ABSTRACT
A monoclonal antibody against carazolol (CRZ), 2F5, was generated for the construction of an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) and an immunochromatographic strip for detection of CRZ in milk and pig kidney samples. The antibody had a half-maximal inhibitory concentration of 0.29 ng/mL; its limit of detection was 0.068 ng/mL, and its linear range of detection was 0.137–0.638 ng/mL. For CRZ in milk samples and porcine kidney samples, the detection limits for this strip were 0.25 and 2.5 ng/mL, respectively. Analysis of CRZ in milk samples showed that the results obtained by immunochromatographic strip were consistent with those obtained by ic-ELISA. Therefore, the CG immunoassay is a sensitive screening method for semi-quantitative and qualitative detection of residual CRZ in milk and porcine kidney samples.
1. Introduction
Carazolol (CRZ) is a beta-adrenoceptor blocking agent (Wang, Wu, Cheng, & Cai, Citation2011) that is used in pigs to prevent stress and death during transport to the slaughterhouse (Nocentini et al., Citation2018; Qiu, Citation2006) . Stress during transport can cause premature death in pigs or can degrade meat quality (Mitrowska, Posyniak, & Zmudzki, Citation2009). With the widespread use of sedatives and CRZ, the possibility of accumulation of their residues in animal-derived foods increases, thus, potentially leading to toxic effects, such as embryo toxicity and genotoxicity to consumers (Jiangeng et al., Citation2011). However, CRZ overuse can cause residues to accumulate in animal products, such as milk and pork, and can be harmful to consumer health (Dubois, Citation2004; Tan, Yang, Yang, & Li, Citation2017). The European Union established the recommended maximum residue limit for CRZ at 5 µg/kg in swine muscle and 25 µg/kg in liver and kidney (He, Wang, Zhang, Liu, & Fang, Citation2012). Therefore, it is necessary to be able to quickly detect CRZ in food.
Recently, various analytical methods have become available for detecting CRZ, such as high performance liquid chromatography (Fan, Hong, Zhao, Chen, & Wu, Citation2013; Zhou et al., Citation2015), gas chromatography-mass spectrometry (Qiu, Citation2006), liquid chromatography–mass spectrometry (LC-MS) (Bock & Stachel, Citation2013; Hui, Zhang, Wang, Zhang, & Wang, Citation2014; Wang et al., Citation2013; Zhang, Shao, Yin, Wu, & Duan, Citation2009), ultra-performance liquid chromatography (Cheng, Shen, Zhang, Zhang, & Zhang, Citation2015), radioimmunoassay methods (Zheng, Zhang, & Feng, Citation2009), and electrokinetic chromatography (Aranas, Guidote, Haddad, & Quirino, Citation2011). However, these methods with high sensitivity, high precision, and an ability to simultaneously detect multiple samples require skilled operators and are expensive. Enzyme-linked immunosorbent assay (ELISA) is a sensitive high-throughput method for sample screening and quantification (Cooper, Delahaut, Fodey, & Elliott, Citation2004). However, its antibody sensitivity is insufficient, and the detection ability is only 5 μg/kg, which cannot meet the detection requirements. Therefore, the content of this paper is very necessary. Lateral flow immunochromatography (ICA) strips, another fast and easy diagnostic tool, can be used for rapid on-site screening with qualitative or semi-quantitative testing, and do not require instrumentation (Isanga et al., Citation2016; Xie, Kong, Liu, Song, & Hua, Citation2017). Compared with ELISA, strip testing takes less time and is easier to operate, thus making it suitable for on-site measurements and high-throughput processing of samples. Therefore, in this study, a high-affinity anti-CRZ monoclonal antibody was developed on the basis of a CRZ-specific antigen, and an immunochromatographic strip based on the mAb was developed to analyse CRZ in milk and porcine kidney samples.
2. Materials and methods
2.1. Chemicals
CRZ, carvedilol, atenolol, nadolol, propranolol, carteolol, lcvobunolol, acepromazine, chlorpromazine, propionylpromazine, ovalbumin (OVA), bovine serum albumin (BSA), N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimeth-ylaminopropyl) carbodiimide hydrochloride (EDC), and keyhole limpet hemocyanin (KLH) were obtained from Sigma (St. Louis, MO, USA). Goat anti-mouse immunoglobulin (IgG) antibody was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Other reagents and chemicals were supplied by the National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China).
