ABSTRACT
Fifteen strains of lactic acid bacteria isolated from artisanal Cocido Mexican cheese were evaluated for their probiotic potential and capacity to modulate the immune system. The results revealed that the strains J20, J23, J24, J25, J27, J28, J32 and J37 presented the highest potential probiotic. Some strains showed resistance to antibiotics; however, they did not exhibit haemolytic activity or mucin degradation. The auto-aggregation capacity ranged from 9.54% to 47.80%, and the hydrophobicity was 21.1%, 63.4% and 78.8% for J27, J24 and J37, respectively. The strains showed high survival capacity (>80%) under different storage conditions and the β-galactosidase activity was 241.77–249.25 MU. Furthermore, the administration of fermented milk by specific strains of Lactobacillus enhanced IL-10 levels and upregulated IL-6 and IgA levels in serum samples of rats. Therefore, the evaluated strains may modulate the immune system and may be good candidates for their inclusion in the manufacture of probiotic-fermented milk.
Introduction
It has been widely reported that the different microbial groups present in Mexican cheese have an important role in the development of characteristics such as flavour, ripening time, texture and rheological properties (González-Córdova, Yescas, & Ortiz-Estrada, Citation2016; Guerra-Martínez, Montejano, & Martín del Campo, Citation2012; Tunick, van Hekken, Call, Molina-Corral, & Gardea, Citation2007; Van Hekken, Drake, Corral, Guerrero-Prieto, & Gardea, Citation2006). For example, in Mexican Fresco cheese, Lactobacillus casei, Lactococcus lactis and Enterococcus faecium were reported as the dominant microbial groups (Saxer, Schwenninger, & Lacroix, Citation2013; Torres-Llanez et al., Citation2006). Meanwhile, in artisanal Cocido cheese, the microbiota varied, lactic acid bacteria (LAB) such as Lactobacillus spp., Lactococcus spp. and Streptococcus spp. being the predominant microbial groups (Cuevas-González et al., Citation2017). Lactobacillus spp. specifically have demonstrated the potential effect on the health to produce bacteriocins (Heredia-Castro et al., Citation2015).
In this sense, over the past decade, increasing interest has been placed on artisanal cheeses as sources of LAB with probiotic potential. According to the International Scientific Association for Probiotics and Prebiotics (ISAPP), probiotics are live microorganisms that, when administered in adequate amounts, confer health benefits to a host (Hill et al., Citation2014). In this sense, expert panels have specifically linked probiotic microorganisms to health claims. However, it is important to examine the potential of specific probiotic strains to determine their viability, efficacious doses and shelf-lives (Bartazzoni, Donelli, Midtvedt, Nicoli, & Sanz, Citation2013).
The positive effects of probiotic consumption have been mainly associated with Lactobacillus and bifidobacterial strains. Specifically, the probiotic effects found have been the following: decreasing the risk of gastrointestinal diseases and diabetes, exhibiting antimicrobial and anticarcinogenic activity, decreasing serum cholesterol levels and modulating the immune system response (Chiang, Liu, Tseng, Mau, & Pan, Citation2012; de Vrese & Schrezenmeir, Citation2008; Karska-Wysocki, Bazo, & Smoragiewicz, Citation2010; Kumar et al., Citation2012; ). In this sense, probiotics may stimulate an innate or adaptive immune response. These effects can be exerted by bacteria including cell wall components or by biomolecules released during fermentation processes such as peptides and exopolysaccharides (Amrouche, Butin, & Fliss, Citation2006; de Moreno de LeBlanc, Matar, Thériault, & Perdigón, Citation2005; Matar, Valdez, Medina, Rachid, & Perdigon, Citation2001; Medici, Vinderola, Weill, & Perdigón, Citation2005).
In this context, dairy products may be good carriers for probiotics, especially fermented milks that maintain concentrations of live cells during storage. From a technological perspective, fermented milks represent a good probiotic product alternative for the dairy industry (Boza-Mendez, Lopez-Calvo, & Cortes-Muñoz, Citation2012). Specifically, fermented milk with probiotics may induce the mucosal response, and up- or down-regulate the production of cytokines, and proliferation of CD4+, CD8+ and IgA+ cells (de Moreno de LeBlanc et al., Citation2005, Citation2008; de Moreno de LeBlanc, Valdez, & Perdigon, Citation2004; Galdeano, de Moreno de LeBlanc, Carmuega, Weill, & Perdigón, Citation2009; Matar et al., Citation2001; Vinderola, Perdigón, Duarte, Farnworth, & Matar, Citation2006).
Based on the existing research, the effects of probiotics appear to be strain dependent. Therefore, the aim of the present study was to evaluate the physiological, technological and functional properties of different Lactobacillus strains isolated from artisanal Cocido cheese.
Materials and methods
Bacterial strains and culture propagation
Fifteen Lactobacillus strains isolated from Mexican Cocido artisanal cheese (Heredia-Castro et al., Citation2015) () were cultured in MRS broth (De Man, Rogosa and Sharpe, Difco). The strains were cultured with 1% inoculum incubated for 18 h, at 37°C in anaerobic jars (Difco, BBL® Gaspak® anaerobic system).
Table 1. Lactobacillus strains isolated from artisanal Cocido cheese for subsequent in vitro and in vivo evaluations of probiotic potential.
Physiological evaluation
Survival under pH and enzyme conditions
The resistance of Lactobacillus strains under distinct pH and enzyme conditions was determined following the methodology described by Maragkoudakis et al. (Citation2006). Briefly, bacterial cells from 18 h were harvested by centrifugation (3600×g, 10 min, 4°C), washed twice with PBS buffer (pH 7.2, 0.2 M) and resuspended in PBS pH 3.0 and incubated for 3 h.
The enzymes lysozyme, pepsin and pancreatin (Sigma-Aldrich) were dissolved in PBS and sterilized by filtration (0.22 µm, Millipore Corporation). Cultures of 18 h in MRS broth were harvested by centrifugation (3600×g, 10 min, 4°C). The pellets were washed twice with PBS, and then resuspended in PBS with lysozyme (pH 6.5, 0.2 mg/mL), PBS with pepsin (pH 2.0, 0.3 mg/mL) or PBS with pancreatin (pH 8.0, 1 mg/mL) and then incubated for 1, 3 and 5 h, respectively. Resistance was evaluated in terms of percentage following the expression: (%) = (N/N 0) × 100, where N 0 represents the initial amount of bacteria and N is the amount of bacteria after incubation.
Bile salt tolerance assay
The ability of the strains to grow in the presence of bile salt was determined according to the method of Thirabunyanon, Boonprasom, and Niamsup (Citation2009) with some modifications. One millilitre of strain cultured for 18 h in MRS broth was transferred to 9 mL of MRS broth with 0.3% (w/v) bile salts (Sigma-Aldrich) and incubated at 37°C for 5 h. After this incubation, one millilitre was placed in warmed MRS agar, pour plated and then incubated for 48 h at 37°C, under anaerobic conditions to determine viable cells. Resistance was evaluated in terms of percentage (%) = (N/N 0)×100.
