ABSTRACT
The work focused on the study of the immunomodulatory and gut-protecting effect of humic substances (HS) in broilers. The diet of experimental chicks was enriched with 0.8% of HS. We noted that HS had a stimulatory effect on the phagocytic activity and the engulfing capacity of phagocytes, however, the level of oxidative burst of phagocytes was not affected. We observed a significant increase of CD4+: CD8+ lymphocyte ratio, an indicator of immune stimulation. HS did not influence the IgA gene expression. In contrast, we observed a significant increase in the expression of MUC-2 (intestinal mucin 2) gene, and a decrease in the expression of IGF-2 (insulin-like growth factor 2) and also AvBD2 (avian beta defensin 2) genes. A decreased Enterobacteriaceae counts in the gut of experimental animals showed a positive effect on intestinal microbiota. We confirmed a gut-protecting and an immunostimulatory effect of HS in broiler chickens.
1. Introduction
In order to maintain the health of the organism, the optimal functionality of the immune system is crucial. Under conditions of intensive farming, animals are constantly exposed to stress, which has a negative impact on their immune system. Mucosal surfaces represent the site of the first and greatest contact of the organism with the antigens, with the gastrointestinal tract mucosa being the most important. To maintain intestinal homeostasis, intestinal mucin 2 (MUC2), a major gel-forming mucin, represents a primary barrier component of mucus layers and a target site for secretory IgA. It has evolved to form the first line of defense to limit epithelial contact with and penetration by microbiota and other potentially dangerous antigens (Honda & Takeda, Citation2009; Macpherson & Harris, Citation2004). Defensins primarily show a strong effect against bacterial, viral, and fungal infections. From the three defensin subfamilies, only β-defensins have been identified in birds (Cuperus, Coorens, van Dijk, & Haagsman, Citation2013). Moreover, they have been shown to have a regulatory function in many physiologic processes such as cytokine release, histamine production, chemotaxis, inflammation, and even in the process of wound healing (Proal et al., Citation2011).
The spread of bacterial resistance due to antibiotics being used in therapy is increasingly considered as a hazard, therefore chemical antibiotics used as growth promoters have been banned by EU legislation (Islam et al., Citation2005).
Leonardite is a soft waxy, black or brown, vitreous mineraloid that is easily soluble in alkaline solutions. It is an oxidation product of lignite, which is a rich source of humic substances (up to 90%) (Neuendorf, Citation2005). Humic substances are formed from the decomposition of plants and occur naturally in soil, peat, water, and brown coal. These substances have a complex structure and contain humin, humic and fulvic acids. They have primarily been used to stimulate plant growth (van Rensburg, Citation2015). Moreover, humins are an important mediator of microbial interactions in nature.
Humic substances are used in both human and veterinary medicine for their detoxication, antibacterial, and antiviral effects, but more and more studies confirm their immunomodulatory potential (Gomez-Rosales & Angeles, Citation2015; Joone & van Rensburg, Citation2004). Their immunostimulatory effect on infectious diseases is known, and their anti-inflammatory effect and suppression of an excessive immune response, for example in hypersensitivity reactions, have been reported (Islam et al., Citation2005). The effect of humic substances on the immune system is related to the properties of these substances. They form relatively solid complexes with carbohydrates. Subsequently, these complexes allow the formation of glycoproteins characterised by the ability to bind to NK cells and T lymphocytes. It means that they behave as modulators, and enable subsequent communication between these cells. The ability of humic substances to affect the immune system, therefore, lies in the regulation of immune activity (Riede, Zeck-Kapp, Freudenberg, Keller, & Seubert, Citation1991). For poultry husbandry, HS could be a powerful tool in maintaining the gastrointestinal health, thus improving body weight, feed conversion, and ash content of the tibia (Ceylan, Ciftci, & Ilhan, Citation2003; Kocabagli, Alp, Acar, & Kahraman, Citation2002; Taklimi, Ghahri, & Isakan, Citation2012; Windisch, Schedle, Plitzner, & Kroismayr, Citation2008). The effect of HS, as a binding agent for aflatoxin molecules in the gastrointestinal tract and prevention their absorption decreases the risk of aflatoxin toxicity in poultry (Jansen van Rensburg, Van Rensburg, Van Ryssen, Casey, & Rottinghaus, Citation2006). In addition, in laying hens and partridges benefits involving feed intake and egg production have been observed (Dobrzański, Trziszka, Herbut, Krawczyk, & Tronina, Citation2009; Ozturk, Coskun, Ocak, & Erener, Citation2009).
