ABSTRACT
It is long-cherished to develop rapid approaches for the identification and quantification of pesticide and its metabolites. Carbofuran (CBF) and its metabolite 3-hydroxy-carbofuran (3-OH-CBF) are highly toxic to humans and non-target organisms, which makes it mandatory for the detection of their residues in foods and the environment in many countries. Herein, a colloidal gold-strip assay based on a broad-specific monoclonal antibody (mAb) for simultaneous determination of CBF and 3-OH-CBF was developed. The colloidal gold-strips have a runtime within 5 min without complex sample pretreatment and perform a cut-off limit of detection (LOD) of 7–10 ng/mL for both carbofuran and 3-OH-CBF. The developed portable strip assay is a versatile and robust tool for the rapid and simultaneous detection of CBF and 3-OH-CBF in water samples as well as for the determination of illegal addition of CBF in other pesticide preparations.
Introduction
It has been estimated that over 3 million tons of pesticide-active ingredients were used worldwide in 2016, in which about 60% was in China (Caldas, Citation2019). On the other hand, some of their biodegraded products have the same even enhanced toxicity compared with those of their parent pesticides (Rahemi, Garrido, Borges, Brett, & Garrido, Citation2015). To ensure food and environmental safety, simultaneously rapid detection of pesticides and their toxic metabolites in the real samples is required (Chen et al., Citation2019; Hao, Suryoprabowo, Song, Liu, & Kuang, Citation2018). Carbofuran (CBF), a broad-spectrum carbamate pesticide, has been applied for pest control in a variety of crops worldwide for decades. CBF could kill pests by inhibiting the activity of acetylcholinesterase, which prevents the degradation of nervous transmitter acetylcholine and ultimately leads to protracted neural excitation. Meanwhile, when humans were exposed to CBFs, it can adversely cause poisoning signs, such as headache, blurred vision, and aching muscles (Ferslew, Hagardorn, & McCormick, Citation1992; Suzuki & Watanabe, Citation2005). CBF has been classified by EPA as the most toxic Category I. CBF could be degraded into 3-ketocarbofuran and 3-hydroxy-carbofuran (3-OH-CBF, ) (Abad, Moreno, & Montoya, Citation1999). As one of its biodegraded products, 3-OH-CBF is also a potent acetylcholinesterase inhibitor and has equal poisoning effects on humans. (Goncalves et al., Citation2017; Gupta, Citation1994; Ogada, Citation2014; Otieno, Lalah, Virani, Jondiko, & Schramm, Citation2010a). Although it has been banned to use CBF for pest control in vegetables, fruits, tea, Chinese herbal medicines and sugarcane productions in China, there are still plenty of CBF applications in agriculture around the world.
The inappropriate and illegal use of pesticides such as regulated pesticides hiddenly added to other pesticide preparations is still often happening, which makes an extremely increasing exposure risk to public. Therefore, quick and accurate detection of illegal addition is urgently needed for the quality control of pesticide preparations. CBF had been reported to be hiddenly added to other pesticide preparations intended to improve their efficacy in China (Qiao et al., Citation2015), which is prohibited by law and would result in more exposure risk of CBF. Based on the Standards for Drinking Water Quality of China (GB5749-2006), CBF is in the list and has a guideline value of 0.007 mg/L. In our previous study, CBF would be gradually degraded to 3-OH-CBF in agricultural products (Lan et al., Citation2019), which might prolong the duration of carbofuran residues in a degradation way and be harmful to public health. It is urgently required to establish a rapid and accurate method for detecting CBF and its metabolite 3-OH-CBF simultaneously.