Auto-aggregation and hydrophobicity assay
Auto-aggregation assays were performed and evaluated following the methodology described by Collado, Meriluoto, and Salminen (Citation2008). The cultures were centrifuged (3600×g, 10 min, 4°C), washed twice with PBS (pH 7.2, 0.2 M) and suspended in PBS. The samples were incubated at 37°C for 5 h. Finally, 0.2 mL of the upper suspension was carefully removed at 0, 2 and 5 h, and OD600nm was measured (SpectraMax M Series Multi-Mode Microplate Readers, Molecular Devices, CA, USA). The auto-aggregation was expressed as 1 − (OD600nm upper culture/OD600nm total) × 100.
Hydrophobicity assays were also performed. Cell suspensions were harvested by centrifugation (3600×g, 10 min, 4°C), washed twice with PBS and finally the pellet was resuspended in 4 mL of PBS. Then, 0.8 mL of ρ-xylene (Fluka, Sigma-Aldrich) was added to the cell suspension and thoroughly mixed for 2 min. The aqueous phase was removed after 20 min of incubation at 37°C, and the absorbance at OD600 nm was measured. The decrease in absorbance of the aqueous phase was taken as a measure of cell surface hydrophobicity and was calculated as [(A 0 − A)/A 0] × 100, where A 0 and A denote absorbance before and after the addition of xylene, respectively (Collado et al., Citation2008).
Mucin degradation
Mucin degradation assays were performed using liquid medium and degradation assay in a Petri dish, according to previous reports with modifications. The composition of the basal medium was prepared using the methodology previously reported by Abe et al. (Citation2010), Fernandez, Boris, and Barbes (Citation2005) and Zhou, Gopal, and Gill (Citation2001) with some modifications.
The basal medium was modified by adding 0.5% (w/v) of porcine gastric mucin (HGM type III, Sigma-Aldrich). Briefly, 100 µL of fresh cultures (12 h) was transferred to basal media broth (10 mL) and incubated at 37°C for 24 h. Escherichia coli ATCC 25922 was used as a positive control and the results are reported as OD600nm.
Haemolytic activity
Haemolytic activity was tested on Columbia agar (BD Difco) plates added with human blood (5% v/v). At 18 h, cultures were streaked on agar plates and incubated at 37°C for 48 h under anaerobic conditions. The characteristics of haemolysis were evaluated on blood agar plates by examining the β-, α- and γ-haemolysis zones. Staphylococcus aureus ATCC 29213b was used as a positive control (Thirabunyanon et al., Citation2009).
Antibiotic susceptibility testing
Antibiotic testing was performed according to the manufacturer’s instructions (Multidisc Gram positives II, BIO-RAD). The utilized antibiotics were ampicillin (AM, 10 µg), gentamicin (GE, 10 µg), cephalothin (CF, 30 µg), cefotaxime (CTX, 30 µg), cefepime (FEP, 30 µg), cefuroxime (CL, 30 µg), dicloxacillin (DC, 1 µg), erythromycin (E, 15 µg), levofloxacin (LEV, 5 µg), penicillin (PE, 10 U), tetracycline (TE, 30 µg) and trimethoprim-sulfamethoxazole (SXT, 25 µg).
The cultures were inoculated in Mueller-Hinton broth (BD Difco) and incubated at 37°C for 3 h. Afterwards, the suspensions were applied to the surface of Mueller-Hinton agar plates using sterile swabs. Antibiotic strips were placed on the surface of each plate, and plates were subsequently incubated at 37°C for 24 h. The inhibition halos were measured and considered to demonstrate either resistance or susceptibility.
Technological evaluation
β-Galactosidase activity
β-Galactosidase activity was determined according to the method of Miller (1972) with slight modifications proposed by Vinderola and Reinheimer (Citation2003). Briefly, cultures were harvested by centrifugation, washed twice with PBS and inoculated in MRS broth modified with lactose as carbon source. Cultures were incubated at 37°C for 24 h. Finally, the cells were harvested by centrifugation and washed with PBS, and adjusted to OD600nm 1.0 with the same buffer. One millilitre of cells suspension was permeabilized with 50 µL of toluene/acetone (1:9 v/v) solution, vortexed for 7 min. Posteriorly, 100 µL of permeabilized cell suspension was mixed with 900 µL of PBS and 200 µL of o-nitrophenyl-β-d-galactopyranoside (4 mg/mL, ONPG, Sigma-Aldrich). The samples were incubated at 37°C for 15 min, and finally, 0.5 mL of Na2CO3 (1M) was added to stop the reaction. The samples were evaluated at both 420 and 600 nm and the β-galactosidase activity was calculated in MU as follows: 1000 × [((A 420 − 1.75) + A2600)/(15 min + 1 mL + A1600)], where A1600 was the absorbance just before assay and A2 was the value of the reaction mixture.
Preparation of fermented milk
Fermented milk was prepared from skim milk (10% w/v) (Organic Valley USDA Organic Grade A) that was sterilized (110°C, 10 min). The milk was inoculated at 1% (107 CFU/mL) and incubated for 12 h; afterwards, an aliquot of 3% was added to 20 mL of milk.
Survival of probiotics in milk under cold, lyophilizing and freezing conditions
Fresh cultures of 18 h of growth were centrifuged (3600×g, 10 min, 4°C), washed twice with PBS, suspended in 10 mL of sterile milk (10% w/v) and then stored at 4°C or −20°C; other samples (10 mL) were lyophilized. The percentage of survival was evaluated after 15 and 30 days of storage. Cell concentrations were evaluated on MRS agar plates incubated at 37°C for 48 h under anaerobic conditions (Zacarías, Binetti, Laco, Reinheimer, & Vinderola, Citation2011).
Survival of probiotics in milk at pasteurization temperatures
Heat resistance was evaluated under conditions of pasteurization (63°C, 30 min and 75°C, 1 min). Strains were cultivated in 10 mL of MRS broth, harvested by centrifugation (3600×g, 10 min, 4°C) and washed twice with PBS. Pellets were resuspended in 10 mL of skim milk. Initial cell concentrations were determined before heat treatments on MRS agar plates incubated at 37°C for 48 h under anaerobic conditions. Additionally, the heating time required to generate a 1 log reduction on viable cells counts (Dt) at specific temperatures was calculated as follows: Dt = [t/(Log10 N before − Log10 N after)], where t is the time (min) of pasteurization and N is the amount of bacteria before and after treatment.
Immunomodulatory effect
Animal studies and administration of fermented milk
The J20, J23, J24 and J28 strains with high proteolytic activity and the J24, J27 and J28 strains with high adhesion capacity in vitro were selected for the preparation of fermented milk. For this, sterile milk was inoculated (3%) and incubated for 48 h at 37°C.