There are only a few studies focused on the immune response or intestinal microbiota of poultry, therefore our work was aimed at the study of the immunomodulatory and gut-protecting effect of natural humic substances in broilers.
2. Material and methods
2.1. Animals and experimental design
The Ethical committee of the University of Veterinary Medicine and Pharmacy in Košice and State Veterinary and Food Administration of the Slovak Republic approved the experimental protocol number 3040/14-221, and the animals were handled and sacrificed in a humane manner. We received 100 Cobb500 male broiler chicks from Mach Hydina Budmerice s.r.o. (Hydina Slovensko s.r.o., Slovakia). The one-day-old chicks were divided into 2 groups – control (C; n = 50) and experimental (HS; n = 50). Chicks were reared in a controlled atmosphere on deep bedding with free access to water and feed. For the first 10 days, they were fed a starter diet – BR-1 (CP 221 g, Fat 38 g, Fibre 36 g, Ash 90 g, Methionine 5.6 g, Vit. A 10800 IU, Vit. D3 2400 IU, Vit. E 36 mg, Cu 12.3 mg, Sodium salinomycinate 70 mg; De Heus, Bučovice, Czech Republic). From day 11 to day 27, birds consumed a grower diet – BR-2 (CP 186 g, Fat 34 g, Fibre 36 g, Ash 60 g, Methionine 5.3 g, Vit. A 10800 IU, Vit. D3 2400 IU, Vit. E 42 mg, Cu 12 mg, Sodium salinomycinate 70 mg; De Heus, Bučovice, Czech Republic), and from day 28 to day 38, a finisher diet – BR-3 (CP 180 g, Fat 43 g, Fibre 37 g, Ash 100 g, Methionine 4.9 g, Vit. A 8100 IU, Vit. D3 1800 IU, Vit. E 37 mg, Cu 11 mg; De Heus, Bučovice, Czech Republic). Feed mixtures BR-2 and BR-3 for the experimental chicks were supplemented with 0.8% of natural humic substances prepared from leonardite (). The treatment dose was selected on the basis of a pilot experiment where 0.6%, 0.8% and 1% of HS was administered to broilers, with the best immunological response after 0.8% (unpublished data). On day 38, fifteen birds from both groups were randomly selected for sampling and subsequent immunological and microbiological analyses. Blood samples were collected from vena jugularis and gastrointestinal tracts were immediately removed from the birds, which were sacrificed by cervical dislocation.
Table 1. Composition of humic supplement.
2.2. Homogenisation of tissue and isolation of total RNA
Tissue samples from the caudal part of the caecum were cut into 20 mg pieces, placed immediately into RNA Later solution (Qiagen, UK), and stored at –70°C, prior to RNA purification. Single tissue fragments were transferred into 1 ml of TRIzol Reagent (Molecular Research Center, USA), and homogenised with 2.3 mm of zirconium silica beads (BioSpec Products, USA) in a vortex mixer (Labnet, USA). To separate the phases, 50 μl of 4-bromanisole (Molecular Research Center, USA) was added. The whole content of the tube was centrifuged and the upper aqueous phase was collected for total RNA purification with the RNAeasy mini kit (Qiagen, UK), according to the manufacturer's instructions. Turbo DNA-free kit (Ambion, USA) was used for the treatment of RNA samples to remove genomic DNA. Both the purity and concentration of RNA were determined spectrophotometrically on NanoPhotometer (Implen, Germany) and 1 μg of the total RNA immediately underwent reverse transcription with iScript cDNA Synthesis Kit (Bio-Rad, USA) and oligo-dT primers. The resulting cDNA was diluted 10-fold in UltraPure™ DNase/RNase-Free distilled water (Invitrogen, USA) and used as a template for real-time PCR, or stored at −20°C until used.
2.3. Quantitative real-time PCR
The mRNA level of IgA, MUC-2, IGF-2 and AvBD2 was determined. In addition, mRNA relative expression of the reference gene, coding for GAPDH (glyceraldehyde-3- phosphate dehydrogenase), was used for data normalisation. The primer sequences used for qPCR are listed in . All primer sets allowed DNA amplification efficiencies between 94% and 100%. Amplification and detection of specific products were performed via the CFX 96 RT system (Bio-Rad, USA). The cycling conditions included an initial denaturation at 94°C for 3 min, followed by 36 cycles at 93°C for 45 s. The optimal annealing temperature and time for each primer is shown in , and the final extension step was carried out for 10 min at 72°C. A melting curve ranging from 50°C to 95°C, with readings taken every 0.5°C, was charted for each individual RT–PCR plate. Each sample was subjected to real-time PCR in duplicate, and mean values of tiplicates were used for subsequent analysis. We also confirmed that the efficiency of amplification of each target gene (including GAPDH) was essentially 100% in the exponential phase of the reaction, where the quantification cycle (Cq) was calculated. The Cq values of the genes studied were normalised to an average Cq value of the reference gene (ΔCq), and the relative expression of each gene was calculated as 2–ΔCq. The relative expression of IgA, MUC-2, IGF-2 and AvBD2 in the caecum was determined in fifteen animals individually.
