ABSTRACT
Two seed storage proteins, glycinin and β-conglycinin, account for most of the proteins in soymeal, while a valuable source of protein, soybean, has also been reported to be allergenic in some animals. Glycinin and conglycinin have been reported as allergens in multiple species, including humans. Allergic responses to soy proteins are due to hypersensitivity immunological reactions upon consumption of protein. We have isolated antibodies from chicken eggs to demonstrate that chickens mount an immunogenic response to soy glycinin. All three classes of chicken immunoglobulins bound glycinin protein. We identify immunogenic regions unique to each glycinin protein that bind unique repertoires of IgA, IgM and IgY. This knowledge can be used to identify variants of immunogenic glycinin epitopes to determine the role these immunogenic sites play in the allergic response of chickens to soy protein.
Introduction
Soybean (Glycine max (L.) Merril) meal supplements the protein content of food for humans and feed for animals. In 2015, soybean was planted on 82.7 million acres and produced a crop worth 35.2 billion dollars (USDA ERS) and animals consume almost 28 million tons of soymeal. The poultry industry is an important consumer of soybean meal. Broilers consumed about 11.3 million tons of soymeal in 2015 (http://www.animal.ag/economics/book/index.html#6).
The soybean seed storage proteins, glycinin and conglycinin, account for 40–60% of the protein content of soymeal (Liu et al., Citation2007). Conglycinin is a 7S trimer composed of different combinations of β, α and α′ subunits (Vu Huu & Shibasaki, Citation1978). Glycinin is an 11S hexamer divided into two groups based on similarity (Nielsen, Citation1985; Nielsen et al., Citation1989; Utsumi, Kohno, Mori, & Kito, Citation1987). Each glycinin subunit is composed of an acidic and basic region (Badley et al., Citation1975; Nielsen et al., Citation1989). Soybean 5 genes encoding glycinin are expressed in the seed. Each gene encodes both a basic and acidic subunit of the protein. The acidic and basic subunits are cleaved during the maturation of the protein.
While soybean also is a valuable source of protein, soy protein has also been reported to have anti-nutrition properties (Codina, Ardusso, Lockey, Crisci, & Medina, Citation2003; Helm, Citation2002; Helm et al., Citation2000; Krishnan, Kim, Jang, & Kerley, Citation2009; Taliercio & Kim, Citation2013, Citation2014). One cause of the anti-nutritional properties of soy protein is that they can be allergenic (Bessler, Ricki, & Ogawa, Citation2000). Allergies to food proteins are caused by immunological hypersensitivity responses. Over 19 soybean peptides have been shown to be allergenic including all subunits of glycinin and conglycinin. In the United States, about 4.7% of people are allergic to soy protein and allergies to soy protein in post-weaning pigs have been associated with a growth lag (Li et al., Citation1990; Li, Blecha, et al., Citation1991; Li, Giesting, et al., Citation1991). To deal with the growth lag in post-weaned pigs, soymeal can be fermented or pretreated with proteinases. These treatments presumably degrade the epitopes that cause the inappropriate immune response. An increase in broiler carcass weight has been reported for chickens fed fermented or enzymatically digested soy (Fernando Guilherme Perazzo Costa, Figueiredo, Oliveira, & Silva, Citation2008). This increase in production associated with fermented or enzymatically digested soy protein suggests that chickens may also have allergic responses to soy protein like piglets. We demonstrate that the basic and acidic subunits of A1aBx and A5A4B3 glycinin proteins are antigenic in chicken by evaluating the occurrence of IgY, IgA and IgM from individual eggs which are able to bind glycinin. These immunoglobulins are derived from the same precursor cell linages but play different roles in the immune response (Kim et al., Citation2016; Masteller & Thompson, Citation1994; Tizard, Citation2002). Using a tiled peptide array representing the A1aBx and A5A4B3 glycinin proteins, we identify specific epitopes that are antigenic in chickens and identify differences in the repertoire of IgA, IgM and IgY that the antigenic epitopes bind.
Materials and methods
Isolation of antibodies from eggs
Chicken eggs were obtained from six commercial sources, two sources designated their eggs as organic. The source of the eggs was not considered as a dependent variable in the analysis because no statistically significant differences in amounts of immunoglobulins or immunogenic epitopes were observed among the eggs from different sources or between conventional and organic eggs.
