ABSTRACT
Insecticides containing furan ring have attracted more attention owing to their unique mechanism of action. 25 furanone analogues were used to conduct a preliminary screening on insect cell line SL2 and these compounds exhibited good cytoactivity. Particularly, compound 14 presented good inhibitory effect with an IC50 value of 28.14 μM. Subsequently, we investigated the selectivity against insect cells by testing the cytotoxicity of it on human cell line HEK293 and we noticed that compound 14 was less toxicity than the reference insect cells. Finally, we attempted to illustrate the act mechanism of compound 14 on SL2 cells at the cellular level and found it initiated apoptosis through a caspase-dependent mitochondrial pathway that down-regulated mitochondrial membrane potential level, increased the activity of caspase-3 and altered the cell cycle. The result showed that compound 14 could be considered as a new potent insecticide to be used for further optimization.
1. Introduction
At present, agricultural insect pests are becoming one of the major economic damages in agriculture around the world (Partida-Martinez & Hertweck, Citation2005; Sparks & Lorsbach, Citation2017). Many methods are applied to control insect pests and one of them is the usage of insecticides which has been the main alternative choice to minimize economic losses (Lamberth, Jeanmart, Luksch, & Plant, Citation2013). In vivo studies on insecticides’ activities are carried so as to evaluate the insecticides’ actual effects by simulating natural environment, however, some of these assays are labour intensive and time-consuming (Huang, Qian, Song, & Cao, Citation2003). In contrast, in vitro studies are becoming a useful assay method in cytotoxicity screening of insecticides, not only because they can greatly short screening time but also provide more useful information, such as insecticides’ mechanisms of action (Zhang, Tao, & Hou, Citation2012). Through cytotoxicity screening in vitro, some potent compounds with good insecticidal activities can be detected at cellular level.
Our group is involved in the discovery, synthesis and screening of novel insecticide (Lu, Chen, & Hou, Citation2007 and Chen et al., Citation2007). On the basis of previous studies, it was found that compounds containing furan ring had good insecticidal activities (Jin et al., Citation2009 and Liu et al., Citation2009). Furanone analogues are widely used in agricultural chemical field as insecticidal, fungicidal, antimicrobial agents (Cui et al., Citation2010, Hu et al., Citation2012 and Ren et al., Citation2015). However, to our knowledge, there was a few reports about toxic effect and mechanism of furanone analogues on insect cells (Ren et al., Citation2015). Therefore, the toxicity of a series of furanone analogues on Drosophila melanogaster cells (SL2) is of interest in the current study.
Apoptosis is a programmed cell death associated with cell shrinkage, plasma membrane blebbing, chromatin condensation, DNA fragmentation and formation of apoptotic bodies that can be taken up and degraded by neighbouring cells without triggering any inflammatory reaction. Apoptosis occur either by extrinsic or intrinsic (mitochondrial-dependent) pathways (Xia et al., Citation2016). In extrinsic apoptosis, external signals or ligands interact with a receptor present in the plasma membrane, initiating a cascade of events that lead to apoptosis. In the intrinsic pathway, mitochondria irreversibly commit cells to apoptosis by releasing death factors into cytosol (Kroemer et al., Citation1995). The apoptosis process is strictly regulated and the Bcl-2 gene families play a key role in the process. Among them, the Bax/Bcl-2 is the key factor in regulating apoptosis. Cytochrome c, a death factor, can form a complex with Apaf-1 in the presence of dATP in cytosol. This is followed by activation of caspase-3, which results in the activation of a cascade of caspases (Zhang et al., Citation2017). Finally, these activated caspases degrade key structural and nuclear proteins and irreversibly commit the cells to death.
In the present study, Drosophila SL2 cells were used as a model for typical insect cells to evaluate the toxicological effects of furanone analogues, and we found that compound 14 has significant cytotoxicity to it in vitro. We used different methods to verify the mechanism of action of compound 14 against SL2 cells, which showed that it can induce apoptosis. Our results indicate that compound 14 induced DNA fragmentation, down-regulated mitochondrial membrane potential (ΔΨm) level, up-regulated the expression level of caspase-3, altered the cell cycle. In conclusion, this evidence shows that the SL2 cells were undergoing caspase-dependent mitochondrial apoptosis.
