previous studies, the interference of matrix proteins to ELISA has been fully confirmed by its possible competitive interaction with antibodies (Wang et al., Citation2016). In this study, we have verified the influence of proteins on GICA. After 5-sulfosalicylic acid (2% m/v) treatment, more than 99% proteins were removed (), and correspondingly the matrix effect was observed somewhat slighter than that in the original extracts (). Similar to the previous study for ELISA (Wang et al., Citation2016), in which some matrix protein of fishery extracts were found to react with IgG (especially enzyme-labelled antibodies), here the protein-induced matrix effect may also be attributed to its non-specific binding with the gold-labelled antibodies. This protein-IgG interaction demonstrated no significant affluence on the gold-labelled antibody for its reaction with the secondary antibody on the control line, however, significantly reduced its efficiency to bind with the antigens on the test line. The pre-treatment of the samples with 5-sulfosalicylic acid was demonstrated to have a potential for removing proteins in the extracts, therefore effectively diminish the matrix interference and improve the efficiency of the GICA.
Figure 2. GICA results for crude extracts after 5-sulfosalicylic acid treatment. From left: Blank control; 1, Scophthalmus maximus; 2, Cyprinus carpio; 3, Siniperca chuatsi; 4, Aristichthys nobilis; 5, Ctenopharyngodon idella.
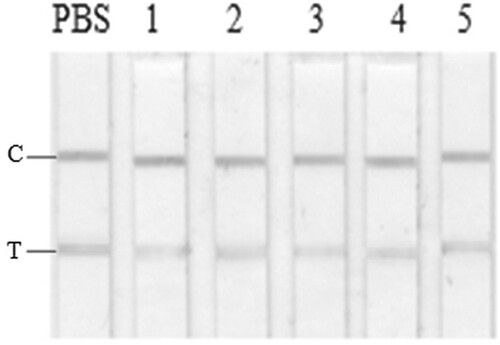
Table 1. The protein content in different fish samples.
Even after the complete removal of all the proteins, the colour darkness on the test line was still significantly lighter in comparison to that on the control line, which indicated the presence of other sources of matrix effect besides proteins. Such a result was quite different from that observed in the case of ELISA, in which the influence of proteins seemed dominant in comparison to other water-soluble components, and little interference remained after the elimination of proteins by the 5-sulfosalicylic acid treatment (Cui et al., Citation2015; Qu et al., Citation2016).
Effect of the lipid and sugars on the GICA
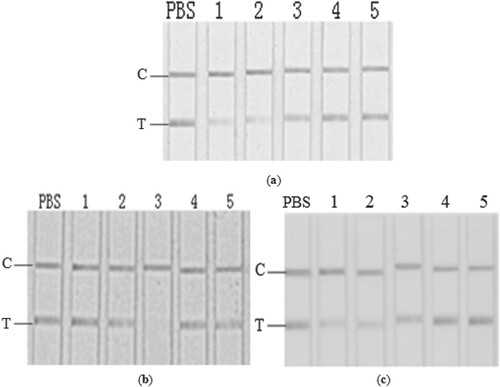
A considerable amount of lipids were there in the crude extracts, but after the removal of most of the lipids by diatomite filtration (), no significant difference was demonstrated on the test line (a) in comparison to the crude extracts (b). Therefore the effect of lipid components in these fish samples were considered negligible for the matrix interference investigated.
Figure 3. GICA results for sample extracts removed lipid component (a), and for fish polysaccharide simulated solutions (b). From left: PBS; 1, Scophthalmus maximus; 2, Cyprinus carpio; 3, Siniperca chuatsi; 4, Aristichthys nobilis; 5, Ctenopharyngodon idella.
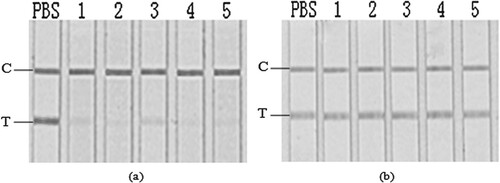
Table 2. The lipid and sugar content in different fish samples.