Immunoglobulins were extracted using modified methods of Hamal, Burgess, Pevzner, and Erf (Citation2006). Briefly, Igs were extracted from egg whites by adding two volumes of PBS (137 mM sodium chloride, 2.7 mM potassium chloride, 10 mM phosphate, pH 7.4 (Sigma, St. Louis, MO, USA)) to the whites of each egg and mixed thoroughly. Pulverized PEG-8000 (Sigma) was dissolved into the egg white solution to a final concentration of 3.5% (wt/vol). The supernatant fluid containing Ig was collected after centrifugation at 14,000 × g for 10 min at room temperature. Aliquots were stored at −20°C.
Intact yolks were rinsed in distilled water to remove egg whites. After rinsing with water, the yolk membrane was pierced, and the yolk was suspended in 2 volumes of PBS. An equal volume of chloroform (Fisher Scientific, Fair Lawn, NJ, USA) was added and mixed vigorously. After centrifugation at 1000 × g for 30 min at room temperature, three distinct layers were formed. The top aqueous layer contained Ig. This layer was aliquoted and stored at −20°C. Amounts of IgY, IgA and IgM were measured using ELISA quantitative kits (Bethyl Laboratories, Montgomery, TX, USA). MyAssays software generated bestfit, 4-parameter or 5-parameter logistics regression standard curves for quantitative analysis. Dilutions of 10−3–10−6 were used to measure IgY. Dilutions of 10−1–10−3 were used to measure IgA and IgM. In most cases, values that are consistent across consecutive dilution are reported to assure that measurements are taken in the accurate and reproducible range of the assay. Calculations to adjust for background and correlations were done in EXCEL.
Analysis of glycinin
Glycinin was purified by the method of Kwanyen and Burton (Citation1998). Ten picogram of glycinin was dried onto a 96-well microtiter plate. ELISA was performed on total glycinin as described in Taliercio and Kim (Citation2013). Horse-radish peroxidase (HRP) labelled species-specific antibodies against chicken IgY, IgA or IgM were used to identify which eggs had antibodies against glycinin. HRP labelled antibodies were obtained from Bethyl Laboratories, Inc. (Montgomery, TX, USA). A control with no primary serum was used to assess the background.
Analysis of peptide library
The series of 16 biotinylated amino acid peptides with an 11 amino acid overlap represented that glycinin proteins, A1aBx and A5A4B3, were synthesized (Mimotopes, Raleigh, NC, USA). The acidic region of A1aBx is represented by 56 peptides and the basic region is represented by 35 peptides. The acidic region of the other subunit, A5A4B3, is represented by 70 peptides and the basic region is represented by 35 peptides. Detailed information about the peptides used in this study are available in Taliercio and Kim (Citation2013). One nanogram of each peptide was attached to a well in a streptavidin-coated Nunc Maxisorp microtiter plate (Roskilde, Denmark) by incubating at room temperature for 1 h. Each plate included a well without bound peptide to assess the background. Purified IgY, IgA or IgM from eggs shown to bind glycinin were used to screen the peptide library using the protocol previously described in Taliercio and Kim (Citation2013). Only samples with a signal greater than 1.5 times the background were considered positive. Running two sets of samples (IgY, IgA and IgM from two eggs) in duplicate assured the reliability of the measurements, so the remaining eight sets were not replicated since we report peptides that were antigenic in more than three eggs.
Results
ELISA methods using IgY, IgA and IgM isolated from 42 eggs were used to identify Igs that bind glycinin. To improve the comparison across different plates, the same chicken reference serum was included in each plate and the reported values are the signal relative to the same reference sera after the background was removed. shows the values for glycinin-specific IgY, IgA and IgM. shows the correlations (r value) between and among total Ig classes and the glycinin-specific Ig classes. Total IgA and IgM are positively correlated with the glycinin-specific amount of each Ig class and the glycinin-specific IgA and IgM are positively correlated with each other as well (p < 0.05).
Table 1. Eggs from seven local sources were used to isolate total IgA, IgM and IgY.
Table 2. The correlation between total Ig levels and Ig classes that recognize glycinin is shown above the diagonal.