2. Materials and methods
2.1. Chemicals
25 furanone analogues were shown in , which were synthesized in our laboratory (Liu et al., Citation2009 and Zhou et al., Citation2012). Rhodamine 123 (Rh 123), dimethyl sulfoxide (DMSO), thiazolyl blue tetrazolium bromide (MTT), phosphate buffer saline (PBS, pH = 7.4), agarose, propidium iodide (PI) and Schneider’s insect medium were the products of Sigma Chemical Co., Ltd. (St. Louis, USA). Dulbecco’s modified eagle medium (DMEM) was purchased from Gibco (Bethesda, USA). Cultured cells genomic DNA extraction kit and caspase-3 assay kit were purchased from Tiangen Biotech Co., Ltd. (Beijing, China). Fetal bovine serum (FBS) was the products of Thermo Fisher Scientific Co., Ltd. (Utah, USA).
Table 1. Structures of 25 furanone analogues and proliferation inhibition rates (%).
2.2. Cell culture
SL2 cells derived from China Center for Type Culture Collection (CCTCC) were cultured in Schneider’s insect medium supplemented with 10% FBS at 28°C in the culture flask. Human embryonic kidney cells (HEK293) were obtained from the Key Laboratory of Bio-Resource and Eco-environment of Ministry of Education and cultured in DMEM with 10% FBS and 1% penicillin/streptomycin at 37°C in a humidified incubator (5% CO2). The cells were grown to a density of about 1×105 cells per millilitre in a culture flask and subcultured every 7 days.
2.3. Cytotoxicity assay
Cytotoxicity assay was conducted by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphe nyltetrazolium bromide (MTT) method (Mosmann, Citation1983). Cells were cultured into 96-well culture plates at a density of 1 × 105 cells per millilitre. After overnight incubation, the cells were treated with compounds (100 μM) dissolved in DMSO (<1%) for another 48 h. Then, MTT was added to cell cultures at a final concentration of 5 g/L. After 4 h incubation at 28°C, culture supernatants were removed, and formazan crystals formed in the cells were dissolved in DMSO. The absorbance of the resulting solution was measured at 490 nm in enzyme standard instrument (BioTek, USA). The cell viability was calculated as a percentage from the viability of the control (untreated) cells.
2.4. DNA fragmentation assay
After overnight incubation, SL2 cells (1 × 106 cells per millilitre) were treated with compound 14 at 50 μM for 24 and 48 h. The cells were collected by centrifugation at 6000 rpm at 4°C for 10 min and washed twice by PBS. Then TIANamp genomic DNA extraction kit was used to extract the DNA fragments. Subsequently, the extracted DNA samples were run on a 1.5% agarose gel and visualized by ethidium bromide staining.
2.5. Mitochondrial membrane potential (ΔΨm) analysis
To analyse mitochondrial membrane potential (ΔΨm), cells were stained with Rh 123. Rh 123 is a probe for mitochondrial membrane potential that was determined by mean aggregate fluorescence of the fluorochrome Rh123. The loss of mitochondrial membrane potential resulted in a diminished ability to accumulate the fluorochrome Rh 123. After being treated with compound 14 at 50 μM for 12 h, the cells (1 × 106 cells per millilitre) were harvested and washed twice with PBS, and then incubated with Rh 123 (2 μM) for a further 60 min at 28°C in the dark, before immediately analysing them by FACScan® flow cytometry (Becton Dickinson, USA) at an excitation wavelength of 507 nm and an emission wavelength of 529 nm (Zhou et al., Citation2010).
2.6. Activation of caspase-3
Cells were treated with at 50 μM compound 14 for 0, 4, 8, 12 and 24 h, and control cells were treated with an equivalent amount of vehicle (DMSO). Then the cells were collected by centrifugation at 10,000 rpm for 10 min at 4°C and subsequently resuspended in cell lysis buffer and incubated on ice for 1 h; the resulting supernatant (50 μl) was mixed in 2× Reaction Buffer (50 μl) and Caspase-3 Substrate (5 μl) and incubated at 28°C for 4 h. Finally, caspase-3 activity was measured at 405 nm with a microplate reader (BioTek, USA).