Instrumental methods such as HPLC and GC are expensive, time-consuming and required tedious pretreatment of samples (Petropoulou, Tsarbopoulos, & Siskos, Citation2006; Zhang et al., Citation2016). Immunoassays, including enzyme-linked immunosorbent assay (ELISA) (Abad et al., Citation1999; Gui, Jin, Sun, Guo, & Zhu, Citation2009; Lan et al., Citation2019; Yao, Liu, Song, Kuang, & Xu, Citation2017) and immunochromatographic strip assay (Guo, Liu, Gui, & Zhu, Citation2009; Zhao et al., Citation2019; Zhou et al., Citation2004), are time-saving, sensitive, and high-throughput. Colloidal gold-based strip immunoassay provides a portable detection with visual observation in the field (He et al., Citation2019; Jawaid et al., Citation2015; Sun, Liu, Song, Cui, & Kuang, Citation2018; Wang et al., Citation2015; Wang et al., Citation2017), which requires relatively short development time that brings applications faster to the market. Strip-based immunoassays are specific, sensitive and sample pre-treatment simplified and could obtain the result within 5 min. With the advantage of detecting structural analogues, broad-specific antibodies could be used in the immunochromatography assay to improve the efficiency in practice (Cliquet, Goddeeris, Okerman, & Cox, Citation2007; Franek, Diblikova, Cernoch, Vass, & Hruska, Citation2006; Guo et al., Citation2009; Jiang, He, Gong, Gao, & Xu, Citation2019; Jin, Guo, Wang, Wu, & Zhu, Citation2009; Lan et al., Citation2019; Pagkali et al., Citation2018; Sun et al., Citation2018).
The purpose of the present study is to develop a quick detection method for CBF and 3-OH-CBF to facilitate the quality control of water and pesticide preparations. The broad-specific monoclonal antibody (mAb)-based colloidal gold-strip immunochromatographic assays were established for the detection of CBF and 3-OH-CBF in water samples, as well as for the detection of illegal addition of CBF in other pesticide preparations. The whole detection procedure is time-saving and without tedious sample pre-treatments. To our knowledge, this is the first report on the rapid detections of regulated pesticides hiddenly added to other pesticide preparations by using immunochromatographic assays.
Materials and methods
Reagents
CBF, 3-OH-CBF, bovine serum albumin (BSA), ovalbumin (OVA), colloidal gold nanoparticles (30 nm), and QuEChERS-dispersive SPE tubes were purchased from Sigma-Aldrich (St. Louis, MO, USA). Goat anti-mouse IgG was purchased from Jackson Immunoresearch Laboratories (West Grove, PA, USA). All strip materials including absorbance pad, sample pad, and nitrocellulose membrane were obtained from Schleicher & Schuell (Dassel, Germany). The monoclonal antibody against both CBF and 3-OH-CBF and CBF-OVA were produced by our laboratory as previously described (Lan et al., Citation2019).
Preparation of colloidal gold-mAb conjugate
The pH of the colloidal gold suspension was adjusted to 8.2 with 0.2 M K2CO3, and the mAb solution was dropwise added with stirring to make a final antibody concentration at 2 µg ml−1. The resulting solution was incubated for 30 min followed by the addition of 1% (w/v) BSA and stirred for another 20 min to stabilize the colloidal gold. The mixture was centrifuged at 12,000 g at 4 °C for 15 min, and the precipitate was washed 3 times with PBS (1.5 mM KH2PO4, 8.3 mM Na2HPO4 12H2O and 154 mM NaCl, pH 7.4) containing 1% BSA. The resulting pellet was suspended in PBS containing 1% BSA and 0.05% sodium azide and stored at 4 °C.
Preparation of colloidal gold-strips
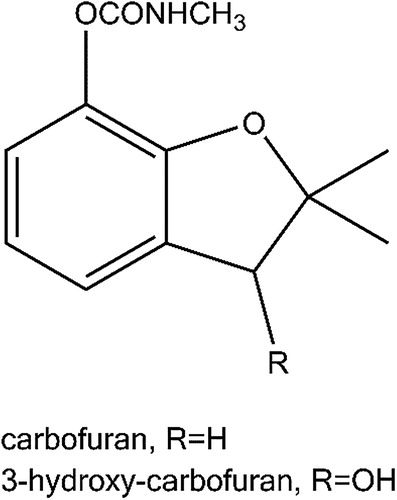
The strips are composed of a sample pad, conjugate pad, nitrocellulose membrane and absorbent pad with flow direction (). The CBF-OVA and goat anti-mouse IgG were dispensed onto the nitrocellulose membrane by BioDot Quanti3000 dispenser as the test line and control line, respectively. The prepared colloidal gold-mAb conjugate was immobilized onto the conjugate pad using AirJet Quanti3000 dispenser. The colloidal gold-strips were then assembled and stored at 4 °C.