Table 2. List of primers used in RT-PCR for IgA, MUC-2, IGF-2 and AvBD2 mRNA detection in chicks.
2.4. Phagocytic activity and oxidative burst of phagocytes
To test the activity of phagocytes we used heparinised blood (5–10 IU/ml). To evaluate the activity of phagocytes were used commercial tests for flow cytometry: Phagotest® (Glycotope Biotechnology, Germany) for phagocytic activity and Bursttest® (Glycotope Biotechnology, Germany) for the metabolic burst activity of phagocytes.
2.5. Lymphocyte phenotyping
For the lymphocyte phenotyping, mononuclear leukocytes (MNL) were isolated from heparinised blood as follows: 600 μl of blood was diluted in a 1:1 ratio with PBS (MP Biomedicals, France) and carefully underlayered with 2 ml of lymphocyte separating medium LSM 1077 (PAA Laboratories GmbH, Austria). Tubes were centrifuged for 30 min at 600× g. MNL were recovered by aspirating white layer in plasma-LSM interphase, and the acquired sample was washed twice with PBS by centrifugation for 5 min at 250× g. Selected lymphocyte subpopulations were identified by direct staining with a subsequent combination of conjugated anti-chicken monoclonal antibodies (MoAb): CD4-FITC/CD8a-R-PE/CD45-APC (Southern Biotech, USA). Samples containing 5.105 cells in 50 µl were stained with 2 µl anti-CD4 MoAb (clone: CT-4; concentration: 0.5 mg/ml), 1 µl anti-CD8a MoAb (clone: CT-8; concentration: 0.1 mg/ml), and 5 µl anti-CD45 MoAb (clone: LT-40; concentration: 0.1 mg/ml) for 20 min in the dark at laboratory temperature. Then tubes were washed twice with 1 ml PBS by centrifugation for 5 min at 250× g and subsequently 100 μl of PBS was added to each tube. Flow cytometric analysis was performed on a six colour BD FACSCantoTM flow cytometer equipped with blue (488 nm) and red (633 nm) lasers (Becton Dickinson Biosciences, USA). Data were analysed using the BD FACS DivaTM software. Position of cells was gated in FSC vs. SSC dot plot. Contaminating chicken thrombocytes were differentiated from lymphocytes based on their different side scatter profiles. Cells with the increased side-scatter correspond to thrombocytes and those with lower SSC are lymphocytes (Bertram, Citation1998). For the analysis, only cells with high expression of CD45 antigen were used. Proportions of lymphocytes are expressed in percentage.
2.6. Intestinal bacteria analysis
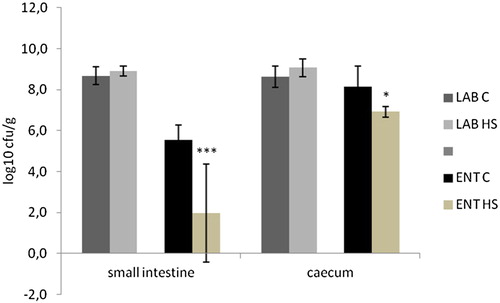
Lactic acid bacteria and enterobacteria were found, by the plate count method, in the contents of the small intestine and caecum. Lactic acid bacteria were counted on MRS agar plates (Merck, Germany) after 48 h incubation at 37°C under anaerobic conditions (GasPak system, Becton Dickinson, USA). Endo agar plates (HiMedia, India) incubated aerobically for 24 h at 37°C were used to determine numbers of enterobacteria. The bacterial counts are expressed in log10 of colony forming units per gram of content (log10 cfu/g) ± standard deviation.
2.7. Statistical analysis
The data were evaluated with an unpaired T-test in the statistical program Graph Pad Prism version 3.00. The results are expressed as mean ± standard deviation.
