?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Using immunological and cell fusion technology, group-specific monoclonal antibodies were produced against cyproheptadine. The 50% inhibitory concentration of the antibody for cyproheptadine was 0.37 ng/mL, the linear range was 0.16–0.85 ng/mL, and recoveries of antibody in spiked negative pig urine were 80–120%. An ultrasensitive immunochromatographic strip, based on the antibody, was developed for the rapid detection of cyproheptadine in pig urine. The test strip had a cut-off value of 5 ng/mL.
Introduction
Cyproheptadine is an anti-allergy drug that antagonizes the effects of histamine on blood vessels and bronchial smooth muscle, thus eliminating allergic symptoms (Lin et al., Citation2016; Szepietowski & Reszke, Citation2016). Despite being banned for veterinary use, some animal feed products used in China have been found to contain cyproheptadine. This is because cyproheptadine can promote weight gain in livestock by increasing appetite and has a similar effect to β-agonists which can promote pig growth by reducing fat content and raising lean meat percentage., such as clenbuterol (Lulebo et al., Citation2016; Martin-Galvez & Soler, Citation2017). In humans, high concentrations of cyproheptadine can cause coma, or even death, with children and the elderly being the most sensitive groups (Merhar et al., Citation2016). The veterinary use of cyproheptadine is, therefore, strictly prohibited both in China and other countries. On December 27 2010, the Chinese Ministry of Agriculture issued Announcement 1519, which made it clear that the use of cyproheptadine was forbidden in animal feed products and in animal drinking water.
At present, traditional chromatographic analysis methods, such as high performance liquid chromatography (Yang et al., Citation2015), gas chromatography-mass spectrometry (Hasegawa et al., Citation2006) and liquid chromatography-mass spectrometry(Sun et al., Citation2017), are used for the detection of cyproheptadine residues. Electrochemical methods, such as capillary electrophoresis (Li, Xu, Albano, & You, Citation2011; Mendes et al., Citation2012; Zhu, Xia, Sun, & You, Citation2012) and electrochemical sensors have also been used. Although these methods are highly sensitive and accurate, they require expensive instruments and time-consuming pretreatment processes and, since they also incur high detection costs, they are unsuitable for screening large quantities of samples(Yan, Liu, Xu, Kuang, & Xu, Citation2015). Enzyme-linked immunosorbent assay (ELISA) is a fast, sensitive, and efficient method that is suitable for rapid detection of drug residues, but it is inappropriate for large-scale sample testing (Gu, Liu, Song, Kuang, & Xu, Citation2016). At present, testing for cyproheptadine residues in pigs intended for the market is mainly carried out using porcine urine, but matrix interference seriously affects the analysis. The identification of highly sensitive monoclonal antibodies that are unaffected by matrix interference and the development of a colloidal gold immunoassay paper test strip that can be used for rapid analysis in the field is thus of great significance for the maintenance of national food safety in animal-derived products.
Materials and methods
Chemicals
Cyproheptadine hydrochloride was purchased from J&K Scientific (Shanghai, China), keyhole limpet hemocyanin (KLH), ovalbumin(OVA), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), 2-methylpropyl carbonochloridate, N-hydroxysuccinimide (NHS), N,N-dimethylformamide (DMF),enzyme immunoassay grade horseradish peroxidase-labeled goat anti-mouse immunoglobulin, 3,3′,5,5′-tetramethylbenzidine (TMB), Tween-20, and horseradish peroxidase (HRP)were purchased from Sigma Chemical Company(St. Louis, MO, USA). Hypoxanthine-aminopterin-thymidine supplement, hypoxanthine-thymidine supplement, and 1640 cell culture medium were purchased from Gibco (Shanghai, China).
Glass fiber membrane for the sample pad, nitrocellulose (NC) membrane for immobilizing the coating antigen, H5076 filter paper for the absorbent pad and Ahlstrom 8964 glass microfiber filter paper for the conjugated pad were purchased from JieYi Biotech. Co., Ltd. (Shanghai, China).