2.7. Cell cycle analysis
SL2 cells were treated with 50 μM compound 14 and 1% DMSO (the control) for 24 h then subsequently harvested by centrifugation at 1000 rpm for 5 min at 4°C and washed twice with PBS. The cells were then fixed with 70% ethanol at 4°C overnight, washed twice with PBS, then incubated with RNAase (20 mg/L for the final concentration) for 30 min at 37°C. Finally, the cells were stained with 50 mg/L PI in the dark for 30 min at 4°C and then filtered with cell strainers (75 μm). Cell cycle analysis was carried out with flow cytometry (Beckman Coulter Inc, USA). The proportion of each phase was calculated using the ModFit LT software (Verity Software House, USA).
2.8. Statistical analysis
All experiments were performed in triplicate. Data were expressed as mean values ± SD. Statistical differences were analysed by Student’s t-test and were considered significantly (*P < .05) or extremely significantly different (**P < .01).
3. Results
3.1. Cytotoxicity screening
As shown in , 25 furanone analogues had good inhibitory effects in SL2 cells. Among them, compounds 2, 9, 13 and 14 exhibited better inhibitory effects and their inhibition rates were (70.7 ± 3.05%), (71.5 ± 3.02%), (73.7 ± 2.514%) and (85.2 ± 2.25%), respectively. At the same time, it was observed that compound 14, containing two bromine atoms and one nitro group, had the highest cytotoxicity which was slightly better than chlorfenapyr (84.1 ± 2.57%). The result () was showed that IC50 value of compound 14 was 28.14 μM. In the present study, it was observed that 25 furanone analogues exhibited potent inhibitory effects in SL2 cells at 100 μM. Particularly, compound 14 which IC50 was 28.14 μM had the best inhibitory effect in cell line SL2. Subsequently, for investigating the selectivity of the most active derivatives (compound 14) against insect cells, we tested the cytotoxicity of compound 14 on the human cell line HEK293. As result shown (), IC50 value of compound 14 was 110.78 μM against HEK293, which displayed cytotoxicity towards human cells at much higher concentrations than those required for the pharmacological effect towards insect cells. Accordingly, we surmised that the compound 14 possessed a very low toxicity against human cells with respect to insect cells.
Table 2. IC50 value of compound 14 against SL2.
Table 3. IC50 values for compounds 14 against HEK293
3.2. Apoptosis-inducing effect of compound 14
3.2.1. Morphological changes of SL2
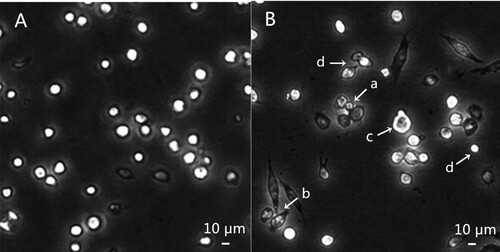
The SL2 cells apoptosis was confirmed by studying apoptotic body formation and apoptotic DNA degradation. Usually, the formation of apoptotic bodies is considered as one of the terminal events of apoptosis (Kumarswamy et al., Citation2009). As displayed in , SL2 cells observed under inverted phase contrast microscope were the adherent growth, the most round and the uniform size in the control group. However, after treatment with compound 14, apoptotic bodies were formed and cell morphological changed. The cell morphological changes including an apoptotic body (a), fusiform shape (b), membrane blebbing (c) and smaller proportion (d) were demonstrated by arrows in .