Female Wistar rats (6–8 weeks old, weighing 140 ± 20 g) were obtained from Harlan Laboratories (Mexico City). Animals were fed a conventional diet and water ad libitum and maintained in a room with a 12-h light/dark cycle at 23 ± 2°C. The Animal Protection Committee of the Food and Development Research Center (Centro de Investigación en Alimentación y Desarrollo A.C. [CIAD]) approved the animal protocols. The rats were randomized (p > .05) and allocated in nine groups of five rats/group.
The rats were administered 1 mL of fermented milk (109 CFU/mL). Two control groups were administered 1 mL of unfermented milk and water via intragastric feeding. The treatment period lasted for 21 days, after which rats were euthanized.
Sampling procedures
Blood samples from each group were obtained at days 7 and 21 and were centrifuged (1800×g, 10 min, 4°C) to obtain serum. Serum samples were stored at −20°C.
ELISAs of serum samples
Interleukin (IL)-10 and IL-6 cytokine concentrations in serum were evaluated using commercial ELISA kits (BD, Invitrogen). Immunoglobulin-A (IgA) concentrations in serum were also determined with an ELISA kit (GenWay Biotech Inc., San Diego, EU). The results were expressed as concentrations of cytokine (pg/mL) or immunoglobulin (μg/mL).
Statistical analysis
Experimental data were expressed as mean values ± standard deviation for the in vitro assays. For the in vivo evaluations, the results were expressed as average standard error. Multiple comparisons were performed by one-way analyses of variances (ANOVAs) followed by Tukey’s tests (p < .05). For mucin degradation, the results were performed by paired t-test (p < .05).
Results
Physiological evaluation
Survival under distinct pH and enzyme conditions
The LAB exhibited viability under different gastrointestinal conditions (). In general, the strains under distinct pH conditions showed the highest percentage of cell survival ranging from 81.67% to 99.17%. Only one strain (J32) presented a low percentage of cell survival under pepsin conditions. After exposure to lysozyme, the strains’ survival rates were 88.22–99.51%. With respect to bile salts, the most tolerant strains were J20, J24 and J32, which had tolerances of 96.95%, 96.23% and 98.82%, respectively. Together, these results show that the strains selected have great capacity to survive the harsh conditions of the gastric and intestinal environment.
Table 2. Evaluation of different physiological properties of Lactobacillus strains in vitro.
Auto-aggregation and hydrophobicity assay
The auto-aggregation and hydrophobicity assays are indicative of adhesion capacity for the selection of probiotics. In this sense, the J24, J25 and J32 strains presented the highest auto-aggregation values of 47.8%, 42.92% and 36.29%, respectively (). These results were compared with those from other studies where different strains of Bifidobacterium and Lactobacillus showed values of 12.3 ± 2.7% to 63.2 ± 8.0% after 20 h of incubation. Collado et al. (Citation2008) found that Lactobacillus presented the highest rates of auto-aggregation, yet, comparatively, our strains showed better values during the first 5 h of incubation. The hydrophobicity values were 63.45%, 21.1% and 78.85% for the strains J24, J27 and J37, respectively.
Mucin degradation
Mucin degradation is a non-desirable characteristic for probiotics, as this could alter the intestinal mucosal barrier. In addition, the production of mucin has been suggested as a virulence factor for some enteropathogens and facilitates the mucosal penetration of other pathogens and toxic agents (Ruseler-van Embden, van Lieshcut, Gosselink, & Marteau, Citation1995; Zhou et al., Citation2001).
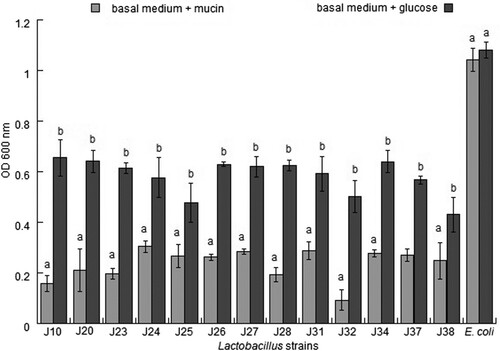
In this assay, mucin degradation was evaluated on agar plates and in the basal medium. None of the strains evaluated in this study exhibited mucolytic activity on plates or in medium supplemented with mucin compared with the control strain. In contrast, Escherichia coli showed growth in the medium supplemented with mucin or glucose, which was the only carbon source with OD values of 1.042 ± 0.047 and 1.081 ± 0.033, respectively, after 24 h of incubation (). However, Escherichia coli was able to produce a clear lysis zone on plates. These results imply that the evaluated LAB strains were unable to degrade mucin in vitro.
Haemolytic activity
The absence of haemolytic activity is an important property to consider upon selecting probiotics. In general, probiotic strains have been recognized as safe and have not demonstrated virulence in hosts (de Vuyst, Foulquie, & Revets, Citation2003; Morandi, Brasca, Andrighetto, Lombardi, & Lodi, Citation2006). In the present study, the examined strains did not present haemolytic activity when grown on a blood agar medium.
Antibiotic resistance
The evaluation of resistance to different antibiotics showed that strain J23 was susceptible to CXM, strain J26 to GE and CXM and strain J25 to LEV. Other strains were moderately susceptible to DC (strains J26 and J25), to GE (strains J25, J31, J32 and J34) and to LEV (strains J23, J32 and J34). No strains were susceptible to all antibiotics, and multiple resistances to most antibiotics were observed. In fact, all strains were resistant to PE, CF, E, AM, FEP, CL and TE.
Technological evaluation
β-Galactosidase activity
The β-galactosidase activities ranged from 137.64 ± 16.36 to 249.25 ± 0.54 MU for strains J24, J26, J27, J34, J31 and J37 (). In comparison, Zago et al. (Citation2011) reported a β-galactosidase activity of 600 MU for L. plantarum, which was much higher than that obtained in the present study. Another study reported values of 147–860 MU for Bifidobacterium and 675–1301 for L. acidophilus strains (Vinderola & Reinheimer, Citation2003). According to these results, β-galactosidase activity is dependent on the analysed strain, and Lactobacillus appears to present the highest activities.
Table 3. Evaluation of different technological properties of Lactobacillus strains in vitro.
Survival capacity under cold, lyophilizing and freezing conditions
All strains had a survival capacity of 80% under conditions of refrigeration, freezing and lyophilization. The percentages of survival under freezing conditions differed significantly among strains per bacterial concentration (p > .05) (). In comparison, the results of Zacarías et al. (Citation2011), who evaluated Bifidobacterium strains, did not show significant differences (p > .05) in cell viability under frozen and refrigeration conditions.