Solutions
The solutions used for the assays were: PBS (0.01 M phosphate, pH 7.4), MES (0.1 M 2-(N-morpholino) ethanesulfonic acid, pH 4.7), PBST (0.05% Tween-20 (v/v) in 0.01 M PBS), coating buffer (CB:0.05 M carbonate–bicarbonate buffer, pH 9.6), antibody dilution buffer (PBS containing 0.1% gelatin), blocking buffer (coating buffer containing 0.2% gelatin), solution A (9.33 g citric acid, 180 μL H2O2, and 36.8 g Na2HPO4 12H2O in 1L H2O) and solution B (0.06% w/v TMB in glycol). Pig urine was obtained from a local farm (Wuxi, China).
Synthesis of hapten
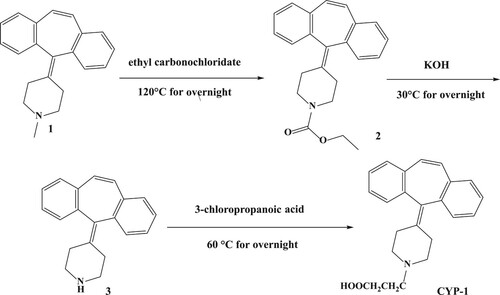
The hapten was synthesized by nucleophilic substitution on the piperidine ring N atom of cyproheptadine, followed by demethylation and subsequent alkylation with 3-chloropropanoic acid to provide CYP-1, which has a free carboxyl group (). Compound 1 (5 g, 17.4 mmol) in toluene (50 mL) was added dropwise to ethyl carbonochloridate (3.8 g, 34.8 mmol) and the mixture was stirred at 120°C overnight. The mixture was cooled to room temperature, treated with 10 mL aqueous HCl and extracted with EtOAc (3 × 50 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo to give compound 2 (4 g, 66.6%). A solution of compound 2 (4 g, 11.6 mmol) in EtOH (64 mL) was added dropwise to aqueous KOH solution (10.3 g dissolved in 16 mL H2O, 174 mmol, 15 equiv) and the mixture was stirred at 30°C overnight. The mixture was then concentrated in vacuo and extracted with EtOAc (3 × 50 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo to give compound 3 (2.5 g, 80.6%). A solution of compound 3 (2.5 g, 9.2 mmol) and 3-chloropropanoic acid (1 g, 9.2 mmol)in THF (30 mL) was added dropwise to Et3N (1 g, 9.2 mmol) and the mixture was stirred under N2 at 60°C overnight. The mixture was then concentrated in vacuo and extracted with EtOAc (3 × 30 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo to give the crude product, which was purified by chromatography on silicagel eluting with dichloromethane/MeOH (20:1) to afford the hapten, CYP-1(400 mg, 12.7%).
Antigen preparation
The immunogenic antigen (CYP-1-EDC-KLH) was prepared by coupling the hapten to KLH. Firstly, hapten (2.1 mg), EDC(4.2 mg), and NHS(2.6 mg) were mixed in a small bottle. The mixture was dissolved in DMF (1 mL) and MES (200 μL) and the solution was stirred overnight at room temperature in the dark, to provide solution I. KLH (6 mg) was dissolved in CB (2 mL) and solution I was added slowly to the protein solution, maintaining the pH of the solution at 9–10. Next, the solution was stirred overnight at room temperature in the dark. The immunogenic antigen was dialyzed in 0.01 M PBS for two days to remove foreign substances. After confirming its immunogenicity by ultraviolet spectroscopy, the antigen was stored at −20°C. The coating antigen (CYP-1–EDC-OVA) was prepared in a similar manner.