3.2.2. DNA fragmentation
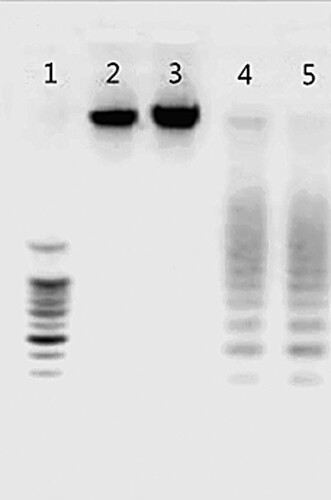
As presented in , DNA ladders were observed after compound 14 treatment at 24 and 48 h. The formation of apoptotic body and apoptotic DNA degradation indicated that compound 14 caused SL2 cells apoptosis. Apoptosis is an active process of cell death, which includes morphogenesis, DNA fragmentation, cell cycle arrest, inhibition of caspase-3 activity and reduction of the mitochondrial membrane potential. Changes in morphogenesis have been defined as “proofs of physiological cell death,” characterized by cell shrinkage, membrane blebbing, formation of apoptotic bodies and fragmentation of nuclear DNA. In the current study, we saw morphological changes typical of apoptosis in compound 14-treated cells. Meanwhile, we also clearly detected a fragmentation in the DNA ladder. Taken together, these results suggested that the compound mediates apoptosis.
3.2.3. Change of mitochondrial membrane potential (ΔΨm)
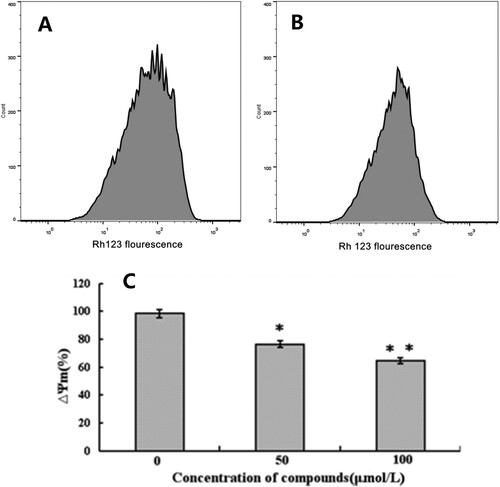
Mitochondrial membrane potential (ΔΨm) change as a marker of mitochondrial function is one of the events frequently associated with apoptosis (Huang et al., Citation2011). The loss of ΔΨm was indicated in (A,B) by using flow cytometry. The results showed that the peak value of Rh 123 fluorescence in treated cells with 50 μM compound 14 reduced by 23.26% (*P < .05). Furthermore, as illustrated in (C), a significant decrease (32.73% (*P < .01)) of mitochondrial membrane potential (ΔΨm) was observed after treatment with 100 μM compound 14 for 12 h.
3.2.4. Activation of caspase-3
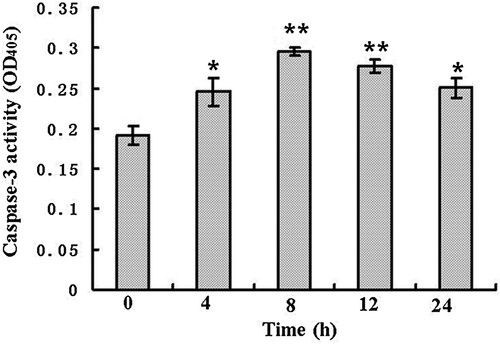
As an apoptosis executioner, caspase-3 plays a key role in the apoptotic process (Chipuk et al., Citation2005). There is evidence that caspase-3 is required for DNA fragmentation and the morphological changes during apoptosis (Perchellet et al., Citation2004). Many reports suggest that activation of caspase system may induce cell apoptosis (Jänicke et al., Citation1998). showed that after treated by 50 μM compound 14 for different times, the activities of caspase-3 increased significantly at 4 h (*P < .05), then reached the maximum value at 8 h (**P < .01). Finally, decreased from 12 h (**P < .01).
3.2.5. Cell cycle analysis
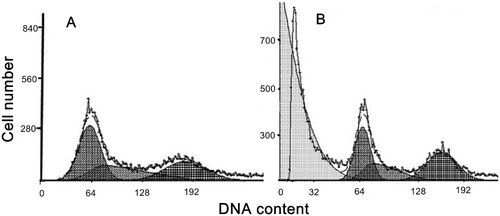
Incubation of compound 14 (50 μM) for 24 h produced an increase in the percentage of cells in S phase as compared with the control (13.42% (*P < .05)). A concomitant increase in cells during the G2/M phase was also observed (17.43% (*P < .05)). These results indicated that the inherent cells hyperplastic cycle was disrupted after treatment; some cells were blocked in S phase and some cells failed to go through mitosis ().