Under lyophilization conditions after 30 days of storage, the survival percentages of J31, J32, J34, J37 and J38 differed significantly (p < .05), corresponding with 71.54%, 63.81%, 81.11%, 81.00% and 66.70%, respectively.
Survival at pasteurization temperatures
The D values were evaluated for thermostability of the probiotic. Cell concentrations of all strains reduced after pasteurization and no viable cells of the J23, J27, J31 and J38 strains were detected after pasteurization (63°C, 30 min). The D values at 63°C were 5.43 and 5.63 min for strains J34 and J37, respectively (). Notably, the D values at 73°C were 11.50 and 13.76 s for strains J25 and J34, respectively. Together, these results indicate that the strains.
From the biotechnological and physiological characteristics, the strains J20, J23, J24, J25, J28 and J37 show the best probiotic capability and were selected for evaluation of the immune response.
Evaluation of the immune system
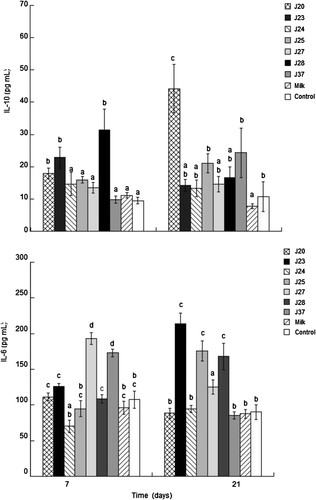
To evaluate the capacity of the selected strains to modulate the immune system, cytokines IL-6, IL-10 and the systemic IgA levels were determined in the serum of rats fed with fermented milk with the different strains. Our results revealed a significant increase (p < .05) in IL-10 in the groups that received milk fermented by strains J20, J23 and J28 after 7 days (17.93 ± 1.70, 22.85 ± 3.27 and 31.48 ± 6.45 pg/mL, respectively). Furthermore, the concentration of IL-10 was constant at 21 days after administration of the J20 strain (44.22 ± 7.47 pg/mL). Meanwhile, IL-6 increased significantly (p < .05) in groups administered with milk fermented by J27 and J37 during the first 7 days (193.33 ± 8.28 and 172.88 ± 5.42 pg/mL, respectively) but decreased after 21 days. However, in strains J23, J25 and J28, the IL-6 concentration increased significantly (p < .05) (213.77 ± 14.65, 176 ± 14.01 and 168 ± 18.86 pg/mL, respectively) with respect to the control group ().
Figure 2. Evaluation of IL-10 and IL-6 in serum following the administration of fermented milk. Different letters between bars indicate significant differences between treatment up to 7 and 21 days (p < .05).
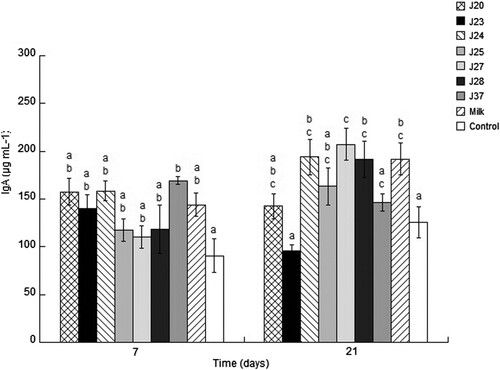
The IgA have a role in the protection mechanism of mucosal immunity, and confer protection pathogens; hence, high levels of IgA are considered as a health benefit. The concentration of IgA in serum did not significantly differ between the evaluated groups. However, between strains, the IgA concentration increases from 118.56 to 191.48, 158.35 to 193.94 and 110.35 to 207.12 for the strains J28, J24 and J27 at 21 days, J27 being the better strain with respect to the control group ().
Discussion
The important characteristics for selection of probiotics include resistance to acid and bile salts, adhesion capacity, resistance to technological process and conferring health benefits, and thus modulating the immune system (Vasiljevic & Shah, Citation2008). The results for pepsin and pancreatin displayed high viability, contrasting with the results of Maragkoudakis et al. (Citation2006), who found that different lactobacilli strains did not present viability after pepsin exposure. In the latter study, only L. rhamnosus ACA-DC 112 and L. paracasei subsp. paracasei ACA-DC 130 were viable after pepsin exposure. The results for pepsin and pancreatin displayed high viability, contrasting with the results of Maragkoudakis et al. (Citation2006), who found that different lactobacilli strains did not present viability after pepsin exposure. In the latter study, only L. rhamnosus ACA-DC 112 and L. paracasei subsp. paracasei ACA-DC 130 were viable after pepsin exposure. Resistance to bile salt is an important factor for the selection of potential probiotic bacteria, as strains with this characteristic can better colonize the small intestine of a host and carry out their metabolic activities (Liong & Shah, Citation2005; Strompfova & Laukova, Citation2007). In comparison to our results, L. rhamnosus isolated from Parmigiano Reggiano cheese showed better survival (Succi et al., Citation2005). In addition, different strains of L. plantarum and paraplantarum isolated from fermented Portuguese olives exhibited comparatively greater tolerance in conditions of acid and bile salt stress (Peres et al., Citation2014). Our results showed better tolerance under conditions of pH and enzymes, which confers them the potential to be considered probiotic.
The properties of adhesion to epithelial cells and mucosal surfaces have been suggested as characteristics for the selection of probiotics (Collado et al., Citation2008). The adherence of bacterial cells has been related to cell surface characteristics as well as complex processes involved in the interactions between bacterial cell membranes and other surfaces. Several researchers have reported varying interactions between epithelial cells and mucus depending on the composition and structure of different bacteria (Collado, Gueimonde, Hernandez, Sanz, & Salminen, Citation2005; del Re, Sgorbati, Miglioli, & Palenzona, Citation2000; Ouwehand, Salminen, & Isolauri, Citation2002). On the other hand, the adhesion characteristics to cell surface hydrophobicity influence non-specific interactions between microbial and host cells. Initial interactions may be weak and often reversible and often precede subsequent adhesion processes mediated by specific mechanisms, as the binding of macromolecular protein complexes and amphophile lipoteichoic acids (LTAs) to bacterial surfaces, which then serves to bind cells to tissue surfaces (Rojas, Ascencio, & Conway, Citation2002; Roos & Jonsson, Citation2002). In this sense, proteins, LTA and deacylated teichoic acid combine via hydrophobic and hydrophilic domains and mutually interact to form a linear layer terminated by an LTA molecule (Percy & Gründling, Citation2014).
Furthermore, the adhesion of microbes in polar solvents may be affected by cell surfaces and the presence of proteins on cell surfaces. Meanwhile, hydrophilic surfaces are associated with the presence of polysaccharides (Perez, Minnaard, Disalvo, & De Antoni, Citation1998; Rojas & Conway, Citation1996), and the surface layer (S-layer) proteins detected in some Lactobacillus strains may be involved in adherence (Mukai & Arihara, Citation1994; Schneitz, Nuotio, & Lounatma, Citation1993).