Compared to protein and lipid contents, sugars accounted for a small portion of the total water-soluble contents in the crude fish extracts (). No significant effect was observed on the GICA by using some polysaccharides to simulate the sugar components (b), thereby indicating a minor contribution of sugars to the matrix interference. These results didn’t agree with the previous study on Apostichopus japonica, in which some japonicus polysaccharides were proved to significantly interfere the GICA of furazolidone metabolite (Li et al., Citation2015). Though detail information is still limited, the reasons may be attributed to the differences in the species and the properties of the polysaccharides in fish and Apostichopus japonica.
Effect of metal ions on the GICA
Ash constitute approximately 1.2–1.8 g/kg of the total fish weight, which mainly include Mg, Al, Na, K and Ca, with little variations among fish species (Wang, Citation2014). In the crude aqueous fish extracts, Na was determined to be the highest with concentration ranging from 3.18–3.22 g/kg, followed by K, Ca, Mg and Al (1.72–1.76, 0.07–0.11, 0.01–0.05, and 0–0.01 g/kg, respectively). Based on the results, simulated salt solutions (pH 7.0) were prepared and their influence on the GICA was investigated. Similar to the crude extracts, obvious false positive results were exhibited, and the extent of interference seemed significantly dependent on the concentration of these ions (a). For further evaluation of single ions, only magnesium was demonstrated significant and a strong correlations were observed between magnesium concentration and the degree of interference, while the results with other ions seemed the same as that in blank (b and c).
Figure 4. GICA results for simulated total metal ions in PBS and their gradient solutions (a, from left: PBS; 1–5: diluted with PBS to 0, 10, 50, 100 folds), single salt in PBS (b, from left: PBS; 1–5: K+, Na+, Mg2+, Al3+, Ca2+), and gradient magnesium in PBS (c, from left: PBS; 1–5: 1 g/kg, 0.5 g/kg, 0.1 g/kg, 0.05 g/kg, 0.01 g/kg).
Previous works have revealed the affluence of ions (such as Na+, Mg2+, Al3+, K+, and Ca2+) on the ELISA (Wang, Citation2014), which was observed much weaker than the interference of proteins and assumed to be induced by the change of ion strength or electronic properties in the immunological reactions. But here the results for GICA were quite different, though it has been confirmed that colloids may agglomerate at high ion strength (Lazzari, Nicoud, Jaquet, Lattuada, & Morbidelli, Citation2016). Among the ions investigated, only magnesium exhibited significant interference to GICA, while its ion strength was in fact much lower than that of Na solutions. All these allowed us to suggest that some ions (especially Mg2+) are important sources of the matrix interference to GICA and even contribute more than proteins, but the reasons could not simply be attributed to the change of ion strength or electronic properties of the reaction system.
Though the detail mechanism is still unclear, Liu (Citation2014) proved significant interaction between magnesium ions and immunoglobulin. Therefore, it can be reasonably assumed that here magnesium could effectively bind with the gold-labelled antibody and then prohibited its reaction with the antigen on the test line, through the conformational distortion of epitopes or the blocking of the binding sites. In comparison to the relatively fragile interaction between antibody and antigen, the multiple binding sites between the antibody and the secondary antibody may effectively diminish such an affluence of magnesium on the reaction at the control line. In contrast, the presence of magnesium may promote the reaction by its binding with both molecules to form some kind of linkage like a “bridge” () (Lazzari et al., Citation2016).
Approaches to eliminate the matrix interference
A lot of techniques seem interesting to diminish the magnesium-induced interference, such as the cation-exchange membrane (Yan et al., Citation2017). Considering the simplicity and rapidity of the performance, the magnesium ions were removed as magnesium hydroxide with proper pH adjustment (12.0 and higher), in which NaOH, KOH and Ca(OH)2 were used as pH regulators.