Sample fortification and recovery
Mineral water and some pesticide samples were purchased from the local market, the other pesticide samples were provided by Hainan Plant Protection Station, and the tap water was obtained from the laboratory. A series of concentrations of CBF were fortified to water samples with a final concentration of 3.5, 7, 14 and 28 ng/mL, respectively. Determinations of pesticide preparations were performed by strip test with a 1000-fold sample dilution in PBSTG (0.1% (v/v) Tween-20 and 0.1% (w/v) gelatin in PBS), ddH2O, or tap water.
Validation by UPLC-MS/MS
Quantitative analysis of CBF in pesticide samples was performed by following the previous report with some minor modifications (Lan et al., Citation2019). Briefly, a 1-ml suspension was added to 10 ml acetonitrile and then extracted with saturated sodium chloride solution. The organic phase was then applied to the QuEChERS-dispersive SPE tube and centrifuged at 12,000 rpm for 5 min. The resultant solution was then transferred to UPLC-MS/MS for quantitative analysis (Zhou, Y., Guan, Gao, Lv, & Ge, Citation2018). The UPLC-MS/MS system with Nexera X2 (Shimadzu, Japan) and SCIEX QTRAP 5500 (Applied Biosystems, USA) equipped with a positive mode electrospray ionization source was applied. A C18 column (Shim-pack XR-ODS, 100 mm × 2.0 mm, 2.2 μm, Japan) was used for the chromatographic separation. The acquired data were then analysed using Analyst software (AB SCIEX, Foster, CA, USA).
Results and discussion
Development of colloidal gold-strips
The monoclonal antibody (mAb) used in this study was produced by immunizing mice with 3-OH-CBF-KLH conjugate, which has strong affinities with both CBF and 3-OH-CBF (Lan et al., Citation2019). The mAb had been demonstrated no cross-reactivities with other compounds, such as carbofuran-phenol, carbosulfan, and carbaryl as well as pesticides with potentially positive addition of CBF, including omethoate, isocarbophos, and acephate.
Concentrations of gold NPs-mAb conjugate, hapten-OVA and goat anti-mouse IgG had influences on line intensity and visible determination and were optimized as 10 µg/mL, 0.5 and 1.5 mg/mL, respectively. The schematic diagram of colloidal gold-strips based on broad-specific mAb was shown in . The goat anti-mouse IgG and hapten-OVA were coated onto the control line and test line, respectively. Colloidal gold-mAb was immobilized on the conjugate pad. Following the application of samples containing CBF or 3-OH-CBF, the mAb labelled with colloidal gold would bind with CBF or 3-OH-CBF, the intensity of the test line was weaker than control. For negative samples, both the control line and test line will emerge with the same intensity. For samples that gave no observation on the control line should be judged as invalid samples.
Sensitivity of colloidal gold-strips
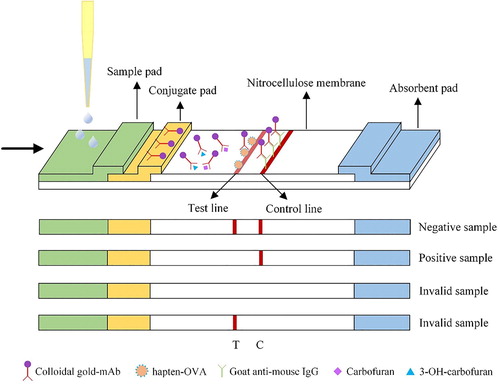
The cut-off limit of detection (LOD) for colloidal gold-strip was defined as the minimum concentration that made the test line completely invisible. A series of concentrations of standard solutions diluted in PBSTG were deployed into the strips, including 0–20 ng/mL CBF or 3-OH-CBF (). The cut-off LOD was 7–10 ng/mL for both CBF and 3-OH-CBF. While the visible LOD was defined as the lowest CBF or 3-OH-CBF concentration that gave a significantly weaker test line than the control line, and it was determined as 1 ng/mL for both CBF and 3-OH-CBF.
Figure 3. Colloidal gold-strip-based immunoassay of CBF and 3-OH-CBF. Standard solutions of CBF and 3-OH-CBF at 0, 0.25, 0.5, 1, 2.5, 5, 10, and 20 ng/mL were prepared respectively: + means positive and − means negative samples.