3. Results
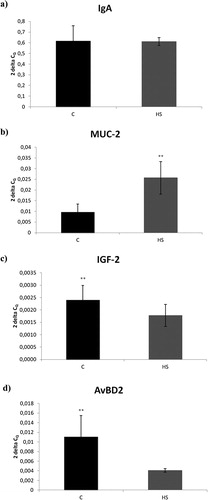
The relative expression of the genes coding for IgA, MUC-2, IGF-2 and AvBD2 in the ceacal mucosa were evaluated to study the effect of humic substances on the humoral immune response and gut protection. HS did not influence the IgA gene expression ((a)). In contrast, we observed a significant increase in the relative expression of the MUC-2 gene in the HS group compared to the control group (p < .01) ((b)). On the other hand, the relative expressions of the IGF-2 and AvBD2 genes were down-regulated in the HS group compared to the control group (p < 0.01) ((c,d)).
Figure 1. Relative expression level of (a) IgA, (b) MUC-2 (c) IGF-2 and (d) AvBD2 in the caecum of broiler chickens (n = 15) receiving 0.8% of natural humic substances prepared from leonardite in feed. Results at each time point are the median of 2–ΔCq. Means with different superscripts are significantly different **p < .01.
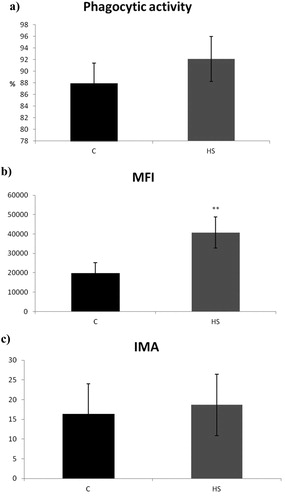
The effect of humic substances on the innate cellular immunity of broilers has been studied by analysing the influence of phagocytosis, including oxidative burst. In the experimental group, we noted a significantly higher phagocytic activity ((a)), as well as a higher mean fluorescence intensity ((b)), which expresses the phagocyte uptake capacity, measured as the average number of fluorescein-labelled E. coli engulfed per phagocyte. The index of metabolic activity, indicating the oxidative burst of phagocytes, was not significantly affected by the addition of HS to the feed ((c)).
Figure 2. Influence of 0.8% of natural humic substances prepared from leonardite on the activity of phagocytes in broiler blood evaluated as: (a) percentage of active phagocytes, (b) engulfing capacity of phagocytes (expressed as mean fluorescence intensity – MFI) and (c) oxidative burst activity of phagocytes (expressed as index of metabolic activity – IMA). Means with different superscripts are significantly different **p < .01.
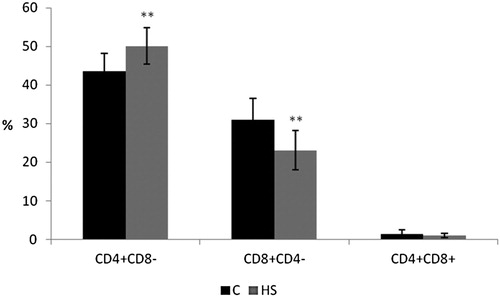
The proportions of CD4+ and CD8+ lymphocyte subpopulations and their ratio, which is an indicator of immunostimulation, were selected as parameters for monitoring the effect on the acquired cellular immunity. After the application of HS, the proportion of CD4+ lymphocytes was significantly increased and CD8+ lymphocytes were decreased (). This resulted in a statistically higher CD4:CD8 ratio (2.28 ± 0.59), in comparison to that of the control group (1.48 ± 0.43; p < .01). Double positive lymphocytes (CD4+ CD8+) were not affected.
Figure 3. The effect of 0.8% of natural humic substances prepared from leonardite in feed on the percentage of CD4+ CD8–, CD8+ CD4– and CD4+ CD8+ lymphocytes in broiler peripheral blood. Means with different superscripts are significantly different **p < .01.
The influence of HS on the gut microbiota was evaluated on the basis of counts of lactic acid bacteria (LAB) and enterobacteria. LAB represents beneficial microbiota whereas counts of enterobacteria (ENT) represent symbionts, as well as many enteropathogens. Despite the fact that the numbers of LAB were only slightly higher in HS group than in the control, without any statistical significance, the numbers of ENT were significantly lower in the small intestine as well as in the caecum (). Therefore ENT:LAB ratio was significantly lower in the HS group in the small intestine (0.23 ± 0.21; p < .001) and also in the caecum (0.69 ± 0.22; p < .05) when compared to those in control group in the small intestine and the caecum − (0.70 ± 0.09) and (0.90 ± 0.14), respectively.