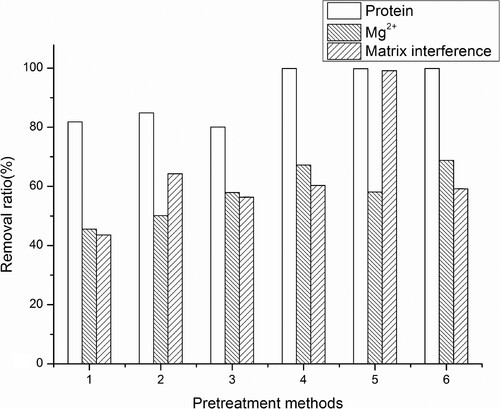
In comparison with the pH adjustment directly after acid extraction, the processes including centrifugation after acid-extraction exhibited a much higher efficiency to remove protein of samples (), which indicated that some protein precipitates were re-dissolved with the addition of alkali. Among the three pH regulators investigated, the KOH showed approximately 2 times higher efficiency compared to the other alkalis for the removal of matrix interference. With the optimized pH-adjustment procedures (using KOH as the regulator after a centrifugation step), no significant difference was demonstrated in the relative brightness on test line between sample extracts and blank controls (, P > 0.05), indicating a complete elimination of matrix interference of GICA. For quantitative analysis of ciprofloxacin, the LOD was evaluated as 1.24 ng/ml according to the standard curve after the developed pre-treatment, almost 8 times lower than that in the crude extracts.
Figure 6. The efficiency of different pre-treatments to remove protein, magnesium and matrix interference of Scophthalmus maximus. 1–3: the pH of the samples was directly adjusted with NaOH, KOH, Ca(OH)2 after acid extraction. 4–6: the pH of the samples was adjusted with NaOH, KOH, Ca(OH)2 after acid extraction and centrifugation.
Conclusion
The interference of matrix components in fishery products on GICA using five fish species as samples was investigated comprehensively for the first time. Some water solvable proteins were proved to be able to reduce the colour density of the test line and therefore lead to false-positive results. Some ions, especially magnesium, could also result in significant matrix effect to an extent even higher than proteins, which has not been indicated in previous studies. The interaction between magnesium and some IgG, especially gold-labelled antibodies, was considered an important source of the matrix effect on GICA. Other main components such as lipids and sugars showed no significant effect on GICA. Based on these results, corresponding approaches to remove the interference were also explored. Pre-treatment with 5-sulfosalicylic acid dehydrate followed by pH adjustment using KOH solution as the regulator, the matrix interference was fully eliminated, and the sensitivity of GICA was increased about 8 times for the quantitative analysis of ciprofloxacin. Further studies need to be conducted to explore the effect of magnesium ion on interaction among biomacromolecules in GICA and specific interference mode, and to provide the guidance for further explaining the mechanism of matrix interference.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Boris, B. D., Nadezhda, A. B., Alexandr, E. U., & Anatoly, V. Z. (2014). Immunochromatographic methods in food analysis. TrAC Trends in Analytical Chemistry, 55, 81–93. doi: https://doi.org/10.1016/j.trac.2013.11.007
- Carla, L., Davide, B., & Carola, L. (2009). Fatty acid composition of meat and perirenal fat in rabbits from two different rearing systems. Meat Science, 83, 135–139. doi: https://doi.org/10.1016/j.meatsci.2009.04.011
- Chen, T. Y., & Huang, D. F. (2002). Electrophoretic identification of muscle proteins in 7 puffer species. Food Chemistry and Toxicology, 67(3), 936–942.