Nitrocellulose high-flow membrane (PuraBind™ RP) was obtained from Whatman-Xinhua Filter Paper Co. (Hangzhou, China). The sample pad (CB-SB08), polyvinylchloride backing card, and absorption pad (SX18) were supplied by Goldbio Tech Co. (Shanghai, China).
All buffer solutions were prepared with ultrapure water (Milli-Q Water Purification System, Millipore Co., Bedford, MA, USA). The hand-held strip scan reader was from Huaan Magnech Bio-Tech Co., Ltd (Beijing, China).
2.2. Derivatization of CRZ
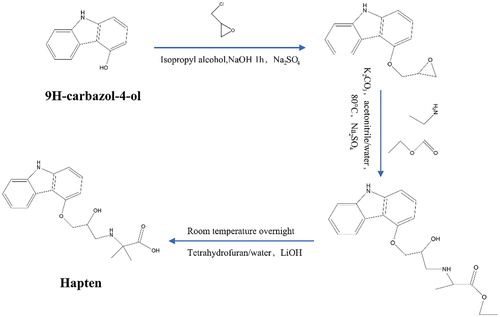
CRZ has only one reactive group hydroxyl group, which was derivatized into a compound with a carboxyl group. The structure of the hapten synthesized in this study is shown in .
A solution of 9H-carbazol-4-ol (2.00 g, 10.9 mmol) in isopropyl alcohol (20.0 mL) was added dropwise an aqueous solution of NaOH (476 mg, 11.9 mmol) dissolved in 10 mL water, and the mixture was stirred at room temperature for 1 h. Then, epichlorohydrin (2.32 g, 25.1 mmol) was added all at once, and the solution was stirred at room temperature overnight. The resulting mixture was diluted with water and extracted with ethyl acetate (EA). The combined organic layer was dried over Na2SO4. After filtration, the filtrate was concentrated under reduced pressure, and the residue was purified with a silica column to yield 4-(oxiran-2-ylmethoxy)-9H-carbazole (1.70 g) as a white solid (yield: 65.4%).
A solution of 4-(oxiran-2-ylmethoxy)-9H-carbazole (1.70 mg, 7.11 mmol) and K2CO3 (491 mg, 3.56 mmol) in a mixture of solvent acetonitrile (20.0 mL) and water (5.00 mL) was added to ethyl 2-amino-2-methylpropanoate (1.19 g, 7.11 mmol), and the mixture was stirred at 80°C overnight. The resulting mixture was cooled to room temperature and diluted with water and extracted with EA. The combined organic layer was dried over Na2SO4. After filtration, the filtrate was concentrated, and the residue was purified with a silica column to yield ethyl 2-((3-((9H-carbazol-4-yl)oxy)-2-hydroxypropyl)amino)-2-methylpropanoate (1.00 g) as a yellow oil (yield: 38.5%).
A solution of ethyl 2-((3-((9H-carbazol-4-yl)oxy)-2-hydroxypropyl)amino)-2-methylpropanoate (1.00 g, 2.69 mmol) in a mixture of the solvent Tetrahydrofuran (THF) (10.0 mL) and water (2.00 mL) was added to LiOH (170 mg, 4.04 mmol). The reaction was stirred at room temperature overnight. Water was added, and the aqueous layers were extracted with EA. The aqueous phase was acidified with 6 N aqueous HCl until pH 7 was reached and was then concentrated to dried. The crude product was purified with a silica column to yield ethyl 2-((3-((9H-carbazol-4-yl)oxy)-2-hydroxypropyl)amino)-2-methylpropanoate(CRZ-S1) (200 mg) as a white solid (yield: 21.6%).
2.3. Antigen preparation
Antigen 1 (A1): CRZ-S1 was conjugated with the carrier protein (KLH or BSA or OVA) with the NHS ester method, as previously reported (Lei, Xu, Song, Liu, & Hua, Citation2017), with some modifications. CRZ-S1 (15 mg) was dissolved in 0.45 mL of dimethylformamide, 6.8 mg of NHS was added to the mixture, and 9.3 mg of EDC was added after 10 min. The reaction solution was stirred for 8 h and added dropwise to 8.1 mg of BSA (MW: 67,000 Da) in 3 mL of carbonate buffer solution (CBS). The solution was allowed to react overnight in the dark at room temperature with continuous stirring. The CRZ-S1-BSA conjugate was dialyzed against phosphate buffer saline (PBS) for 2 days with three changes of dialysis solution per day, and was stored at −20°C until use (Xing, Hao, Liu, Xu, & Hua, Citation2014).