Monoclonal antibody (mAb) preparation
Healthy BALB/c mice (5, female), which were 6–8 weeks of age, were selected for immunization. CYP-1-EDC-KLH (1 mg/mL) was emulsified with the same amount of Freund’s adjuvant and the mice were immunized by subcutaneous multi-point injection with antigen (100 μg). The first immunization used Fuchs complete adjuvant and Freund’s incomplete adjuvant to strengthen the immune response. The dose for subsequent intraperitoneal injections was half of the previous dose, and the intraperitoneal injections were carried out directly using synthetic antigen mixed with normal saline. The immunization intervals were three weeks. After the third immunization, blood titers and the 50% inhibitory concentration were measured at intervals of one week. The best mice were selected for intraperitoneal injection with synthetic antigen on day 21 and, after the fourth immunization, cells from these mice were removed for fusion. Following an appropriate cell fusion and hybridoma screening program, hybridoma cells that could produce antibodies against cyproheptadine were obtained. The cell strain that could secrete monoclonal antibodies was expanded and cultured, and the cells were collected and injected into the abdominal cavity of mice. After 7–10 days, the ascites in the abdominal cavity of mice was extracted using a syringe. The monoclonal antibody was purified using the caprylic acid-saturated ammonium sulfate method (Kong et al., Citation2015).
Characterization of mAbs
Indirect competitive (IC)-ELISA can be used to evaluate the cross-reactivity and sensitivity of mAbs. After dilution with coating buffer, CYP-1-OVA (100 μL/well)was added to standard 96-well ELISA plates, which were then incubated in an oven at 37°C for 2 h. The plates were washed three times with PBST and patted dry using absorbent paper. Blocking buffer (200 μL/well) was added to the plates, followed by incubation in an oven for 2 h at 37°C. The plates were subsequently washed three times and dried using absorbent paper. Diluted monoclonal antibody (50 μL/well) and standard diluent were added to each well and the plate was incubated in an oven for 30 min at 37°C. The plates were washed and patted dry. HRP, diluted three thousand times with antibody dilution buffer (100 μL/well), was added to the plates, which were then incubated in an oven for 30 min at 37°C. The plates were washed three times with PBST and dried on absorbent paper. Fresh color buffer (100 μL, solution A/solution B = 5:1, v/v) was added to each well, and the plate was incubated in an oven for 15 min at 37°C. Sulfuric acid (50 μL, 2 M) was pipetted into all wells to stop the reaction and absorbance values (at 450 nm) were measured using the enzyme marker.
Specificity of mAbs
The specificity of the mAbs was defined by cross-reactivity measurements. If there was <10% cross-reactivity, there was assumed to be no cross-reaction or negligible cross-reaction. Cross-reactivities between the antibody and a series of β-agonists (salbutamol, clenbuterol, clenpenterol hydrochloride, mabuterol, terbutaline, clenpenterol hydrochloride, mapenterol, and pirbuterol) were confirmed by IC-ELISA:
Preparation of mAb-labeled gold nanoparticles
The average diameter of the gold particles used in the experiment was 20–30 nm. The specific method was as follows: chloroauric acid solution (100 mL, 0.01 g/L HAuCl4) was heated to boiling point with magnetic stirring for 30 min and sodium citrate solution (2 mL, 1%) was then quickly added. The color of the reaction solution rapidly became wine-red, and heating and stirring were continued for 15 min when the reaction was complete. The solution was then fixed by addition of ultra-pure water (100 mL) and stored at 4°C (Guo et al., Citation2015; Sun, Liu, Song, Kuang, & Xu, Citation2016). The presence of colloidal gold was confirmed by ultraviolet spectroscopy.
Firstly, the volume of 0.1 M K2CO3 was optimized to adjust the pH of the solution, then the 0.1 M K2CO3 was added to 1 mL colloidal gold solution. The purified monoclonal antibody, at an optimized concentration, was then slowly added to the reaction over 2 h with stirring. BSA solution (100 μL, 10%) was mixed with the solution, which was then shaken for 30 min to avoid non-specific adsorption between the mAb-labeled gold nanoparticles and protein loci. The clear supernatant was separated by centrifugation at 6200 rpm for 10 min to eliminate redundant mAbs and BSA, and the precipitate was dissolved in ultra-pure water (1 mL). After two centrifugations, borate buffer (0.1 mL) was added to the precipitate, which was stored at 4°C for future use (Peng, Song, Liu, Kuang, & Xu, Citation2015a).