4. Discussion
The 25 furanone analogues are newly synthesized by our group. Previous research had revealed that furanone compounds had antibacterial and antifungal activity (Cui et al., Citation2010 and Hu et al., Citation2012). To our knowledge, most of the studies about furanone analogues were focused on their antimicrobial activity. However, compounds containing furan ring also attracted much attention owing to their unique mechanisms of action against insects (Paula et al., Citation2008 and Kagabu et al., Citation2010). Our group had optimized the diphenyl ketones’ structures and obtained some furanone analogues. The results showed that they had good insecticidal activities in vivo. This status induced us to further study the cytotoxicity and action mechanism of furanone analogues against SL2 cells.
As is known, apoptosis relies on two major pathways, the cell death receptor-mediated extrinsic pathway and the mitochondrial-mediated intrinsic pathway (Evan, Citation1997). The two most important characteristics of the mitochondrial-mediated apoptotic pathway are loss of mitochondrial membrane potential (ΔΨm) and release of cytochrome c (Garrido et al., Citation2006). Most studies have confirmed that mitochondria play a decisive role in apoptosis by controlling the membrane potential (Zhou and Zhi-Yuan, Citation2006). Actually, loss of mitochondrial membrane potential is considered the earliest events in process of apoptosis cascade and is one of the specific signs of apoptosis. Our results clearly show that mitochondrial membrane potential (ΔΨm) reduced. Furthermore, many apoptotic responses are initiated by the delivery of cytochrome-c from mitochondria, leading to the activation of caspase-3 and subsequent apoptosis. However, the role of cytochrome c in insect apoptosis is still an unresolved issue (Liu et al., Citation2012). For Drosophila SL2 cells apoptosis, evidence supports the hypothesis that there is cytochrome c-independent mechanisms for apoptosis regulation. Cytochrome c is not required for apoptosis and caspase activation in most Drosophila cells. The regulation of apoptosis in cytochrome c-independent Drosophila cells may follow a more primary mechanism, which may be the precursor to the apoptosis signalling pathway in mammals.
Caspases are a family of cysteine proteases that play a key role in modulating apoptosis. Caspases begin as inactive precursors, but upon receiving an apoptotic signal, the procaspase will undergo proteolytic processing to generate an active enzyme. Finally, the executed caspase is activated to make the relevant substrate degradation and induce apoptosis (Zhang et al., Citation2017). Among them, caspase-3 is the major effector protein of apoptosis that is activated by an initiator caspase as caspase-9. In our system, we detected a significant increase in the activity of caspase-3 during the early phase of apoptosis suggesting that this caspases contributed to compound 14 induced SL2 cell apoptosis.
The control of the cell cycle is the major regulatory mechanism involved in cell growth. Many cytotoxic agents arrest the cell cycle at G1, S or G2/M phase causing apoptotic cell death. In the present study, we found that compound 14 induces an accumulation of SL2 cells in the S and G2/M phase of the cell cycle thus leading to an overall inhibition in proliferation.
Taken together, the 25 furanone analogues exhibited well inhibitory effects in SL2 cells. In particular, the highly active compound, 2,3-dibromo-1-(2-furanyl)-3-(3-nitrophenyl) propanone (compound 14) demonstrate that it induces the death of Drosophila SL2 via the caspase-dependent mitochondrial apoptotic pathways. Meanwhile, we also observed that compound 14 possessed lower toxicity against human cells. This will contribute to a better understanding of the apoptotic process induced by furanone analogues and promote the development of furanone analogues as new lead compounds for the development of promising insecticidal drugs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen, L., Wang, X. L., Lu, T., & Hou, T. P. (2007). Lead optimization and insecticidal activity of analogues of daphneolone isolated from Stellera chamaejasme L. Pest Management Science, 63, 1428–1434.