On the other hand, safety characteristics such as mucin degradation, haemolytic activity and antibiotic resistance were determined. In our study, for mucin degradation, none of our evaluated strains showed degradation significantly with respect to strain control. However, studies have been reported that Bifidobacterium spp. and Lactobacillus spp. (Abe et al., Citation2010; Monteagudo-Mera et al., Citation2012; Peres et al., Citation2014) were also unable to degrade mucin. In contrast, another study demonstrated that Bifidobacterium bifidum, Bifidobacterium breve and Bifidobacterium longum possessed mucin degradation activity and genes that encode mucin-decomposing enzymes (Ruas-Madiedo, Gueimonde, Fernández-García, de los Reyes-Gavilán, & Margolles, Citation2008). The negative effect of mucin degradation facilitates translocation of bacteria to lamina propria and extraintestinal tissues (Zhou et al., Citation2001) and our results suggest that bacteria are non-invasive to host.
Haemolytic activity is correlated with the production of exotoxins and induces lysis of blood cells. Our results were similar to those found by Kalui, Mathara, Kutima, Kiiyukia, and Wongo (Citation2009); Mami, Henni, and Kihal (Citation2008), Peres et al. (Citation2014) and Thirabunyanon et al. (Citation2009), who reported that L. plantarum isolated from fermented maize porridge, raw goat’s milk, LAB fermented dairy and fermented Portuguese olives, respectively, does not exhibit haemolytic activity.
Liasi et al. (Citation2009) and Boulares et al. (Citation2012) found that strains isolated from fermented fish products and Tunisian fresh fish were resistant to different antibiotics. In contrast, the findings of Banwo, Sanni, and Tan (Citation2013) demonstrated that Enterococcus faecium CM4 had a high susceptibility to vancomycin but was moderately sensitive to other antibiotics. Another study reported resistance to vancomycin and teicoplanin and sensitivity to chloramphenicol and tetracycline in different Lactobacillus strains (Maragkoudakis et al., Citation2006). Furthermore, L. plantarum strains isolated from Italian and Argentinean cheeses presented resistance to tetracycline and sensitivity to gentamicin, erythromycin and chloramphenicol (Zago et al., Citation2011). The antibiotics’ resistance is important for the use of probiotics due to the possibility of transferring antibiotic resistance genes to pathogens.
The technological properties and resistance of probiotics to pasteurization conditions demonstrated their possible usage as starter cultures at an industrial level. Some strains showed survival under pasteurization conditions and D values were strain dependent. In a study reported by Crow et al. (Citation2002), D value for a strain of L. paracasei was 5.2 s at 72°C, whereas the D value of L. paracasei DPC2103 isolated from Irish Cheddar cheese was 4.45 s at 72°C (Jordan & Cogan, Citation1999). Also, L. paracasei isolated from milk had a D value of 2 s at 73°C (Christiansen, Waagner, Vogensen, Brogren, & Ardö, Citation2006). Compared with our results, the strains evaluated showed higher D values which showed a better stability for heat treatment.
Some characteristics of heat resistance could be affected by different conditions such as physiological status, pH, water activity and salt content (Casadei, Ingram, Hitchings, Archer, & Gaze, Citation2001). The presence of surface S-layers formed by proteins, which are found in different strains of Lactobacillus, may be responsible for heat resistance. In other bacteria such as L. acidophilus NCFM, L. casei LC301, L. helveticus LH212 and L. plantarum DPC2739, different proteins have been found to play a role in stress response mechanisms (Broadbent, Oberg, Wang, & Wei, Citation1997; de Angelis & Gobbetti, Citation2004). The expression of heat-shock proteins (DnaK and GroEL) also varies depending on the species. Angelis et al. (Citation2004) reported that the protein expression of L. plantarum DPC2739 was related with some physiological processes in the cell (e.g. chaperone activity, ribosome stability, stringent response mediation, temperature sensing and control of ribosomal functions). Our study shows evidence of possible adaptations in the heat responses, which may be related to the expression of some proteins on the cell surfaces.
Several researchers have demonstrated the beneficial effects of fermented milk on the immune system when consumed for a prolonged period, showing the importance of doses and cell viability for the activation of macrophages and mucosal response (de Moreno de LeBlanc et al., Citation2005; Medici et al., Citation2005). The results showed the potential effect on the immune system systemic to enhance IL-10 cytokine, which has an important role as a regulatory cytokine in different processes of inflammation for maintaining the homeostasis of tissue epithelial layers, also facilitating the tissue-healing process and repressing the pro-inflammatory response (Ouyang, Rutz, Crellin, Valdez, & Hymowitz, Citation2011). On the other hand, the regulation of IL-6 has an important role during chronic inflammation and autoimmunity, as the administration of fermented milk might decrease the negative effect of IL-6 when it is synthesized in high concentrations (Tanaka, Narazaki, & Kishimoto, Citation2014).
The administration of fermented milk for 98 days increased the CD4+ and CD8+ cells in the lamina propria of the intestine; cytokines IL-10, TNFα, IFNγ and IL-12 and IgA+ cells in the mucosal immune system; and the activity of macrophages (de Moreno de LeBlanc et al., Citation2008). Furthermore, milk fermented with probiotics and biomolecules or free-cell fractions may become involved in different systemic and mucosal responses (Galdeano et al., Citation2009; Medici et al., Citation2005). The interaction between lamina propria and probiotics is mainly generated at the gut level. Several models have been considered for this interaction, including one based on the maintained contact of probiotics with gut-associated immune cells of the small intestine. Dendritic cells and macrophages are the first cells that interact with bacteria and may induce the production of cytokines and favour lymphocyte proliferation (Galdeano, de Moreno de LeBlanc, Vinderola, Bonet, & Perdigón, Citation2007). The response of the small intestine’s immune system is associated with Peyer’s patches, which are the principal inductive sites after oral administration of an antigen. Anatomically, these are connected to the systemic immune system by the mesenteric lymphatic node. Their interaction with probiotics induces an increase in IgA+ cells in distant mucosal sites. The IgA+ cells migrate to the mesenteric lymphoid node and then, via the thoracic duct, through the circulatory system. A response generated in the intestine may therefore spread throughout the systemic immune system and reach gastrointestinal mucosal system (Galdeano et al., Citation2009; Vinderola et al., Citation2006).