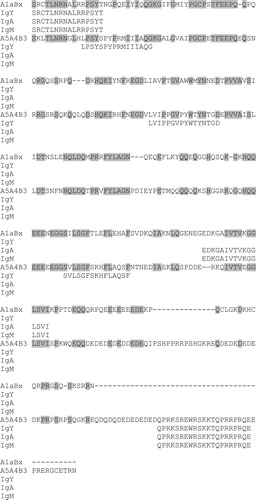
The glycinin epitopes bound by antibodies from eight eggs are highlighted in bold in and two other eggs not included in the data in were identified using ELISA to query a tiled peptide array representing acidic and basic subunits of two glycinin proteins. shows the alignment of the acidic subunits from the two glycinin proteins queried and the epitopes that bind IgA, IgM and/or IgY from at least three eggs. shows the alignment of the basic subunits of the two glycinin proteins and the epitopes that bind IgA, IgM and/or IgY from at least three eggs. Epitopes SRCTLNRNALRRPSYT and QPRKSREWRSKKTQPRRPRQE in the acidic subunit of A1aBx and A5A4B3, respectively, bind all Ig classes from at least three eggs. Epitope ALRQFQLSAQYVVLYKNGIYSPHWNLNANSVIYVTR in the A5A4B3 basic subunit binds Ig from all three classes of multiple eggs. There are no glycinin-specific epitopes in the basic subunit of A1aBx that bind all three classes of Ig, but LDFPALSWLRLSAEF is adjacent to relevant epitope in A1aBx and only binds IgY.
Discussion and conclusion
We have evaluated the immunogenicity of the soybean seed storage proteins with the goal of improving the value of soy protein in animal feed. The fact that animals can have an allergic response to soybean seed storage proteins is well established (Helm, Citation2002). Determining which epitopes are immunogenic is the first step in identifying allergenic epitopes because not all immunogenic epitopes are allergenic. It is well established for several species, including fish, pigs, rabbits and dogs, that soy protein is immunogenic (Taliercio, Loveless, & Turano, Citation2015; Taliercio, Loveless, Turano, & Kim, Citation2014). In fact, most individual animals fed soy protein in these studies developed an immunogenic response to soybean seed storage proteins. It is extremely unlikely that all or even most of these immunogenic events lead to an allergic response given the demonstrated value of soy protein in animal feed. Evaluating the immunogenicity of glycinin epitopes in chickens has allowed us to evaluate the repertoire of Ig classes that bind specific epitopes. There are clear patterns of immunogenicity. Some epitopes bind all three classes of Ig while others bind only one or two Ig classes. Using the same approach presented in this publication, it is possible to identify the immunogenic epitopes in vivo, classify the immunogenicity of each epitope in a tractable number of animals and determine if the animal is allergic to the epitope when challenged. Variants of allergenic epitope can be identified in the glycine germplasm or created through Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and a less allergenic version of the seed storage proteins is identified. If the modified protein improves growth of animals, it can be deployed to the elite germplasm by standard soybean breeding practices. These experiments suggest that glycinin is a more promising target for generating less allergenic variants than conglycinin because similar experiments with conglycinin showed that multiple subunits had the same immunogenic epitopes making it very difficult to eliminate the targeted epitope. For example, if multiple chicken were identified that are allergic to EDKGAIVTVKGG when IgA and IgM antibodies against it are present, a mutant lacking the A1aBx subunit would eliminate the EDKGAIVTVKGG epitope that only binds IgA and IgM making it possible to determine if chickens grow more efficiently on a soy protein diet depleted of this subunit. This may allow the release of soybean varieties that improve the performance of chickens on feed that includes soy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Badley, R. A., Atkinson, D., Hauser, H., Oldani, D., Green, J. P., & Stubbs, J. M. (1975). The structure, physical and chemical properties of the soy bean protein glycinin. Biochimica et Biophysica Acta (BBA) – Protein Structure, 412, 214–228. doi: 10.1016/0005-2795(75)90036-7
- Bessler, M. H., Ricki, M., & Ogawa, T. (2000). Allergen data collection – Update: Soybean (Glycine max). International Symposium of Food Allergens, 2(3), 1–35.
- Codina, R., Ardusso, L., Lockey, R. F., Crisci, C., & Medina, I. (2003). Allergenicity of varieties of soybean. Allergy, 58, 1293–1298. doi: 10.1046/j.1398-9995.2003.00301.x
- Fernando Guilherme Perazzo Costa, C. C. G., Figueiredo, D. F., Oliveira, C. F. S., & Silva, J. H. V. (2008). Economic and environmental impact of using exogenous enzymes on poultry feeding. International Journal of Poultry Science, 7(5), 311–315.