- Chipuk, J., Green, E., & R, D. (2005). Do inducers of apoptosis trigger caspase-independent cell death?. Nature Reviews Molecular Cell Biology, 6, 268–275. doi: https://doi.org/10.1038/nrm1573
- Cui, Z., Zhang, L., & Huang, J. (2010). Synthesis, insecticidal activity and 3D-QSAR studies on diacylhydrazine derivatives containing furan. Chinese Journal of Organic Chemistry, 30, 1482–1491.
- Evan, G. (1997). Cancer-A matter of life and cell death. International Journal of Cancer, 71, 709–711. doi: https://doi.org/10.1002/(SICI)1097-0215(19970529)71:5<709::AID-IJC2>3.0.CO;2-V
- Garrido, C., Galluzzi, L., Brunet, M., Puig, P. E., Didelot, C., & Kroemer, G. (2006). Mechanisms of cytochrome c release from mitochondria. Cell Death and Differentiation, 13, 1423–1433. doi: https://doi.org/10.1038/sj.cdd.4401950
- Hu, Y., Gao, H., & Wang, G. (2012). Synthesis and antitumor activity of some 2-amino-furo[2,3-d]- yrimidin-4(3H)-one derivatives. Chinese Journal of Organic Chemistry, 32, 1468–1472. doi: https://doi.org/10.6023/cjoc201203003
- Huang, Q. C., Qian, X. H., Song, G. H., & Cao, S. (2003). The toxic and anti-feedant activity of 2H-pyridazin-3-one-substituted 1,3,4-oxadiazoles against the armyworm Pseudaletia separata (Walker) and other insects and mites. Pest Management Science, 514, 1433–1439.
- Huang, J. F., Tian, M., Lv, C. J., Li, H. Y., Muhammad, R. H., & Zhong, G. H. (2011). Preliminary studies on induction of apoptosis by abamectin in Spodoptera frugiperda (Sf9) cell line. Pesticide Biochemistry and Physiology, 100, 256–263. doi: https://doi.org/10.1016/j.pestbp.2011.04.010
- Jänicke, R. U., Sprengart, M. L., Wati, M. R., & Porter, A. G. (1998). Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. Journal of Biological Chemistry, 273, 9357–9360. doi: https://doi.org/10.1074/jbc.273.16.9357
- Jin, H., Geng, Y. C., Yu, Z. Y., Tao, K., & Hou, T. P. (2009). Lead optimization and anti-plant pathogenic fungi activities of daphneolone analogues from Stellera chamaejasme L. Pesticide Biochemistry and Physiology, 93, 133–137. doi: https://doi.org/10.1016/j.pestbp.2009.01.002
- Kagabu, S., Ohno, M. I., Tomizawa, K. A., Durkin, R., Nagae, N., & Kumazawa, S. (2010). Furan-2,5-dimethylene-tethered bis-imidacloprid insecticide conferring high potency. Journal of Agricultural and Food Chemistry, 58, 11832–11836. doi: https://doi.org/10.1021/jf102819n
- Kroemer, G., Petit, P., Zamzami, N., Vayssiere, J. L., & Mignotte, B. (1995). The biochemistry of programmed cell death. The FASEB Journal, 9, 1277–1287. doi: https://doi.org/10.1096/fasebj.9.13.7557017
- Kumarswamy, R., Seth, R. K., Dwarakanath, B. S., & Chandna, S. (2009). Mitochondrial regulation of insect cell apoptosis: Evidence for permeability transition pore-independent cytochrome-c release in the Lepidopteran Sf9 cells. The International Journal of Biochemistry & Cell Biology, 41, 1430–1440. doi: https://doi.org/10.1016/j.biocel.2008.12.009
- Lamberth, C., Jeanmart, S., Luksch, T., & Plant, A. (2013). Current challenges and trends in the discovery of agrochemicals. Science, 341, 742–746. doi: https://doi.org/10.1126/science.1237227
- Liu, W., Shi, H. M., Jin, H., & Hou, T. P. (2009). Design, synthesis and antifungal activity of a series of novel analogs based on diphenyl ketones. Chemical Biology & Drug Design, 73, 661–667. doi: https://doi.org/10.1111/j.1747-0285.2009.00820.x
- Liu, K. Y., Yang, H. P., Jian, X., & Hong, H. Z. (2012). Cytochrome c and insect cell apoptosis. Insect Science, 19, 30–40. doi: https://doi.org/10.1111/j.1744-7917.2011.01431.x
- Lu, T., Chen, L., & Hou, T. P. (2007). Synthesis and structure–activity study of botanical aphicides 1,5-diphenyl-1-pentanone analogues. Pesticide Biochemistry and Physiology, 814, 60–64.
- Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65, 55–63. doi: https://doi.org/10.1016/0022-1759(83)90303-4
- Partida-Martinez, L. P., & Hertweck, C. (2005). Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature, 437, 884–888. doi: https://doi.org/10.1038/nature03997
- Paula, V. F., Barbosa, L. C., Teixeira, R. R., Picanco, M. C., & Silva, G. A. (2008). Synthesis and insecticidal activity of new 3-benzylfuran-2-yl N,N,N’,N’-tetraethyldiamidophosphate derivatives. Pest Management Science, 64, 863–872. doi: https://doi.org/10.1002/ps.1559
- Perchellet, E. M., Wang, Y., Weber, R. L., Sperfslage, B. J., Lou, K., Crossland, J., … Perchellet, J. P. (2004). Synthetic 1,4-anthracenedione analogs induce cytochrome c release, caspase-9, -3, and -8 activities, poly (ADP-ribose) polymerase-1 cleavage and internucleosomal DNA fragmentation in HL-60 cells by a mechanism which involves caspase-2 activation but not Fas signaling. Biochemical Pharmacology, 67, 523–537. doi: https://doi.org/10.1016/j.bcp.2003.09.012
- Ren, Y. H., Jin, H., Tao, K., & Hou, T. P. (2015). Apoptotic effects of 1,5-bis-(5-nitro-2-furanyl)-1, K]4-pentadien-3-one on Drosophila SL2 cells. Molecular & Cellular Toxicology, 11, 187–192. doi: https://doi.org/10.1007/s13273-015-0017-3
- Sparks, T. C., & Lorsbach, B. A. (2017). Perspectives on the agrochemical industry and agrochemical discovery. Pest Management Science, 73, 672–677. doi: https://doi.org/10.1002/ps.4457
- Xia, Y., Bo, A., Liu, Z., Chi, B., Su, Z., Hu, Y., et al. (2016). Effects of magnesium sulfate on apoptosis in cultured human gastric epithelial cells. Food and Agricultural Immunology, 27, 171–181. doi: https://doi.org/10.1080/09540105.2015.1079596
- Zhang, Y., Chang, Y., Cao, H., Xu, W., Li, Z., & Tao, L. (2017). Potential threat of chlorpyrifos to human liver cells via the caspase-dependent mitochondrial pathways. Food and Agricultural Immunology, 29, 294–305. doi: https://doi.org/10.1080/09540105.2017.1373271
- Zhang, X. G., Tao, K., & Hou, T. P. (2012). Investigation of 1-(4-morpholinophenyl)-3-(4-fluoro-phenyl)-propenone and 1-(4-morpholinophenyl)-3-(3-fluorophenyl)-propenone as new apoptosis inducers on Spodoptera frugiperda (Sf9) cells. Toxicology Mechanisms and Methods, 22, 315–320. doi: https://doi.org/10.3109/15376516.2012.658979
- Zhou, G. P., Liu, W., Jin, H., Tao, K., & Hou, T. P. (2012). Synthesis and screening for potential against phytopathogenic fungi activity of novel amides. Journal of Sichuan University, 49, 871–878.
- Zhou, B. H., Wang, H. W., Xue, F. Q., Wang, X. Y., & Fei, C. Z. (2010). Effects of diclazuril on apoptosis and mitochondrial transmembrane potential in second-generation merozoites of Eimeria tenella. Veterinary Parasitology, 168, 217–222. doi: https://doi.org/10.1016/j.vetpar.2009.11.007
- Zhou, Y. Q., & Zhi-Yuan, G. U. (2006). Mitochondria and apoptosis. Prog Anatomy Science, 281, 13014–11312.