Antigen 2(A2): CRZ was conjugated to CDI to OVA (CRZ-OVA) and used as a heterologous coating antigen (Sun, Liu, Song, Kuang, & Xu, Citation2016). Then, 8 mg of CDI was dissolved in 0.4 mL of anhydrous dimethylformamide, and 5.7 mg of CRZ was added and stirred at room temperature for 3 h. The reaction solution was then added to 5 mg of OVA (molecular weight 45,000 Da) in 1.5 ml of CBS and stirred at room temperature for another 24h. Finally, the solution was dialyzed against PBS for 2 days and stored at −20°C (Wang, Xie, et al., Citation2017).
2.4. Production of monoclonal antibodies
Fifteen female BALB/c mice (8–10 weeks old) were injected with different reaction specific antigens (CRZ-S1-BSA) and tested by ic-ELISA with different coating antigens (Li, Zhi, Jia, Hui, & Ai, Citation2017). Mice with the highest serum antibody titre and a half-maximal inhibitory concentration (IC50) under optimal conditions were selected for cell fusion, as described previously (Chen et al., Citation2015). After the third immunization, the mice were tail-bled 1 week after each boost and examined by ic-ELISA. BALB/c mice that tested positive and had the highest inhibition were selected for injection of 25 μg of CRZ-S1-BSA as the final immunization. After 21 days, the mouse cells were fused, and three rounds of subcloning were performed with the limiting dilution method to isolate the target cell strain that best inhibited the production of CRZ. The strain was expanded and intraperitoneally injected into BALB/c mice 8–10 weeks of age (Yan, Liu, Xu, Hua, & Xu, Citation2015). Ascites was purified by precipitation of caprylic acid and ammonium sulfate and dialyzed against PBS at 4°C for 3 days.
The IC50 value and limit of detection (LOD) of the mAb were determined by a standard curve to reflect its sensitivity, and the cross-reactivity (CR) of the mAb was determined to assess its specificity.
The CR values were calculated as follows: CR (%) = (IC50 of CRZ/IC50 of competitor) × 100% (Mercader et al., Citation2014).
All animal studies in this work were performed according to institutional ethical guidelines and were approved by the Committee on Animal Welfare of Jiangnan University.
2.5. Preparation of CG particles
Preparation of colloidal gold nanoparticles was performed according to previous reports with some modifications (Xu et al., Citation2010). Briefly, 80 mL of chloroauric acid solution (HAuCl4, 0.01%, w/v) was vigorously stirred in an Erlenmeyer flask to boiling, and this was followed by the addition of 3 ml of a freshly prepared 1% trisodium citrate solution under continuous stirring. The colour of the solution changed from colourless to black in half a minute and then became burgundy. The solution was boiled for 15 min, then cooled to room temperature and stored at 4°C for further use (Wang, Zheng, et al., Citation2017).
2.6. Labelling the McAb with CG
The anti-CRZ McAb-labelled CG particles were prepared according to previously reported methods (Le, Yi, Zhao, & Wei, Citation2011; Yun-Jie, Liu, & Yu-Xiang, Citation2010). Briefly, 8 μg of purified mAb 2F5 was diluted with PBS solution, and the pH of the CG solution was adjusted to 8.8 with 0.1 M K2CO3. The mixture was stirred at room temperature for 50 min, and after blocking of free CG with 1 mL of 10% aqueous BSA solution, the solution was further incubated at room temperature for 2 h. After centrifugation at 8000 rpm for 30 min at 4°C, the supernatant was removed and the soft precipitate was collected and washed with gold-labeled resuspension buffer (20 mM Tris, 0.1% PEG, and 0.1% Tween, 5% sucrose, 5% trehalose, 0.2% BSA, and 5% Brij) three times.
2.7. Preparation of immunochromatographic strips
The immunochromatographic test strip consisted of a sample pad, a nitrocellulose membrane, an absorbent pad, and a polyvinyl chloride backing card. As shown in Scheme 1, the coating antigen (1 mg/mL CRZ-S1-BSA) and goat anti-mouse IgG (1 mg/mL) were fixed on the nitrocellulose membrane with a dispenser for the test and control lines (T/C), respectively. The sample pad was treated with 0.01 M PBS solution (1% BSA, 1% sucrose, and 0.2% Tween 20) and dried at room temperature for 3 h.