Yao et al., Guo et al., and Zhou et al. had reported immunochromatographic strip assays with cut-off LODs of 1, 32 and 250 ng/mL for carbofuran, respectively (Guo et al., Citation2009; Yao et al., Citation2017; Zhou et al., Citation2004). One advantage of our colloidal gold-strips is that they are capable of simultaneously detecting CBF and 3-OH-CBF, which provides practical opportunity to detect CBF and 3-OH-CBF in one step (Cliquet et al., Citation2007; Jiang et al., Citation2019; Kato et al., Citation2007; Mukunzi, Suryoprabowo, Song, Liu, & Kuang, Citation2018).
Samples determined by colloidal gold-strips
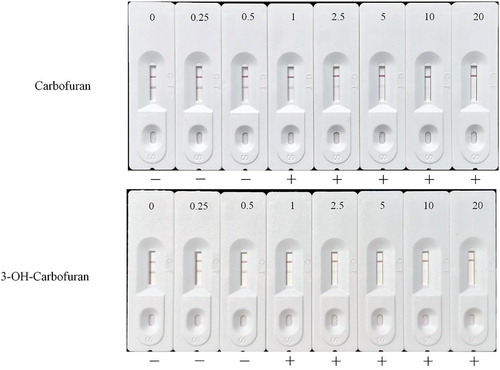
The solubility of CBF in water is 700 mg/L, which is extraordinarily higher than the guideline value of 0.007 mg/L in Standards for Drinking Water Quality of China. Therefore, CBF could easily get into drinking water from agricultural surface runoff, causing contamination of drinking water from surface or groundwater source (Otieno, Lalah, Virani, Jondiko, & Schramm, Citation2010b). CBF residues in water samples were determined by strip tests ((A)). Three types of water sources including tap water, Milli-Q purified water, and mineral water were fortified with 0–28 ng/mL CBF, and then deployed into the strip tests. As shown in (A), regardless of water sources, the colloidal gold-strip in this study could detect CBF residues in drinking water without any redundant operations, and the inspection process would last within 5 min.
Figure 4. (A) Colloidal gold-strip-based immunoassay of different source waters with spiked CBF. (B) Influences of tap water, ddH2O, and PBSTG on the colloidal gold-strip based immunoassays for the CBF detection spiked in five different pesticide preparations. N1 and N2 mean pesticide preparations with negative CBF addition, while P1 to P3 are positive samples.
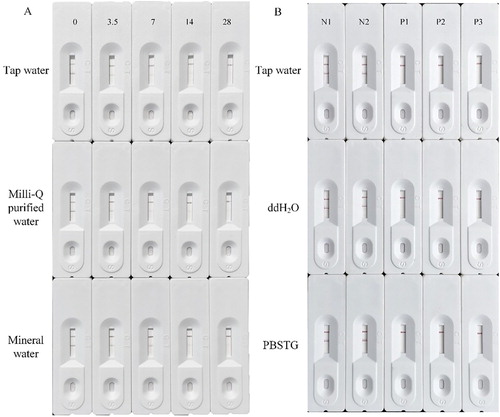
The hidden addition of regulated pesticides into other commercial pesticide preparations sometimes occurred, which requires accurate and fast detection methods for quality control of pesticides. Compared with GC, HPLC, and Fourier transform near-infrared spectroscopies (Lv, Du, Ma, Shen, & Zhou, Citation2018; Ogawa et al., Citation2006; Soler et al., Citation2007; Yang et al., Citation2016), strip test is more portable with megascopic examination. Colloidal gold-based strip tests have been widely applied for the determination of water, fruits, and vegetables (Guo et al., Citation2018; Luo, Chen, Xiao, Zhao, & Wang, Citation2019; Yu, Liu, Song, Kuang, & Xu, Citation2016); however, there were few reports on the specific and rapid detection of illegal addition of one regulated pesticide to another pesticide preparation by using lateral-flow immunochromatographic assay. In the present study, different pesticide preparations were detected by colloidal gold-strips (, ). Among them, nine negative samples were detected as positive followed by spiked with CBF ((A,B)), three samples turned out to be positive and were further confirmed by using UPLC-MS/MS at concentrations of 2.27%, 2.44%, and 3.12%, respectively ((C), ). The process included 1000-fold dilution with PBSTG and loading samples, and the duration was within 5 min. In our study, we have demonstrated that a 1000-fold dilution with PBSTG can make no matrix effect for the strip test performance. From our investigations, the hiddenly added CBF concentration was often over 2% (w/v), and it will still have over 20 mg/L CBF in the pesticide preparations after 1000-fold dilution, which indicates that our developed colloidal gold-strips with 7–10 cut-off LOD are well-qualified for the field applications. Due to variant experimental conditions in the fields, PBSTG sometimes might not be available, so samples diluted with tap water, ddH2O, and PBSTG were tested. As shown in (B), dilution with tap water had no matrix effect on the detection of CBF, which indicated that the colloidal gold-strip-based immunoassay could be employed in the fields with a simple dilution for the rapid detection of CBF hiddenly added to other pesticide preparations.