Preparation of immunochromatographic strip
The test papers were assembled as follows. Suitable concentrations of goat anti-mouse IgG and coating antigen were sprayed onto the NC membrane to form the control line (C) and test line (T), respectively, and the membrane was then dried at 37°C. An absorbent pad was attached to the end of the C line and the sample pad was attached to the end of the T line. The paper was cut into 3.8 mm strips using a cutting machine and the strips were stored in a sealed container at room temperature with the exclusion of light (Peng, Song, Liu, Kuang, & Xu, Citation2015b; Yan et al., Citation2015).
Assay using immunochromatographic strip
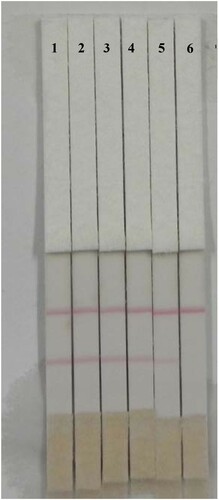
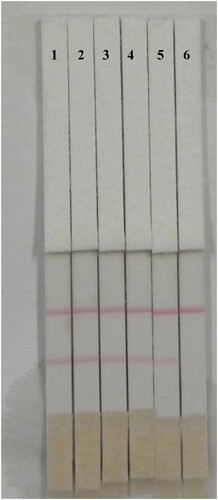
Since the detection of cyproheptadine is currently based mainly on field detection in urine samples, the test method for cyproheptadine residues in pig urine is well-established. Urine samples that tested negative for cyproheptadine were confirmed by HPLC/MS. Before use, the urine was centrifuged at 4700×g for 10 min to remove precipitate. Positive samples, with different concentrations, were prepared by adding cyproheptadine standard to PBS and to negative urine samples. If the control line was darker while the test line was lighter, or had no color, the sample was positive. If both control and test lines appeared dark red, the sample was considered to be negative (Liu et al., Citation2017). The detection line could be judged by the elimination of the line value.
Recovery
Taking into account IC50 values and the linear range of the mAbs, urine was spiked with different concentrations of cyproheptadine and determination values were calculated by the standard curve of the mAbs.
C(Rate of recovery) = Detection concentration/Addition concentration × 100%. When 80% < C < 120%, the antibodies are suitable for the detection of actual samples.
Results and discussion
Antigen preparation
Because the relative molecular mass of cyproheptadine is low, the compound itself is not immunogenic, and carrier proteins must be coupled with the hapten to form a complete immunogenic antigen (Liu, Kuang, Peng, Wang, & Xu, Citation2014). The chemical structure of the hapten is crucial to the quality of their Abs. Since cyproheptadine lacks a reactive group, a derivatization reaction was required to form a reactive species that could couple with carrier proteins. To solve this problem, a carboxyl CYP-1 was designed (). KLH was selected as the carrier protein for the immunogen and OVA was chosen as the carrier protein for the coating antigen. The EDC method was used to couple the hapten and protein. EDC first reacted with the carboxyl group on CYP-1 to form an activated O-acylisourea intermediate and NHS was used to stabilize the next amine condensation, which formed an amide bond.