Conclusion
The physiological and technological characteristics of LAB isolated from artisanal Cocido cheese demonstrate the potential probiotic of these bacteria and their possible applications in the development of functional foods such as fermented milk. An in vivo evaluation was carried out wherein milk was fermented with selected Lactobacillus strains. Milk fermented for longer periods may have the capacity to stimulate the systemic immune system. However, more studies are needed to establish the components contained in fermented milk that stimulate an immunoregulatory response and to assess the effects of this response on other diseases related to the immune system.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abe, F. , Muto, M. , Yaeshima, T. , Iwatsuki, K. , Aihara, H. , Ohashi, Y. , & Fujisawa, T. (2010). Safety evaluation of probiotic bifidobacteria by analysis of mucin degradation activity and translocation ability. Anaerobe , 16 (2), 131–136. doi: https://doi.org/10.1016/j.anaerobe.2009.07.006
- Amrouche, T. , Butin, Y. , & Fliss, I. (2006). Effects of bifidobacterial cytoplasm peptide and protein fractions on mouse lymphocyte proliferation and cytokine production. Food and Agricultural Immunology , 17 , 29–42. doi: https://doi.org/10.1080/09540100600565895
- Banwo, K. , Sanni, A. , & Tan, H. (2013). Technological properties and probiotic potential of Enterococcus faecium strains isolated from cow milk. Journal of Applied Microbiology , 114 , 229–241. doi: https://doi.org/10.1111/jam.12031
- Bartazzoni, E. , Donelli, G. , Midtvedt, T. , Nicoli, J. , & Sanz, Y. (2013). Probiotics and clinical effects: Is the number what counts? Journal of Chemotherapy , 25 , 193–212. doi: https://doi.org/10.1179/1973947813Y.0000000078
- Boulares, M. , Aouadhi, C. , Mankai, M. , Moussa, O. B. , Essid, I. , & Hassouna, M. (2012). Characterisation, identification and technological properties of psychotropic lactic acid bacteria originating from Tunisian fresh fish. Journal of Food Safety , 32 , 333–344. doi: https://doi.org/10.1111/j.1745-4565.2012.00385.x
- Boza-Mendez, E. , Lopez-Calvo, R. , & Cortes-Muñoz, M. (2012). Innovative dairy products development using probiotics: Challenges and limitations. In E. Rigobelo (Ed.), Probiotics (pp. 213–226). London : InTech.
- Broadbent, J. R. , Oberg, C. J. , Wang, H. , & Wei, L. (1997). Attributes of the heat shock response in three species of dairy Lactobacillus . Systematic and Applied Microbiology , 20 , 12–19. doi: https://doi.org/10.1016/S0723-2020(97)80043-4
- Casadei, M. A. R. , Ingram, E. , Hitchings, J. , Archer, J. , & Gaze, J. E. (2001). Heat resistance of Bacillus cereus, Salmonella thyphimurium and Lactobacillus delbrueckii in relation to pH and ethanol. International Journal of Food Microbiology , 63 , 125–134. doi: https://doi.org/10.1016/S0168-1605(00)00465-7
- Chiang, S.-S. , Liu, C.-F. , Tseng, K.-C. , Mau, J.-L. , & Pan, T.-M. (2012). Immunomodulatory effects of dead Lactobacillus on murine splenocytes and macrophages. Food and Agricultural Immunology , 23 , 183–202. doi: https://doi.org/10.1080/09540105.2011.609246
- Christiansen, P. , Waagner, N. E. , Vogensen, F. K. , Brogren, C.-H. , & Ardö, Y. (2006). Heat resistance of Lactobacillus paracasei isolated from semi-hard cheese made of pasteurized milk. International Dairy Journal , 16 , 1196–1204. doi: https://doi.org/10.1016/j.idairyj.2005.10.009
- Collado, M. C. , Gueimonde, M. , Hernandez, M. , Sanz, Y. , & Salminen, S. (2005). Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion. Journal of Food Protection , 68 , 2672–2678. doi: https://doi.org/10.4315/0362-028X-68.12.2672
- Collado, M. C. , Meriluoto, J. , & Salminen, S. (2008). Adhesion and aggregation properties of probiotic and pathogen strains. European Food Research and Technology , 226 , 1065–1073. doi: https://doi.org/10.1007/s00217-007-0632-x
- Crow, V. , Curry, B. , Christison, M. , Hellier, K. , Holland, R. , & Liu, S. Q. (2002). Raw milk flora and NSLAB as adjuncts. Australian Journal of Dairy Technology , 57 , 99–105.
- Cuevas-González, P. F. , Heredia-Castro, P. Y. , Méndez-Romero, J. I. , Hernández-Mendoza, A. , Reyes-Díaz, R. , Vallejo-Cordoba, B. , & González-Córdoba, A. F. (2017). Artisanal Sonoran cheese (Cocido cheese): an exploration of this production process, chemical composition and microbiological quality. Journal of the Science of Food and Agriculture , 97 , 4459–4466. doi: https://doi.org/10.1002/jsfa.8309
- De Angelis, M. , Di Cagno, R. , Huet, C. , Crecchio, C. , Fox, P. F. , & Gobbetti, M. (2004). Heat shock response in Lactobacillus plantarum. Applied and Environmental Microbiology , 70 , 1336–1346. doi: https://doi.org/10.1128/AEM.70.3.1336-1346.2004
- de Angelis, M. , & Gobbetti, M. (2004). Environmental stress responses in Lactobacillus: A review. Proteomics , 4 , 106–122. doi: https://doi.org/10.1002/pmic.200300497
- del Re, B. , Sgorbati, B. , Miglioli, M. , & Palenzona, D. (2000). Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum . Letters in Applied Microbiology , 31 , 438–442. doi: https://doi.org/10.1046/j.1365-2672.2000.00845.x
- de Moreno de LeBlanc, A. , Chavez, S. , Carmuega, E. , Weill, R. , Antoine, J. , & Perdigon, G. (2008). Effect of long-term continuous consumption of fermented milk containing probiotic bacteria on mucosal immunity and the activity of peritoneal macrophages. Immunobiology , 213 , 97–108. doi: https://doi.org/10.1016/j.imbio.2007.07.002
- de Moreno de LeBlanc, A. , Matar, C. , Thériault, C. , & Perdigón, G. (2005). Effects of milk fermented by Lactobacillus helveticus R389 on immune cells associated to mammary glands in normal and breast cancer model. Immunobiology , 210 , 349–358. doi: https://doi.org/10.1016/j.imbio.2005.05.024
- de Moreno de LeBlanc, A. , Valdez, J. , & Perdigon, G. (2004). Inflammatory immune response. European Journal of Inflammation , 2 , 21–31. doi: https://doi.org/10.1177/1721727X0400200104
- de Vrese, M. , & Schrezenmeir, J. (2008). Probiotics, prebiotics, synbiotics. Advances in Biochemical Engineering Biotechnology , 111 , 1–66.