Figure 5. Typical colloidal gold-strip based immunoassay of CBF illegal addition in different pesticide preparations: (A) Negative samples (N1 to N9); (B) negative samples with addition of CBF (N1P to N9P); and (C) three positive samples (P1 to P3).

Table 1. Different pesticide preparations determined by strip test and UPLC-MS/MS.
Conclusion
In the present work, a highly sensitive and broad-specific mAb against CBF and 3-OH-CBF was used to develop the lateral-flow immunochromatographic assay. The established colloidal gold-strip-based immunoassay can be used for the prompt detection of illegal addition of CBF in commercial pesticide preparations, as well as for the simultaneous determination of CBF and 3-OH-CBF residues in water samples. With the characterization that can be directly diluted by tap water, the developed colloidal gold-strips would be convenient to use when no specific buffers and purified water are available in the field investigations. Moreover, it indicated from our results that monoclonal antibody-based strip tests would be a rapid, specific and effective tool to monitor the quality of pesticide preparations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abad, A., Moreno, M. J., & Montoya, A. (1999). Development of monoclonal antibody-based immunoassays to the N-methylcarbamate pesticide carbofuran. Journal of Agricultural and Food Chemistry, 47(6), 2475–2485.
- Caldas, E. D. (2019). Sustainable Agrochemistry (pp. 275–305). Berlin, Heidelberg: Springer.
- Chen, X., Peng, S., Liu, C., Zou, X., Ke, Y., & Jiang, W. (2019). Development of an indirect competitive enzyme-linked immunosorbent assay for detecting flunixin and 5-hydroxyflunixin residues in bovine muscle and milk. Food and Agricultural Immunology, 30(1), 320–332.
- Cliquet, P., Goddeeris, B., Okerman, L., & Cox, E. (2007). Production of penicillin-specific polyclonal antibodies for a group-specific screening ELISA. Food and Agricultural Immunology, 18(3–4), 237–252.
- Ferslew, K. E., Hagardorn, A. N., & McCormick, W. (1992). Poisoning from oral ingestion of carbofuran (Furadan 4F), a cholinesterase-inhibiting carbamate insecticide, and its effects on cholinesterase activity in various biological fluids. Journal of Forensic Sciences, 37(1), 337–344.
- Franek, M., Diblikova, I., Cernoch, I., Vass, M., & Hruska, K. (2006). Broad-specificity immunoassays for sulfonamide detection: Immunochemical strategy for generic antibodies and competitors. Analytical Chemistry, 78(5), 1559–1567.
- Goncalves, V. J., Hazarbassanov, N. Q., de Siqueira, A., Florio, J. C., Ciscato, C. H. P., Maiorka, P. C., … de Souza Spinosa, H. (2017). Development and validation of carbofuran and 3-hydroxycarbofuran analysis by high-pressure liquid chromatography with diode array detector (HPLC-DAD) for forensic veterinary medicine. Journal of Chromatography B, 1065–1066, 8–13.
- Gui, W., Jin, M., Sun, L., Guo, Y., & Zhu, G. (2009). Residues determination of carbofuran in vegetables based on sensitive time-resolved fluorescence immunoassay. Food and Agricultural Immunology, 20(1), 49–56.
- Guo, Y. R., Liu, S. Y., Gui, W. J., & Zhu, G. N. (2009). Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Analytical Biochemistry, 389(1), 32–39.
- Guo, M., Sun, L., Liu, L., Song, S., Kuang, H., & Cui, G. (2018). Ultrasensitive immunochromatographic strip for detection of cyproheptadine. Food and Agricultural Immunology, 29(1), 941–952.
- Gupta, R. C. (1994). Carbofuran toxicity. Journal of Toxicology and Environmental Health, 43(4), 383–418.