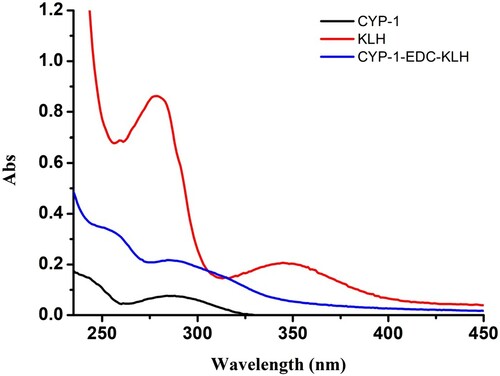
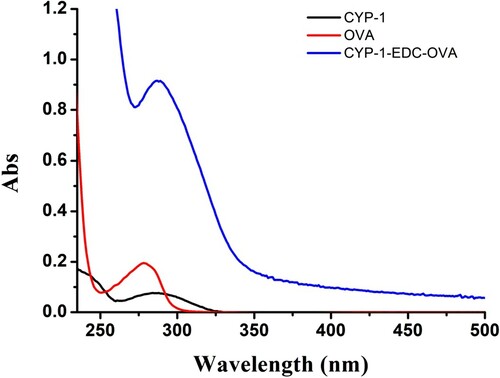
Immunogenicity and coating antigens were confirmed by ultraviolet spectroscopy ( and ). The typical absorption peaks of KLH and OVA were at 280 nm, whereas the typical absorption peak of CYP-1 was at 290 nm. As the extent of the reaction increased, light absorption by the coupled product at 280 nm was obviously enhanced and there was a clear blue shift in the absorption peak of the coupled product at 280 nm, which more closely resembled the peak of CPY-1.
Preparation of mAbs
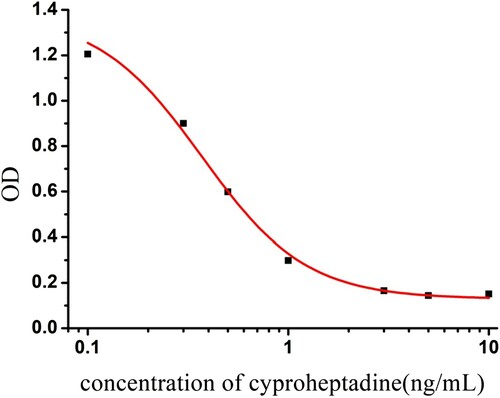
After four immunizations with CYP-1-EDC-KLH, high titer polyclonal serum was obtained, which gave an IC50 of 5 μg/L for cyproheptadine in urine. Next, spleen cells from the tested mouse were hybridized with Sp2/0 myeloma cells. After multiple clonal expansions, strains producing antibodies with high titer and high sensitivity, and which grew more rapidly, were selected. Cell lines cpy-3e10, cpy-4a7, cpy-4b3, cpy-5a6, and cpy-5b4 were expanded, cultured and frozen. mAbs of the cell line were confirmed by IC-ELISA. Among the five cloned strains, the cyp-3e10 cell line was chosen by immune chromatography because of its reactivity and specificity for cyproheptadine. The IC50 of cyp-3e10 for cyproheptadine in urine was 0.37 ng/mL, with a linear range of 0.16–0.85 ng/mL ().
Cross-reactivity (CR)
Because the rapid detection of cyproheptadine in animals intended for the market is mainly carried out by field detection of urine, cross-reactivity with other additives in animal feed was measured by IC-ELISA (). The result in showed that the cross-reactivity with β-agonists from IC-ELISA was <1% and the mAbs is highly specific to cyproheptadine because of its particular structure. The mAbs were thus only susceptible to cyproheptadine and had no cross-reactivity with other additives in animal feed.
Table 1. Cross-reactivity of β-agonists from IC-ELISA.