4. Discussion
In our work, we studied the effect of application of 100% natural humic substances on the immune response in broilers. We observed upregulation of the relative gene expression for MUC-2, which is in agreement with findings that humic substances could facilitate the formation of a protective film on the mucus epithelium of the gastrointestinal tract against infectious agents and toxins, thereby also improving the conversion of animal feed (Santiago-López, Hernández-Mendoza, Mata-Haro, Vallejo-Cordoba, & González-Córdova, Citation2018). In fact, changes in the amount or in the composition of mucus may lead to undesirable inflammatory responses (Wang et al., Citation2018). Moreover, humic substances have a trophic effect, which is manifested by stimulating the proliferation of normal crypt cells, which therefore enhances the maintenance of healthy tissue (Disetlhe, Marume, Mlambo, & Dinev, Citation2017).
IgA’s main role is neutralising antigens on mucosal surfaces. The majority of mucosal IgA positive cells are derived from B-cell activation (Herich, Citation2016). The addition of humic substances to the feed did not show an increase of IgA gene expression. Similarly, Maguey-Gonzalez et al. (Citation2018) observed that humic acids in a concentration of 0.1–0.2% did not influence the total concentration of mucosal IgA in the ileum after Salmonella Enteritidis infection in neonate broiler chickens. The opposite conclusion was drawn by Wang et al. (Citation2008), who found higher relative lymphocyte counts in pigs whose feed was supplemented with 10% HS. In agreement with previous statements, we suppose that the influence of HS on the population of B lymphocytes is dependent on the concentration of the humic substances administered.
Paneth cells are important for the maintenance of mucosal barrier function. They secrete the antimicrobial peptides – defensins (Fasano, Citation2008). Excessive production of defensins such as human beta-defensin 2 (hBD2) by psoriatic keratinocytes in psoriatic patients was reported. For this reason, it serves as a biomarker for psoriasis activity (Jansen et al., Citation2009). Also Feng et al. (Citation2017) found out, that beta-defensin 2 promotes the itch sensation by activating TLR4-expressing cutaneous immune cells in mice. Our results revealed that HS had the ability to decrease the relative expression of AvBD2 gene in caecum, thereby could contribute to the attenuation of inflammatory stimulation. Indeed, several studies in other species confirm that humic substances modulate inflammatory processes (Naude, Cromarty, & van Rensburg, Citation2010; Rath, Huff, & Huff, Citation2006; van Rensburg, Snyman, Mokoele, & Cromarty, Citation2007). The influence of HS on defensins in chicken has not been studied yet.
The concentration of HS used in the broiler feed in this study significantly downregulated the relative expression of the IGF-2 gene. This is interesting because IGF-2 is a primary mediator of growth hormone; thereby it exerts an anabolic action on skeletal and muscular apparatus. In rats, IGF-2 prevents gut atrophy (Sugiura et al., Citation1997). In our experiment, Jaďuttová et al. (Citation2019) studied the effect of HS on fattening performance, carcass yield, biochemical blood parameters and bone mineral profile. They noted a slight increase in final body weight and feed conversion ratio in the supplemented group (p > .05) and no impact on carcass yield. Interestingly, the calcium content in the bones was significantly higher in the experimental group than in the control group, but the phosphorus content was significantly lower. Likewise, Hanafy and El-Sheikh (Citation2008) observed that feed consumption and live body weight were not significantly affected by humic acid supplementation.
In the experimental group, we observed a significant increase in phagocytic activity after the administration of 0.8% HS. This result is consistent with the study by Habibian, Morshedi, and Delirezh (Citation2010), who investigated the effect of humic substances on phagocytic activity of mononuclear cells in rats. In our study, the engulfing capacity of phagocytes was also stimulated by HS, while the respiratory burst was not affected. Sanmiguel and Rondón (Citation2016) studied the impact of humic substances on selected parameters of innate immunity in laying hens. The results changed during the study − the phagocytic index was increased in the groups of laying hens supplemented with the addition of 0.1% and 0.2% humic substances after 8 and 30 days of administration, but on day 60 the phagocytic index was significantly decreased when compared with the control group. These results may suggest that humic substances only have a stimulatory effect on phagocytes for a limited time. The results of the respiratory bursts were also altered during the research; no effect was observed in the experimental group with the addition of 0.1% during the whole research period, but in the group supplemented with 0.2% of humic substances, there was an increase at day 30 and a decrease at day 60.