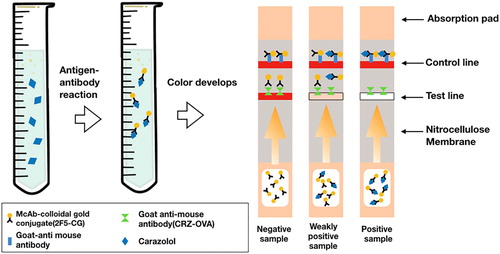
2.8. Principle of the lateral-flow ICA strip
Fifty microliters of anti-CRZ McAb-labelled CG and 150 μL of standard CRZ solution or sample extract were mixed in microwells and incubated for 5 minat room temperature. The mixture was dropped onto the sample pad and migrated toward the absorbent pad, and the results were visually observed after 5 min. When CRZ is present in the sample, the coating antigen immobilized on the test line competes with a limited amount of McAb-labelled CG. Conversely, if CRZ is absent, the McAb-labelled CG is captured by the antigen immobilized on the T-line and eventually forms a red line. Therefore, the colour of the T line is negatively correlated with the amount of CRZ in the sample. As the amount of CRZ in the sample increases, the colour intensity of the T line decreases. Eventually, when the amount of CRZ reaches a certain concentration, all McAb-labelled CG will be reacted, and the T line will disappear.
2.9. Sample analysis
Milk was purchased from a local market and confirmed to be free of CRZ by LC-MS. Samples did not need to be processed before testing, and a range of concentrations of CRZ was added. Each spiked and unspiked sample was analyzed three times in this experiment.
The extraction from kidney was performed as reported by Dubois M. with some modifications (Dubois, Citation2004). The homogenized tissue (porcine kidney) 2.5 g ± 0. 02 g was accurately weighed in a 50 mL centrifuge tube, 1 g of anhydrous sodium sulfate and 10 mL of acetonitrile were added, and the samples were vortexed and shaken at medium speed for 10 min, and centrifuged at 6000 rpm for 5 min. The supernatant was placed into another 50 mL centrifuge tube, 10 mL of n-hexane was added, and the samples were vortexed at medium speed for 10 min, then centrifuged at 6000 r/min for 4 min. The upper layer of n-hexane was discarded, and the residue was re-extracted once with 10 mL of acetonitrile. The extracts were combined and centrifuged at 6000 rpm for 8 min at −10°C. Then, 2 mL of the extract was nearly desiccated at 50°C, and the residue was dissolved in PBS solution.
3. Results and discussion
3.1. Characterization of the hapten and antigen
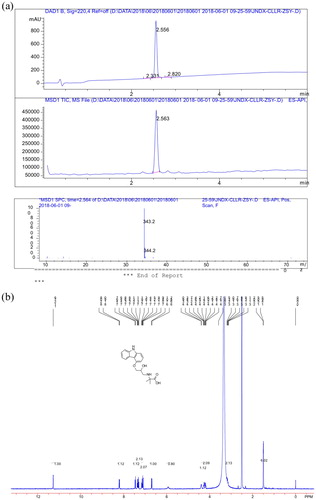
The CRZ derivative was synthesized through a series of reactions such as epichlorohydrin and ethyl 2-amino-2-methylpropionate. As shown in (a), the derivative was analyzed by LC-MS and found to have a main product retention time of 2.6 min; the main UV peak also appeared at 2.6 min, and the yield of CRZ-S1 was found to be high. In addition, MS chromatography detected a fragment with a molecular weight of 343.2 Da, which was consistent with CRZ-S1, thus, indicating that the derivative of CRZ was successfully prepared. As shown in (b), the structure of hapten was confirmed by 1H NMR spectra: 1H NMR (400 MHz, DMSO_d6): δ 11.27 (s, 1 H), 8.19–8.20 (d, 1 H), 7.44–7.46 (d, 1 H), 7.29–7.36 (m, 2 H), 7.08–7.16 (m, 2 H), 6.68–6.70 (d, 1 H), 4.38–4.39 (m, 1 H), 4.16–4.27 (m, 2 H), 3.11–3.19 (m, 2H), 1.50 (s, 6H).
Figure 2. (a) The detection results of derivative reaction of carazolol by LC-MS. (b) 1H-NMR spectra of hapten measured in DMSO at 400MHz.