Cyproheptadine detection using immunochromatographic strip assay
A change in the color of the solution during preparation of mAb-labeled gold nanoparticles can be used to judge whether the gold nanoparticles are aggregated, and allows the optimal concentration of coating antigen and amount of antibody to be determined. The optimized conditions for preparation of the cyproheptadine test strips were 1 mL of gold nanoparticle with 4 µL K2CO3 and 8 µg/mL antibody, with a coating concentration of 0.5 mg/mL. To confirm that the test strips could be used to analyze pig urine spiked with cyproheptadine, urine samples containing different concentrations of cyproheptadine (0, 0.25, 0.5, 1, 2.5 and 5 ng/mL) were added to the sample pad ( and ). When the concentration of cyproheptadine in the urine was 5 ng/mL, the test line disappeared completely. The cut-off limit of the test strips was thus 5 ng/mL in urine and the test strips were shown to be suitable for the field detection of actual samples. Samples of negative pig urine were spiked with cyproheptadine (0.5, 0.1 and 0.2 ng/mL) and analyzed by IC-ELISA. The recovery from pig urine was between 80% and 120% and the coefficient of variation was low. This means that the kit has good accuracy and stability and is suitable for the analysis of actual samples.
Conclusions
We have prepared a monoclonal antibody that can specifically detect cyproheptadine when used in animal feed. Antibodies were prepared based on IC-ELISA and cyproheptadine-antigen, and a new immunochromatographic test strip that is able to detect levels of cyproheptadine in pig urine was developed. Because our newly established detection strip does not require sample pre-processing before analysis, the detection process is greatly simplified and the detection time is greatly shortened. The immunochromatographic strip is thus suitable for rapid detection of cyproheptadine residues.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gu, H., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of an immunochromatographic strip assay for ractopamine detection using an ultrasensitive monoclonal antibody. Food and Agricultural Immunology, 27(4), 471–483. doi: https://doi.org/10.1080/09540105.2015.1126808
- Guo, J., Liu, L., Xue, F., Xing, C., Song, S., Kuang, H., & Xu, C. (2015). Development of a monoclonal antibody-based immunochromatographic strip for cephalexin. Food and Agricultural Immunology, 26(2), 282–292. doi: https://doi.org/10.1080/09540105.2014.907242
- Hasegawa, C., Kumazawa, T., Lee, X. P., Fujishiro, M., Kuriki, A., Marumo, A., … Sato, K. (2006). Simultaneous determination of ten antihistamine drugs in human plasma using pipette tip solid-phase extraction and gas chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry, 20(4), 537–543. doi: https://doi.org/10.1002/rcm.2335
- Kong, N., Guo, L., Guan, D., Liu, L., Kuang, H., & Xu, C. (2015). An ultrasensitive ELISA for medroxyprogesterone residues in fish tissues based on a structure-specific hapten. Food Analytical Methods, 8(6), 1382–1389. doi: https://doi.org/10.1007/s12161-014-0023-4
- Li, X., Xu, X., Albano, D. R., & You, T. (2011). Optimization using central composite design for antihistamines separation by nonaqueous capillary electrophoresis with electrochemical and electrochemiluminescence detections. The Analyst, 136(24), 5294–5301. doi: https://doi.org/10.1039/c1an15730b
- Lin, Y.-H., Hung, S.-K., Chiou, W.-Y., Lee, M.-S., Shen Md, B.-J., Chen, L.-C. … Lin, H.-Y. (2016). Significant symptoms alleviation and tumor volume reduction after combined simultaneously integrated inner-escalated boost and volumetric-modulated arc radiotherapy in a patient with unresectable bulky hepatocellular carcinoma: A care-compliant case report. Medicine, 95(34), e4717. doi: https://doi.org/10.1097/MD.0000000000004717