- de Vuyst, L. , Foulquie, M. R. , & Revets, H. (2003). Screening for enterocins and detection of hemolysin and vancomycin resistance in Enterococci of different origins. International Journal of Food Microbiology , 84 , 299–318. doi: https://doi.org/10.1016/S0168-1605(02)00425-7
- Fernandez, M. F. , Boris, S. , & Barbes, C. (2005). Safety evaluation of Lactobacillus delbrueckii subsp. Lactis UO 004, a probiotic bacterium. Research in Microbiology , 156 , 154–160. doi: https://doi.org/10.1016/j.resmic.2004.09.006
- Galdeano C. M. , de Moreno de LeBlanc, A. , Carmuega, E. , Weill, R. , & Perdigón, G. (2009). Mechanisms involved in the immunostimulation by probiotic fermented milk. Journal of Dairy Research , 76 , 446–454. doi: https://doi.org/10.1017/S0022029909990021
- Galdeano C. M. , de Moreno de LeBlanc, A. , Vinderola, M. E. , Bonet, B. , & Perdigon, G. (2007). Proposed model: Mechanisms of immunomodulation induced by probiotic bacteria. Clinical and Vaccine Immunology , 14 , 485–492. doi: https://doi.org/10.1128/CVI.00406-06
- González-Córdova, A. F. , Yescas, C. , & Ortiz-Estrada, A. M. (2016). Invited review: Artisanal Mexican cheeses. Journal of Dairy Science , 99 , 3250–3262. doi: https://doi.org/10.3168/jds.2015-10103
- Guerra-Martínez, J. A. , Montejano, J. G. , & Martín del Campo, S. T. (2012). Evaluation of proteolytic and physicochemical changes during storage of fresh Panela cheese from Queretaro, Mexico and its impact in texture. CyTA Journal of Food , 10 , 296–305. doi: https://doi.org/10.1080/19476337.2011.653791
- Heredia-Castro, P. Y. , Méndez-Romero, J. I. , Hernández-Mendoza, A. , Acedo-Felix, E. , González-Córdova, A. F. , & Vallejo-Cordoba, B. (2015). Antimicrobial activity and partial characterization of bacteriocin-like inhibitory substances produced by Lactobacillus spp. isolated from artisanal Mexican cheese . Journal of Dairy Science , 98 , 8285–8293. doi: https://doi.org/10.3168/jds.2015-10104
- Hill, C. , Guarner, F. , Reid, G. , Gibson, G. R. , Merenstein, D. J. , Pot, B. , … Sanders, M. E. (2014). The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic . Nature Reviews Gastroenterology & Hepatology , 11 , 506–514. doi: https://doi.org/10.1038/nrgastro.2014.66
- Jordan, K. N. , & Cogan, T. M. (1999). Heat resistance of Lactobacillus spp. isolated from Cheddar cheese. Letters in Applied Microbiology , 29 , 136–140. doi: https://doi.org/10.1046/j.1365-2672.1999.00607.x
- Kalui, C. M. , Mathara, J. M. , Kutima, P. M. , Kiiyukia, C. , & Wongo, L. E. (2009). Functional characteristics of Lactobacillus plantarum and Lactobacillus rhamnosus from ikki a Kenyan traditional fermented maize porridge. African Journal of Food Microbiology , 67 , 147–152.
- Karska-Wysocki, B. , Bazo, M. , & Smoragiewicz, W. (2010). Antibacterial activity of Lactobacillus acidophilus and Lactobacillus casei against methicillin-resistant Staphylococcus aureus (MRSA). Microbiological Research , 165 , 674–686. doi: https://doi.org/10.1016/j.micres.2009.11.008
- Kumar, D. , Arya, V. , Kaur, R. , Bhat, Z. A. , Gupta, V. K. , & Kumar, V. (2012). A review of immunomodulators in the Indian traditional health care system. Journal of Microbiology, Immunology , 45 , 165–184.
- Liasi, B. , Asmi, S. A. , Hassan, T. I. , Shuhaimi, M. D. , Rosfarizan, M. , & Ariff, A. B. (2009). Antimicrobial activity and antibiotic sensitivity of three isolated of lactic acid bacteria from fermented fish product. Malays Journal Microbiology , 5 , 33–57.
- Liong, M. T. , & Shah, N. P. (2005). Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. Journal of Dairy Science , 88 , 55–66. doi: https://doi.org/10.3168/jds.S0022-0302(05)72662-X
- Mami, A. , Henni, J. E. , & Kihal, M. (2008). Antimicrobial activity of Lactobacillus species isolated from Algerian raw goat’s milk against Staphylococcus aureus. World Journal of Dairy and Food Sciences , 3 , 39–49.
- Maragkoudakis, P. A. , Zoumpopoulou, G. , Miaris, C. , Kalantzopoulos, G. , Pot, B. , & Tsakalidou, E. (2006). Probiotic potential of Lactobacillus strains isolated from dairy products. International Dairy Journal , 16 , 189–199. doi: https://doi.org/10.1016/j.idairyj.2005.02.009
- Matar, C. , Valdez, J. C. , Medina, M. , Rachid, M. , & Perdigon, G. (2001). Immunomodulating effects of milks fermented by lactobacillus helveticus and its non-proteolytic variant. Journal of Dairy Research , 68 , 601–609. doi: https://doi.org/10.1017/S0022029901005143
- Medici, M. , Vinderola, C. G. , Weill, R. , & Perdigón, G. (2005). Effect of fermented milk containing probiotic bacteria in the prevention of an enteroinvasive Escherichia coli infection in mice. Journal of Dairy Research , 72 , 243–249. doi: https://doi.org/10.1017/S0022029905000750
- Monteagudo-Mera, A. , Rodrıguez-Aparicio, L. , Rúa, J. , Martinez-Blanco, H. , Navasa, N. , Garcia-Armesto, M. , & Ferrero, M. A. (2012). In vitro evaluation of physiological probiotic properties of different lactic acid bacteria strains of dairy and human origin. Journal of Functional Foods , 4 , 531–541. doi: https://doi.org/10.1016/j.jff.2012.02.014
- Morandi, S. , Brasca, M. , Andrighetto, C. , Lombardi, A. , & Lodi, R. (2006). Technological and molecular characterization of enterococci isolated from north-west Italian dairy products. International Dairy Journal , 16 , 867–875. doi: https://doi.org/10.1016/j.idairyj.2005.09.005
- Mukai, T. , & Arihara, K. (1994). Presence of intestinal lectin-binding glycoproteins on the cell surface of Lactobacillus acidophilus . Bioscience, Biotechnology, and Biochemistry , 58 , 1851–1854. doi: https://doi.org/10.1271/bbb.58.1851
- Ouwehand, A. C. , Salminen, S. , & Isolauri, E. (2002). Probiotics: An overview of beneficial effects. Antonie van Leeuwenhoek , 82 , 279–289. doi: https://doi.org/10.1023/A:1020620607611
- Ouyang, W. , Rutz, S. , Crellin, N. K. , Valdez, P. A. , & Hymowitz, S. G. (2011). Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual Review of Immunology , 29 , 71–109. doi: https://doi.org/10.1146/annurev-immunol-031210-101312
- Percy, M. G. , & Gründling, A. (2014). Lipoteichoic acid synthesis and function in gram-positive bacteria. Annual Review of Microbiology , 68 , 81–100. doi: https://doi.org/10.1146/annurev-micro-091213-112949
- Peres, C. M. , Alves, M. , Hernandez-Mendoza, A. , Moreira, L. , Silva, S. , Bronze, M. R. , … Malcata, F. X. (2014). Novel isolates of Lactobacilli from fermented Portuguese olive as potential probiotics. LWT-Food Science and Technology , 59 , 234–246. doi: https://doi.org/10.1016/j.lwt.2014.03.003
- Pérez, P. F. , Minnaard, Y. , Disalvo, E. A. , & de Antoni, G. L. (1998). Surface properties of bifidobacterial strains of human origin. Applied and environmental microbiology , 64 , 21–26.