We also analysed the representation of individual subpopulations of lymphocytes. We noted a significant increase in the percentage of CD4+ lymphocytes, and a decrease in CD8+ lymphocytes. The final ratio of CD4+ and CD8+ lymphocytes, as a marker of immune stimulation, showed a significant increase. So far, studies dealing with the effects of HS on individual lymphocyte subpopulations are still low, and previous works have been largely centred on the overall change in the total lymphocytes in blood. An increase in lymphocyte numbers after humic substances supplementation was noted in laying hens (Cetin, Berrin, & Nazmi, Citation2011), in broilers (Rath et al., Citation2006) and in Japanese quails (Ipek, Avci, Iriadam, Kaplan, & Denek, Citation2008).
Within the study of the effect of HS on the intestinal microbiota, we observed a significant decrease in enterobacteria in the intestinal contents, an increase in lactic acid bacteria counts, and the resulting ratio of enterobacteria to lactic acid bacteria showed an increase in the beneficial gut microbiota. Similarly, Aksu and Bozkurt (Citation2009) reported a significant decrease in E. coli numbers in the broiler intestinal contents and only a slight increase in lactobacilli count after HS administration. On the contrary, Shermer, Maciorowski, Bailey, Byers, and Ricke (Citation1998) observed a significant increase in the number of E. coli in caecum of chickens as well as a weaker increase in lactobacilli, after administration of higher humate concentrations (1% and 5%). When chicks were given a lower concentration of humates (0.5%), no significant effect on the caecal microflora was noted.
5. Conclusion
In conclusion, we observed a stimulatory effect of 0.8% HS on phagocytic activity, the engulfing capacity of phagocytes, significant increase of CD4+: CD8+ lymphocytes ratio, as well as upregulation of MUC-2 gene expression. Similarly, there was a decreased ratio of enterobacteria and lactic acid bacteria in the guts of experimental animals showing a positive effect on intestinal microbiota. On the other hand, the level of oxidative burst of phagocytes and IgA gene expression was not affected. These findings indicate that, administration of 0.8% humic substances prepared from leonardite has a gut-protecting, and an immunostimulatory effect on broiler chickens.
Acknowledgements
The authors gratefully acknowledge Humac s.r.o. Slovakia for providing humic substances for the experiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akbari, M. R., Haghighi, H. R., Chambers, J. R., Brisbin, J., Read, L. R., & Sharif, S. (2008). Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar typhimurium. Clinical and Vaccine Immunology, 15, 1689–1693. doi: 10.1128/CVI.00242-08
- Aksu, T., & Bozkurt, A. S. (2009). Effect of dietary essential oils and/or humic acids on broiler performance, microbial population of intestinal content and antibody titres in the summer season. Journal of the Faculty of Veterinary Medicine, University of Kafkas, 15, 180–190.
- Bertram, E. M., Jilbert, A. R., & Kotlarski, I. (1998). Characterisation of duck thromhocytes. Research in Veterinary Science, 64, 267–270. doi: 10.1016/S0034-5288(98)90139-4
- Cetin, E., Berrin, K. G., & Nazmi, C. (2011). Effect of dietary humate and organic acid supplementation on social stress induced by high stocking density in laying hens. Journal of Animal and Veterinary Advances, 10, 2402–2407. doi: 10.3923/javaa.2011.2402.2407
- Ceylan, N., Ciftci, I., & Ilhan, Z. (2003). The effects of some alternative feed additives for antibiotic growth promoters on the performance and gut microflora of broiler chicks. Turkish Journal of Veterinary and Animal Sciences, 27, 727–733.
- Cuperus, T., Coorens, M., van Dijk, A., & Haagsman, H. P. (2013). Avian host defense peptides. Developmental and Comparative Immunology, 41, 352–369. doi: 10.1016/j.dci.2013.04.019
- De Boever, S., Vangestel, C., De Backer, P., Croubels, S., & Sys, S. U. (2008). Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Veterinary Immunology and Immunopathology, 122, 312–317. doi: 10.1016/j.vetimm.2007.12.002
- Disetlhe, A. R. P., Marume, U., Mlambo, V., & Dinev, I. (2017). Humic acid and enzymes in canola-based broiler diets: Effects on bone development, intestinal histomorphology and immune development. South African Journal Of Animal Science, 47, 914–922. doi: 10.4314/sajas.v47i6.19
- Dobrzański, Z., Trziszka, T., Herbut, E., Krawczyk, J., & Tronina, P. (2009). Effect of humic preparations on productivity and quality traits of eggs from greenleg partridge hens. Annals of Animal Science, 9, 165–174.