- Hao, K., Suryoprabowo, S., Song, S., Liu, L., & Kuang, H. (2018). Rapid detection of zearalenone and its metabolite in corn flour with the immunochromatographic test strip. Food and Agricultural Immunology, 29(1), 498–510.
- He, F., Zou, T., Yang, J., Wang, H., Deng, L., Tian, Y., … Shen, Y. (2019). Development of a skeleton-specific antibody and Au nanoparticle-based immunochromatographic sensor for simultaneous detection of various tadalafil adulterants in health food. Food and Agricultural Immunology, 30(1), 349–368.
- Jawaid, W., Meneely, J. P., Campbell, K., Melville, K., Holmes, S. J., Rice, J., & Elliott, C. T. (2015). Development and validation of a lateral flow immunoassay for the rapid screening of okadaic acid and all dinophysis toxins from shellfish extracts. Journal of Agricultural and Food Chemistry, 63(38), 8574–8583.
- Jiang, M., He, J., Gong, J., Gao, H., & Xu, Z. (2019). Development of a quantum dot-labelled biomimetic fluorescence immunoassay for the simultaneous determination of three organophosphorus pesticide residues in agricultural products. Food and Agricultural Immunology, 30(1), 248–261.
- Jin, R. Y., Guo, Y. R., Wang, C. M., Wu, J. X., & Zhu, G. N. (2009). Development of a bispecific monoclonal antibody to pesticide carbofuran and triazophos using hybrid hybridomas. Journal of Food Science, 74(1), T1–T6.
- Kato, M., Ihara, Y., Nakata, E., Miyazawa, M., Sasaki, M., Kodaira, T., & Nakazawa, H. (2007). Development of enrofloxacin ELISA using a monoclonal antibody tolerating an organic solvent with broad cross-reactivity to other newquinolones. Food and Agricultural Immunology, 18(3–4), 179–187.
- Lan, J., Wang, M., Ding, S., Fan, Y., Diao, X., Li, Q. X., & Zhao, H. (2019). Simultaneous detection of carbofuran and 3-hydroxy-carbofuran in vegetables and fruits by broad-specific monoclonal antibody-based ELISA. Food and Agricultural Immunology, 30(1), 1085–1096.
- Luo, P., Chen, X., Xiao, J., Zhao, Y., & Wang, Z. (2019). Rapid detection of iprodione in cucumber and apple using an immunochromatographic strip test. Food and Agricultural Immunology, 30(1), 701–712.
- Lv, G., Du, C., Ma, F., Shen, Y., & Zhou, J. (2018). Rapid and nondestructive detection of pesticide residues by depth-profiling fourier transform infrared photoacoustic spectroscopy. ACS Omega, 3(3), 3548–3553.
- Mukunzi, D., Suryoprabowo, S., Song, S., Liu, L., & Kuang, H. (2018). Development of an indirect enzyme-linked immunosorbent assay and lateral-flow test strips for pefloxacin and its analogues in chicken muscle samples. Food and Agricultural Immunology, 29(1), 484–497.
- Ogada, D. L. (2014). The power of poison: Pesticide poisoning of Africa’s wildlife. Annals of the New York Academy of Sciences, 1322(1), 1–20.
- Ogawa, S., Brito, N. M., Silva, M. R. S., Ribeiro, M. L., Leite, L. A., Dórea, H. S., … Ferreira, J. M. S. (2006). Determination of carbofuran and 3-hydroxycarbofuran residues in coconut water by solid-phase extraction and liquid chromatography with UV detection. Journal of Liquid Chromatography & Related Technologies, 29(12), 1833–1841.
- Otieno, P. O., Lalah, J. O., Virani, M., Jondiko, I. O., & Schramm, K. W. (2010a). Carbofuran and its toxic metabolites provide forensic evidence for furadan exposure in vultures (Gyps africanus) in Kenya. Bulletin of Environmental Contamination and Toxicology, 84(5), 536–544.
- Otieno, P. O., Lalah, J. O., Virani, M., Jondiko, I. O., & Schramm, K.-W. (2010b). Soil and water contamination with carbofuran residues in agricultural farmlands in Kenya following the application of the technical formulation Furadan. Journal of Environmental Science and Health, Part B, 45(2), 137–144.