- Rojas, M. , Ascencio, F. , & Conway, P. L. (2002). Purification and characterization of a surface protein from Lactobacillus fermentum 104R that binds to porcine small intestinal mucus and gastric mucin. Applied and Environmental Microbiology , 68 , 2330–2336. doi: https://doi.org/10.1128/AEM.68.5.2330-2336.2002
- Rojas, M. , & Conway, P. L. (1996). Colonization by lactobacilli of piglet small intestinal mucus. The Journal of Applied Bacteriology , 81 , 474–480.
- Roos, S. , & Jonsson, H. (2002). A high-molecular-mass cell-surface protein from lactobacillus reuteri 1063 adheres to mucus components. Microbiology , 148 , 433–442. doi: https://doi.org/10.1099/00221287-148-2-433
- Ruas-Madiedo, P. , Gueimonde, M. , Fernández-García, M. , de los Reyes-Gavilán, C. G. , & Margolles, A. (2008). Mucin degradation by Bifidobacterium strains isolated from the human intestinal microbiota. Applied and Environmental Microbiology , 74 , 1936–1940. doi: https://doi.org/10.1128/AEM.02509-07
- Ruseler-van Embden, J. G. H. , van Lieshcut, L. M. C. , Gosselink, M. J. , & Marteau, P. (1995). Inability of Lactobacillus casei strain GG, Lactobacillus acidophilus and Bifidobacterium bifidum to degrade intestinal mucus glycoproteins. Scandinavian Journal of Gastroenterology , 30 , 675–680. doi: https://doi.org/10.3109/00365529509096312
- Saxer, S. , Schwenninger, S. M. , & Lacroix, C. (2013). Characterization of the microflora of industrial Mexican cheeses produced without added chemical preservatives. LWT – Food Science and Technology , 53 (1), 314–320. doi: https://doi.org/10.1016/j.lwt.2013.01.016
- Schneitz, C. , Nuotio, L. , & Lounatma, K. (1993). Adhesion of Lactobacillus acidophilus to avian intestinal epithelial cells mediated by the crystalline bacterial cell surface layer (S-layer). Journal of Applied Bacteriology , 74 , 290–294. doi: https://doi.org/10.1111/j.1365-2672.1993.tb03028.x
- Strompfova, V. , & Laukova, A. (2007). In vitro study on bacteriocin production of enterococci associated with chickens. Anaerobe , 13 , 228–237. doi: https://doi.org/10.1016/j.anaerobe.2007.07.002
- Succi, M. , Tremonte, P. , Reale, A. , Sorrentino, E. , Grazia, L. , Pacifico, S. , & Coppola, R. (2005). Bile salt and acid tolerance of Lactobacillus rhamnosus strains isolated from Parmigiano Reggiano cheese. FEMS Microbiology letters , 244 , 129–137. doi: https://doi.org/10.1016/j.femsle.2005.01.037
- Tanaka, T. , Narazaki, M. , & Kishimoto, T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspectives in Biology , 6 (10), a016295. doi: https://doi.org/10.1101/cshperspect.a016295
- Thirabunyanon, M. , Boonprasom, P. , & Niamsup, P. (2009). Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnology Letters , 31 , 571–576. doi: https://doi.org/10.1007/s10529-008-9902-3
- Torres-Llanez, M. J. , Vallejo-Cordoba, B. , Díaz-Cinco, M. E. , Mazorra-Manzano, M. A. , & González-Córdova, A. F. (2006). Characterization of the natural microflora of artisanal Mexican Fresco cheese. Food Control , 17 , 683–690. doi: https://doi.org/10.1016/j.foodcont.2005.04.004
- Tunick, M. H. , van Hekken D. L. , Call J. , Molina-Corral, F. J. , & Gardea, A. A. (2007). Queso Chihuahua: Effects of seasonality of cheesemilk on rheology. International Journal of Dairy Technology , 60 , 13–21. doi: https://doi.org/10.1111/j.1471-0307.2007.00295.x
- Van Hekken, D. L. , Drake, M. A. , Corral, F. J. M. , Guerrero-Prieto, V. M. , & Gardea, A. A. (2006). Mexican Chihuahua cheese: Sensory profiles of young cheese. Journal of Dairy Science , 89 , 3729–3738. doi: https://doi.org/10.3168/jds.S0022-0302(06)72414-6
- Vasiljevic, T. , & Shah, N. P. (2008). Probiotics – From Metchnikoff to bioactives. International Dairy Journal , 18 , 714–728. doi: https://doi.org/10.1016/j.idairyj.2008.03.004
- Vinderola, G. , Perdigón, G. , Duarte, J. , Farnworth, E. , & Matar, C. (2006). Effects of the oral administration of the products derived from milk fermentation by kefir microflora on immune stimulation. Journal of Dairy Research , 73 , 472–479. doi: https://doi.org/10.1017/S002202990600197X
- Vinderola, C. G. , & Reinheimer, J. A. (2003). Lactic acid starter and probiotic bacteria: A comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Research International , 36 , 895–904. doi: https://doi.org/10.1016/S0963-9969(03)00098-X
- Zacarías, M. F. , Binetti, A. , Laco, M. , Reinheimer, J. , & Vinderola, G. (2011). Preliminary technological and potential probiotic characterisation of bifidobacteria isolated from breast milk for use in dairy products. International Dairy Journal , 21 , 548–555. doi: https://doi.org/10.1016/j.idairyj.2011.03.007
- Zago, M. , Fornasari, M. E. , Carminati, D. , Burns, P. , Suarez, V. , Vinderola, G. , … Giraffa, G. (2011). Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiology , 28 , 1033–1040. doi: https://doi.org/10.1016/j.fm.2011.02.009
- Zhou, J. S. , Gopal, P. K. , & Gill, H. S. (2001). Potential probiotic lactic acid bacteria Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) do not degrade gastric mucin in vitro . International Journal of Food Microbiology , 63 , 81–90. doi: https://doi.org/10.1016/S0168-1605(00)00398-6