- Fasano, A. (2008). Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: Living life on the edge of the wall. The American Journal of Pathology, 173, 1243–1252. doi: 10.2353/ajpath.2008.080192
- Feng, J., Luo, J., Mack, M. R., Yang, P., Zhang, F., Wang, G., … Hu, H. (2017). The antimicrobial peptide human beta-defensin 2 promotes itch through Toll-like receptor 4 signaling in mice. The Journal of Allergy and Clinical Immunology, 140, 885–888. doi: 10.1016/j.jaci.2017.03.035
- Gomez-Rosales, S., & Angeles, M. L. (2015). Addition of a worm leachate as source of humic substances in the drinking water of broiler chickens. Asian-Australasian Journal of Animal Sciences, 28, 215–222. doi: 10.5713/ajas.14.0321
- Habibian, R., Morshedi, A., & Delirezh, N. (2010). Effect of humic acid on humoral immune response and phagocytosis. Global Veterinaria, 4, 135–139.
- Hanafy, M. M., & El-Sheikh, A. M. H. (2008). The effect of dietry humic acid supplementation on some productive and physiological traits of laying hens. Egyptian Poultry Science, 28, 1043–1058.
- Herich, R. (2016). Is the role of IgA in local immunity completely known? Food and Agricultural Immunology. doi: 10.1080/09540105.2016.1258547
- Honda, K., & Takeda, K. (2009). Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunology, 2, 187–196. doi: 10.1038/mi.2009.8
- Ipek, H., Avci, M., Iriadam, M., Kaplan, O., & Denek, N. (2008). Effects of humic acid on some hematological parameters, total antioxidant capacity and laying performance in Japanese quails. European Poultry Science, 72, 245–250.
- Islam, K. M. S., Schuhmacher, A., & Gropp, J. M. (2005). Humic acid substances in animal. Agriculture Pakistan Journal of Nutrition, 4, 126–134. doi: 10.3923/pjn.2005.126.134
- Jaďuttová, I., Marcinčáková, D., Bartkovský, M., Semjon, B., Harčárová, M., Nagyová, A., … Marcinčák, S. (2019). Effect of dietary humic substances on the fattening performance, carcass yield, blood biochemistry parameters and bone mineral profile of broiler chickens. Acta Vetrinaria Brno, 88, 307–313. doi.org/10.2754/avb201988030307
- Jansen, P. A., Rodijk-Olthuis, D., Hollox, E. J., Kamsteeg, M., Tjabringa, G. S., de Jongh, G. J., … Schalkwijk, J. (2009). β-Defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PloS ONE, 4, e4725. doi: 10.1371/journal.pone.0004725
- Jansen van Rensburg, C., Van Rensburg, C. E., Van Ryssen, J. B., Casey, N. H., & Rottinghaus, G. E. (2006). In vitro and in vivo assessment of humic acid as an aflatoxin binder in broiler chicken. Poultry Science, 85, 1576–1583. doi: 10.1093/ps/85.9.1576
- Joone, G. K., & van Rensburg, C. E. (2004). An in vitro investigation of the anti-inflammatory properties of potassium humate. Inflammation, 28, 169–174. doi: 10.1023/B:IFLA.0000039563.90066.5d
- Kocabagli, N., Alp, M., Acar, N., & Kahraman, R. (2002). The effects of dietary humate supplementation on broiler growth and carcass yield. Poulty Science, 81, 227–230. doi: 10.1093/ps/81.2.227
- Lammers, A., Wieland, W. H., Kruijt, L., Jansma, A., Straetemans, T., Schots, A., … Parmentier, H. K. (2010). Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Developmental and Comparative Immunology, 34, 1254–1262. doi: 10.1016/j.dci.2010.07.001
- Macpherson, A. J., & Harris, N. L. (2004). Interactions between commensal intestinal bacteria and the immune system. Nature Reviews Immunology, 4, 478–485. doi: 10.1038/nri1373
- Maguey-Gonzalez, J. A., Michel, M. A., Baxter, M. F. A., Soliz-Cruz, B., Hernandez-Patlan, B., Merino-Guzman, R., … Gomez-Rosales, S. (2018). Effects of humic acids on recovery of Salmonella enterica serovar Enteritidis. Annals of Animal Science, 18, 387–399. doi.org/10.1515/aoas-2017-0037
- Mudroňová, D., Karaffová, V., Košcová, J., Bartkovský, M., Marcinčáková, D., Popelka, P., … Marcinčák, S. (2018). Effect of fungal gamma-linolenic acid and beta-carotene containing prefermented feed on immunity and gut of broiler chicken. Poultry Science, 97, 4211–4218. doi: 10.3382/ps/pey306
- Naude, P. J., Cromarty, A. D., & van Rensburg, C. E. (2010). Potassium humate inhibits carrageenan-induced paw oedema and a graft-versus-host reaction in rats. Inflammopharmacology, 18, 33–39. doi: 10.1007/s10787-009-0026-8
- Neuendorf, K. K. E. (2005). Glossary of geology. Alexandria, Virginia: Springer Science & Business Media.