- Christiane, K. F., & Christin, P. (2008). Quantitative sandwich ELISA for the determination of fish in foods. Journal of Immunological Methods, 329, 45–55. doi: https://doi.org/10.1016/j.jim.2007.09.007
- Cui, M. Q., Lin, H., Wang, X. D., Cao, L. M., & Sui, J. X. (2015). 5-sulfosalicylic acid dihydrate-based pretreatment for the modification of enzyme-linked immunoassay of fluoroquinolones in fishery products. Journal of Immunoassay and Immunochemistry, 36(05), 517–531. doi: https://doi.org/10.1080/15321819.2015.1006330
- Du, S. Y., Lin, H., Sui, J. X., Wang, X. D., & Cao, L. M. (2014). Nano-gold capillary immunochromatographic assay for parvalbumin. Analytical and Bioanalytical Chemistry, 406, 6637–6646. doi: https://doi.org/10.1007/s00216-014-8093-0
- Huang, X. L., Zoraida, P. A., Xu, H. Y., Lai, W. H., & Xiong, Y. H. (2016). Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosensors and Bioelectronics, 75, 166–180. doi: https://doi.org/10.1016/j.bios.2015.08.032
- Jiachi, C., Arthur, H. H. L., Hang, W. L., & Wing-tak, W. (2015). Rapid testing methods for food contaminants and toxicants. Journal of Integrative Agriculture, 14(11), 2243–2264. doi: https://doi.org/10.1016/S2095-3119(15)61119-4
- Konstantinos, G., Christos, D. G., & Yves-Jacques, S. (2015). An accurate and sensitive Coomassie Brilliant Blue G-250-based assay for protein determination. Analytical Biochemistry, 480, 28–30. doi: https://doi.org/10.1016/j.ab.2015.03.024
- Lazzari, S., Nicoud, L., Jaquet, B., Lattuada, M., & Morbidelli, M. (2016). Fractal-like structures in colloid science. Advances in Colloid and Interface Science, 235, 1–13. doi: https://doi.org/10.1016/j.cis.2016.05.002
- Li, C. H., Luo, W., Xu, H. Y., Zhang, Q., Xu, H., Zoraida, P. A., … Xiong, Y. H. (2013). Development of an immunochromatographic assay for rapid and quantitative detection of clenbuterol in swine urine. Food Control, 34, 725–732. doi: https://doi.org/10.1016/j.foodcont.2013.06.021
- Li, W. L., Zhuang, S. J., Lin, H., Du, S. Y., Cui, M. Q., Qu, X. L., … Sui, J. X. (2015). The matrix effects and its elimination methods in gold immunochromatography assay of the furazolidone in Apostichopus japonicus. Chinese Fishery Quality and Standards, 5(6), 35–42.
- Liu, H. P. (2014). Study on correlation between the concentration of magnesium ion in serum and immunoglobulin of patients with severe craniocerebral injury. Laboratory Medicine and Clinic, 11(12), 1655–1659.
- Mohammad, J. R., Noor, M. D., Fazlollah, B., Mehrdad, G., Mohammad, R., Khalil, A., & Seyed, M. T. (2016). Lateral flow based immunobiosensors for detection of food contaminants. Biosensors and Bioelectronics, 86, 234–246.
- Qu, X. L., Lin, H., Du, S. Y., Sui, J. X., Zhang, X. L., & Cao, L. M. (2016). Development of a nano-gold capillary immunochromatographic assay for rapid and semi-quantitative detection of clenbuterol residues. Food Analytical Methods, 9(9), 2531–2540. doi: https://doi.org/10.1007/s12161-016-0442-5
- Selby, C. (1999). Interference in immunoassay. Annals of Clinical Biochemistry: An International Journal of Biochemistry and Laboratory Medicine, 36(6), 704–721. doi: https://doi.org/10.1177/000456329903600603
- Tatsuya, M., Akio, M., Norimasa, I., Tokifumi, M., Shin-Ichiro, N., & Yuan, C. L. (2005). Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Analytical Biochemistry, 339, 69–72. doi: https://doi.org/10.1016/j.ab.2004.12.001
- Theodore, V. T., Fotini, N. L., Ioanna, K., Maria, K., Vasso, A., Geoffrey, A. P., … Nikos, K. K. (2005). Synthesis and study of the electrophoretic behavior of mannan conjugates with cyclic peptide analogue of myelin basic protein using lysine-glycine linker. Analytical Biochemistry, 347, 121–128. doi: https://doi.org/10.1016/j.ab.2005.09.014
- Tiwari, R. S., Venkatachalam, M., Sharma, G. M., Su, M. N., Roux, K. H., & Sathe, S. K. (2010). Effect of food matrix on amandin, almond (Prunus dulcis L.) major protein, immunorecognition and recovery. LWT-Food Science and Technology, 43(4), 675–683. doi: https://doi.org/10.1016/j.lwt.2009.11.012
- Wang, X. D. (2014). Effect of matrix components in fish on enzyme-linked immunosorbent assay of drug residue (Dissertation). Ocean University of China.