- Liu, L., Kuang, H., Peng, C., Wang, L., & Xu, C. (2014). Structure-specific hapten design for the screening of highly sensitive and specific monoclonal antibody to salbutamol. Analytical Methods, 6(12), 4228–4233. doi: https://doi.org/10.1039/C4AY00062E
- Liu, R., Liu, L., Song, S., Cui, G., Zheng, Q., Kuang, H., & Xu, C. (2017). Development of an immunochromatographic strip for the rapid detection of 10 β-agonists based on an ultrasensitive monoclonal antibody. Food & Agricultural Immunology, 28(4), 1–14. doi: https://doi.org/10.1080/09540105.2017.1337084
- Lulebo, A. M., Bavuidibo, C. D., Mafuta, E. M., Ndelo, J. D., Mputu, L. S. C. M., Kabundji, D. M., & Mutombo, P. B. (2016). The misuse of cyproheptadine: A non-communicable disease risk behaviour in Kinshasa population, Democratic Republic of Congo. Substance Abuse Treatment, Prevention, and Policy, 11(1), 7. doi: https://doi.org/10.1186/s13011-016-0051-8
- Martin-Galvez, D., & Soler, J. J. (2017). Decoding colouration of begging traits by the experimental addition of the appetite enhancer cyproheptadine hydrochloride in magpie Pica pica nestlings. Journal of Avian Biology, 48(3), 353–361. doi: https://doi.org/10.1111/jav.00641
- Mendes, G. D., Arruda, A., Chen, L. S., Jc, D. A. M., Alkharfy, K. M., & De, N. G. (2012). Quantification of cyproheptadine in human plasma by high-performance liquid chromatography coupled to electrospray tandem mass spectrometry in a bioequivalence study. Biomedical Chromatography, 26(1), 129–136. doi: https://doi.org/10.1002/bmc.1618
- Merhar, S. L., Pentiuk, S. P., Mukkada, V. A., Meinzen-Derr, J., Kaul, A., & Butler, D. R. (2016). A retrospective review of cyproheptadine for feeding intolerance in children less than three years of age: Effects and side effects. Acta Paediatrica, 105(8), 967–970. doi: https://doi.org/10.1111/apa.13477
- Peng, J., Song, S., Liu, L., Kuang, H., & Xu, C. (2015a). Development of Sandwich ELISA and immunochromatographic strip for the detection of peanut allergen Ara h 2. Food Analytical Methods, 8(10), 2605–2611. doi: https://doi.org/10.1007/s12161-015-0163-1
- Peng, S., Song, S., Liu, L., Kuang, H., & Xu, C. (2015b). Rapid enzyme-linked immunosorbent assay and immunochromatographic strip for detecting ribavirin in chicken muscles. Food and Agricultural Immunology, 27(4), 449–459. doi: https://doi.org/10.1080/09540105.2015.1104657
- Sun, X.-L., Li, X.-L., Cao, X.-M., Yang, Y.-F., Wang, J., Wang, S.-T., … Zhao, S.-J. (2017). An ultra-high performance liquid chromatography-tandem mass spectrometric method for fast determination of 30 different species of lean meat agents residues in pig urine. Chinese Journal of Analytical Chemistry, 45(1), 124–132.
- Sun, C., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of a highly sensitive ELISA and immunochromatographic strip to detect pentachlorophenol. Food and Agricultural Immunology, 27(5), 689–699. doi: https://doi.org/10.1080/09540105.2016.1148668
- Szepietowski, J. C., & Reszke, R. (2016). Psychogenic itch management. Current Problems in Dermatology, 50, 124–132. doi: https://doi.org/10.1159/000446055
- Yan, H., Liu, L., Xu, N., Kuang, H., & Xu, C. (2015). Development of an immunoassay for carbendazim based on a class-selective monoclonal antibody. Food and Agricultural Immunology, 26(5), 659–670. doi: https://doi.org/10.1080/09540105.2015.1007446
- Yang, J., Wang, Z., Zhou, T., Song, X., Liu, Q., Zhang, Y., & He, L. (2015). Determination of cyproheptadine in feeds using molecularly imprinted solid-phase extraction coupled with HPLC. Journal of Chromatography B, 990, 39–44. doi: https://doi.org/10.1016/j.jchromb.2015.03.009
- Zhu, D., Xia, L., Sun, J., & You, T. (2012). Chemometrics optimization of six antihistamines separations by capillary electrophoresis with electrochemiluminescence detection. Talanta, 88(1), 265–271. doi: https://doi.org/10.1016/j.talanta.2011.10.040