- Pagkali, V., Petrou, P. S., Makarona, E., Peters, J., Haasnoot, W., Jobst, G., … Kakabakos, S. E. (2018). Simultaneous determination of aflatoxin B1, fumonisin B1 and deoxynivalenol in beer samples with a label-free monolithically integrated optoelectronic biosensor. Journal of Hazardous Materials, 359, 445–453.
- Petropoulou, S.-S. E., Tsarbopoulos, A., & Siskos, P. A. (2006). Determination of carbofuran, carbaryl and their main metabolites in plasma samples of agricultural populations using gas chromatography–tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 385(8), 1444–1456.
- Qiao, C., Huang, Y., Luo, J., Wang, C., Fang, J., & Xie, H. (2015). Determination of 30 hidden ingredients in pesticide products by HPLC-MS/MS. Agrochemicals, 54(5), 340–342.
- Rahemi, V., Garrido, J., Borges, F., Brett, C., & Garrido, E. (2015). Electrochemical sensor for simultaneous determination of herbicide MCPA and its metabolite 4-chloro-2-methylphenol. Application to photodegradation environmental monitoring. Environmental Science and Pollution Research, 22(6), 4491–4499.
- Soler, C., Hamilton, B., Furey, A., James, K. J., Mañes, J., & Picó, Y. (2007). Liquid chromatography quadrupole time-of-flight mass spectrometry analysis of carbosulfan, carbofuran, 3-hydroxycarbofuran, and other metabolites in food. Analytical Chemistry, 79(4), 1492–1501.
- Sun, J., Liu, L., Song, S., Cui, G., & Kuang, H. (2018). Development of an immunochromatographic strip assay for three major capsaicinoids based on an ultrasensitive monoclonal antibody. Food and Agricultural Immunology, 29(1), 930–940.
- Suzuki, O., & Watanabe, K. (2005). Drugs and poisons in humans. Berlin, Heidelberg: Springer.
- Wang, Y., Deng, R., Zhang, G., Li, Q., Yang, J., Sun, Y., … Hu, X. (2015). Rapid and sensitive detection of the food allergen glycinin in powdered milk using a lateral flow colloidal gold immunoassay strip test. Journal of Agricultural and Food Chemistry, 63(8), 2172–2178.
- Wang, R., Zeng, L., Yang, H., Zhong, Y., Wang, J., Ling, S., … Wang, S. (2017). Detection of okadaic acid (OA) using ELISA and colloidal gold immunoassay based on monoclonal antibody. Journal of Hazardous Materials, 339, 154–160.
- Yang, T., Zhou, R., Jiang, D., Fu, H., Su, R., Liu, Y., & Su, H. (2016). Rapid detection of pesticide residues in Chinese herbal medicines by Fourier transform infrared spectroscopy coupled with partial least squares regression. Journal of Spectroscopy, 2016, 1–9.
- Yao, L., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of indirect competitive enzyme-linked immunosorbent and immunochromatographic strip assays for carbofuran detection in fruits and vegetables. Food and Agricultural Immunology, 28(4), 639–651.
- Yu, L., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of an immunochromatographic test strip and ic-ELISA for tetrabromobisphenol: A detection in lake water and rice pudding samples. Food and Agricultural Immunology, 27(4), 460–470.
- Zhang, C. P., He, H. M., Yu, J. Z., Hu, X. Q., Zhu, Y. H., & Wang, Q. (2016). Residues of carbosulfan and its metabolites carbofuran and 3-hydroxy carbofuran in rice field ecosystem in China. Journal of Environmental Science and Health, Part B, 51(6), 351–357.
- Zhao, Y., Tan, G., Wang, M., Lin, H., He, L., Li, L., & Wang, B. (2019). Application of immunoassays for rapid monitor of carbofuran residue in vegetables. Journal of Food Science, 84(11).
- Zhou, Y., Guan, J., Gao, W., Lv, S., & Ge, M. (2018). Quantification and confirmation of fifteen carbamate pesticide residues by multiple reaction monitoring and enhanced product ion scan modes via LC-MS/MS QTRAP system. Molecules, 23(10), 2496.
- Zhou, P., Lu, Y., Zhu, J., Hong, J., Li, B., Zhou, J., … Montoya, A. (2004). Nanocolloidal gold-based immunoassay for the detection of the N-methylcarbamate pesticide carbofuran. Journal of Agricultural and Food Chemistry, 52(14), 4355–4359.