- Wang, B. P., Li, Z. X., Zheng, L. N., Liu, Y. X., & Lin, H. (2011). Identification and characterization of a new IgE-binding protein in mackerel (Scomber japonicus) by MALDI-TOF-MS. Journal of Ocean University of China, 10(1), 93–98. doi: https://doi.org/10.1007/s11802-011-1793-6
- Wang, X. D., Lin, H., Cao, L. M., Cui, M. Q., Du, S. Y., & Sui, J. X. (2016). The matrix interference to the immunoassay of food samples: The effect of some proteins in aquatic products. Food and Agricultural Immunology, 27(2), 230–241. doi: https://doi.org/10.1080/09540105.2015.1086317
- Wang, X. D., Lin, H., Sui, J. X., & Cao, L. M. (2013). The effect of fish matrix on the enzyme-linked immunosorbent assay of antibiotics. Journal of the Science of Food and Agriculture, 93(7), 1603–1609. doi: https://doi.org/10.1002/jsfa.5931
- Wang, X. H., Liu, T., Zhang, Y., & Wang, S. (2007). Enzyme-linked immunosorbent assay and colloidal gold immunoassay for ochratoxin A: Investigation of analytical conditions and sample matrix on assay performance. Analytical and Bioanalytical Chemistry, 389(3), 903–911. doi: https://doi.org/10.1007/s00216-007-1506-6
- Wu, W. D., Li, M., Chen, M., Li, L. P., Wang, R., Chen, H. L., … Chen, H. Z. (2017). Development of a colloidal gold immunochromatographic strip for rapid detection of Streptococcus agalactiae in tilapia. Biosensors and Bioelectronics, 91, 66–69. doi: https://doi.org/10.1016/j.bios.2016.11.038
- Xie, Y. J., Yang, Y., Kong, W. J., Yang, S. H., & Yang, M. H. (2015). Application of nanoparticle probe-based lateral flow immunochromatographic assay in mycotoxins detection. Chinese Journal of Analytical Chemistry, 43(4), 618–628. doi: https://doi.org/10.1016/S1872-2040(15)60821-0
- Xu, R. (2013). Gold immunochromatography technology for the rapid detection of the enrofloxacin in seafood (Dissertation). Ocean University of China.
- Yan, Z., Muhammad, A., Liu, Y., Maria, M., Andriy, Y., & Merlin, L. B. (2017). Adsorption of polyelectrolyte multilayers imparts high monovalent/divalent cation selectivity to aliphatic polyamide cation-exchange membranes. Journal of Membrane Science, 537, 177–185. doi: https://doi.org/10.1016/j.memsci.2017.05.043
- Zeng, N. Y., You, Y., Xie, L. S., Zhang, H., Ye, L. S., Hong, W. X., & Li, Y. R. (2017). A new imaged-based quantitative reader for the gold immunochromatographic assay. Optik - International Journal for Light and Electron Optics, 33(05), 1129–1136.
- Zhang, J. J., Gu, W. G., Yan, Y. F., Yao, Y. J., Chen, J. C., & Ye, X. Q. (2011). Determination of reducing sugars, phosphorylated sugars and sucrose in fish products by HPLC-RI. Journal of Food Science and Biotechnology, 30(4), 576–582.