- Hamal, K. R., Burgess, S. C., Pevzner, I. Y., & Erf, G. F. (2006). Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poultry Science, 85, 1364–1372. doi:10.1093/ps/85.8.1364% doi: 10.1093/ps/85.8.1364
- Helm, R. M. (2002). Food allergy animal models. Annals of the New York Academy of Sciences, 964, 139–150. doi: 10.1111/j.1749-6632.2002.tb04139.x
- Helm, R. M., Cockrell, G., Connaughton, C., Sampson, H. A., Bannon, G. A., Beilinson, V., et al. (2000). A soybean G2 glycinin allergen. International Archives of Allergy and Immunology, 123, 205–212. doi: 10.1159/000024445
- Kim, S. K., Kim, T. H., Lee, S. K., Chang, K. H., Cho, S. J., Lee, K. W., et al. (2016). The use of fermented soybean meals during early phase affects subsequent growth and physiological response in broiler chicks. Asian–Australasian Journal of Animal Sciences, 29, 1287–1293. doi: 10.5713/ajas.15.0653
- Krishnan, H. B., Kim, W.-S., Jang, S., & Kerley, M. S. (2009). All three subunits of soybean β-conglycinin are potential food allergens. Journal of Agricultural and Food Chemistry, 57, 938–943. doi: 10.1021/jf802451g
- Kwanyen, P. W. R. F., & Burton, J. W. (1998). Soybean protein quality. The proceedings of the world conference on oilseed and edible oils processing. Champaign, IL: AOAC Press (pp. 284–289).
- Li, D. F., Blecha, F., Allee, G. L., Hancock, J. D., Nelssen, J. L., Reddy, P. G., et al. (1990). Transient hypersensitivity to soybean meal in the early-weaned pig. Journal of Animal Science, 68, 1790–1799. doi: 10.2527/1990.6861790x
- Li, D. F., Blecha, F., Nelssen, J. L., Reddy, P. G., Klemm, R., & Goodband, R. D. (1991). Interrelationship between hypersensitivity to soybean proteins and growth performance in early-weaned pigs. Journal of Animal Science, 69, 4062–4069. doi: 10.2527/1991.69104062x
- Li, D. F., Giesting, D. W., Blecha, F., Allee, G. L., Hancock, J. D., Nelssen, J. L., et al. (1991). Measuring suitability of soybean products for early-weaned pigs with immunological criteria. Journal of Animal Science, 69, 3299–3307. doi: 10.2527/1991.6983299x
- Liu, C., Wang, H., Cui, Z., He, X., Wang, X., Zeng, X., et al. (2007). Optimization of extraction and isolation for 11S and 7S globulins of soybean seed storage protein. Food Chemistry, 102, 1310–1316. doi: 10.1016/j.foodchem.2006.07.017
- Masteller, E. L., & Thompson, C. B. (1994). B cell development in the chicken. Poultry Science, 73, 998–1011. doi: 10.3382/ps.0730998
- Nielsen, N. C. (1985). The structure and complexity of the 11S polypeptides in soybeans. Journal of the American Oil Chemists’ Society, 62, 1680–1686. doi: 10.1007/BF02541665
- Nielsen, N. C., Dickinson, C. D., Cho, T. J., Thanh, V. H., Scallon, B. J., Fischer, R. L., et al. (1989). Characterization of the glycinin gene family in soybean. The Plant Cell, 1, 313–328. doi: 10.1105/tpc.1.3.313
- Taliercio, E., & Kim, S. W. (2013). Epitopes from two soybean glycinin subunits are antigenic in pigs. Journal of the Science of Food and Agriculture, 93, 2927–2932. doi: 10.1002/jsfa.6113
- Taliercio, E., & Kim, S. W. (2014). Identification of a second major antigenic epitope in the α-subunit of soy β-conglycinin. Food and Agricultural Immunology, 25, 311–321. doi: 10.1080/09540105.2013.791969
- Taliercio, E., Loveless, T. M., & Turano, M. J. (2015). Identification of epitopes of the A1aBx and A5A4B3 subunits of glycinin antigenic in three animal species. Food and Agricultural Immunology, 26, 271–281. doi: 10.1080/09540105.2014.906566
- Taliercio, E., Loveless, T. M., Turano, M. J., & Kim, S. W. (2014). Identification of epitopes of the β subunit of soybean β-conglycinin that are antigenic in pigs, dogs, rabbits and fish. Journal of the Science of Food and Agriculture, 94, 2289–2294. doi: 10.1002/jsfa.6556
- Tizard, I. (2002). The avian antibody response. Seminars in Avian and Exotic Pet Medicine, 11, 2–14. doi: 10.1053/saep.2002.28216
- Utsumi, S., Kohno, M., Mori, T., & Kito, M. (1987). An alternate cDNA encoding glycinin A1aBx subunit. Journal of Agricultural and Food Chemistry, 35, 210–214. doi: 10.1021/jf00074a011
- Vu Huu, T., & Shibasaki, K. (1978). Major proteins of soybean seeds. Subunit structure of beta-conglycinin. Journal of Agricultural and Food Chemistry, 26, 692–695. doi: 10.1021/jf60217a026