- Ozturk, E., Coskun, I., Ocak, N., & Erener, G. (2009). Effects of dietary humic substances on egg production and egg shell quality of hens after peak laying period. African Journal of Biotechnology, 8, 1155–1159.
- Proal, A. D., Albert, P. J., Blaney, G. P., Lindseth, I. A., Benediktsson, C. H., & Marshall, T. G. (2011). Immunostimulation in the era of the metagenome. Cellular and Molecular Immunology, 8, 213–225. doi: 10.1038/cmi.2010.77
- Rath, N. C., Huff, W. E., & Huff, G. R. (2006). Effects of humic acid on broiler chickens. Poultry Science, 85, 410–414. doi: 10.1093/ps/85.3.410
- Riede, U. N., Zeck-Kapp, G., Freudenberg, N., Keller, H. U., & Seubert, B. (1991). Humate induced activation of human granulocytes. Virchows Archiv B. Cell Pathology Including Molecular Pathology, 60, 27–34. doi: 10.1007/BF02899524
- Sanmiguel, R. P., & Rondón, I. B. (2016). Supplementation with humic substances affects the innate immunity in layer hens in posfasting phase. Revista MVZ Córdoba, 21, 5198–5210. doi: 10.21897/rmvz.30
- Santiago-López, L., Hernández-Mendoza, A., Mata-Haro, V., Vallejo-Cordoba, V., & González-Córdova, A. F. (2018). Immune response induced by fermented milk with potential probiotic strains isolated from artisanal Cocido cheese. Food and Agricultural Immunology, 29, 911–929. doi: 10.1080/09540105.2018.1485632
- Shermer, C. L., Maciorowski, K. G., Bailey, C. A., Byers, F. M., & Ricke, S. C. (1998). Caecal metabolites and microbial populations in chickens consuming diets containing a mined humate compound. Journal of the Science and Food Agriculture, 77, 479–486. doi: 10.1002/(SICI)1097-0010(199808)77:4<479::AID-JSFA607>3.0.CO;2-L
- Smirnov, A., Tako, E., Ferket, P. R., & Uni, Z. (2006). Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poultry Science, 85, 669–673. doi: 10.1093/ps/85.4.669
- Sugiura, T., Tashiro, T., Yamamori, H., Morishima, Y., Otsubo, Y., Hayashi, N., … Ito, I. (1997). Effects of insulin-like growth factor-1 on endotoxin translocation in burned rats receiving total parenteral nutrition. Nutrition, 13, 783–787. doi: 10.1016/S0899-9007(97)00189-5
- Taklimi, S. M., Ghahri, H., & Isakan, M. A. (2012). Influence of different levels of humic acid and esterified glucomannan on growth performance and intestinal morphology of broiler chickens. Agricultural Sciences, 3, 663–668. doi: 10.4236/as.2012.35080
- van Rensburg, C. E. (2015). The Antiinflammatory properties of humic substances: A mini review. Phytotherapy Research, 29, 791–795. doi: 10.1002/ptr.5319
- van Rensburg, C. E., Snyman, J. R., Mokoele, T., & Cromarty, A. D. (2007). Brown coal derived humate inhibits contact hypersensitivity: An efficacy, toxicity and teratogenicity study in rats. Inflammation, 30, 148–152. doi.org/10.1007/s10753-007-9031-5
- Wang, Q., Chen, Y. J., Yoo, J. S., Kim, H. J., Cho, J. H., & Kim, I. H. (2008). Effects of supplemental humic substances on growth performance, blood characteristics and meat quality in finishing pigs. Livestock Science, 117, 270–274. doi.org/10.1016/j.livsci.2007.12.024
- Wang, X., Hao, Y., Shan, A., Jin, Y., Fang, H., Zhao, Y., … Zhang, J. (2018). Toxic effects of Zearalenone on intestinal microflora and intestinal mucosal immunity in mice. Food and Agricultural Immunology, 29, 1002–1011. doi: 10.1080/09540105.2018.1503233
- Windisch, W. M., Schedle, K., Plitzner, C., & Kroismayr, A. (2008). Use of phytogenic products as feed additives for swine and poultry. Journal of Animal Science, 86, E140–E148. doi: 10.2527/jas.2007-0459