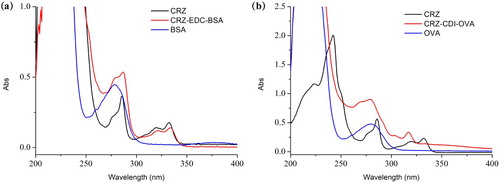
The derivatized hapten was conjugated to BSA through the active ester method and conjugated to OVA through the CDI method. The UV absorption spectra are shown in . As shown in (a), the hapten CRZ-S1 had an absorption peak at 285 nm, and BSA had an absorption peak at 275 nm. At the same concentration, the conjugate had a higher absorption at 275 nm than BSA, and the UV peak shifted at the CRZ and had a distinct absorption peak at 325 nm. Similarly, in (b) CRZ-OVA had a higher absorption at 275 nm than OVA at the same concentration, and the absorption peak broadened. These findings confirmed the successful coupling of the immunogen and the coating to the protein.
3.2. Monoclonal antibody preparation and identification
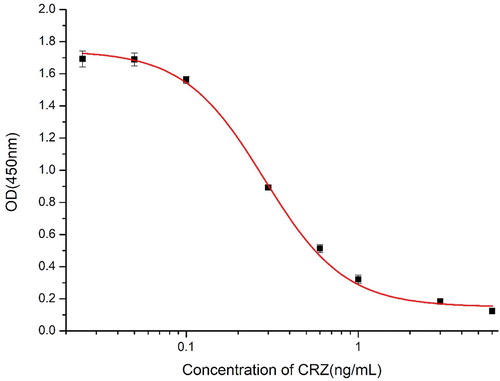
Monoclonal antibodies were prepared through cell fusion techniques. After three rounds of subcloning, the cells were expanded, and ascites was prepared to obtain a cell line secreting 2F5 anti-CRZ, which was identified to have the IgG1 antibody subtype. As shown in , a standard curve was established for CRZ detection with the equation y = 0.14645 + 1.58203/(1 + (x/0.29619) 1.80648) and the correlation coefficient (R2) of 0.9979. From the above formula, the concentration of CRZ that produced 50% inhibition was 0.29 ng/mL, the linear range of detection CRZ was 0.137–0.638 ng/mL, and the LOD was 0.068 ng/mL. Because the complete structure of CRZ was maximally exposed to the immune system, the monoclonal antibodies obtained were also highly specific. As shown in , the cross-reaction with nine other structural analogs and similar drugs did not exceed 11.6%.
Table 1. Cross-reaction results of mAb 2F5.
3.3. Immunochromatographic assay establishment
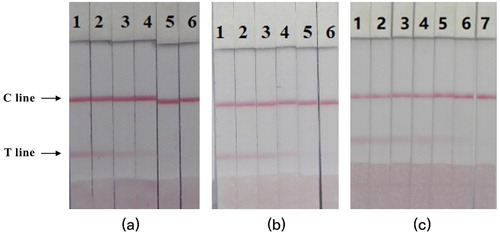
In the PBS system, a series of concentrations of CRZ (0, 0.1, 0.25, 0.5, 1, and 2.5 ng/mL) was added to evaluate the cutoff values under different conditions to test the sensitivity of the strip assay. The standard solution was incubated with McAb-labelled CG to allow the antigenic antibody to be fully reaction. When the concentration of CRZ reached the cut-off value, only when the C line was visible on the strip, it was used to evaluate the sensitivity of the CG strip. As shown in (a), when CRZ was absent, both the control zone and the detection zone had red lines, and when the concentration of CRZ reached 2.5 ng/mL, only the control line was visible. These findings indicated that the CG labelled with the original McAb reacts with the immobilized antigen and goat anti-mouse IgG and becomes reactive with free CRZ in the sample solution and unable to react with the immobilized antigen. The strip reader indicated that the LOD was 0.5 ng/mL at this concentration, because the colour intensity of the T line was significantly weaker than that of the blank PBS.
Figure 5. Image of detection CRZ by CG strip in PBS (a), milk sample (b) and porcine kidney sample (c). (a) 1 = 0 ng/mL, 2 = 0.1 ng/mL, 3 = 0.25 ng/mL, 4 = 0.5 ng/mL, 5 = 1 ng/mL, and 6 = 2.5 ng/mL; Cut-off value was 2.5 ng/mL. (b) 1 = 0 ng/mL, 2 = 0.1 ng/mL, 3 = 0.25 ng/mL, 4 = 0.5 ng/mL, 5 = 1 ng/mL, and 6 = 2.5 ng/mL; Cut-off value was 2.5 ng/mL. (c) 1 = 0 ng/mL, 2 = 0.25 ng/mL, 3 = 0.5 ng/mL, 4 = 1 ng/mL, 5 = 2.5 ng/mL, 6 = 5 ng/ mL, and 7 = 10 ng/mL; Cut-off value was 10 ng/mL.
3.4. Detection of CRZ in milk samples using strip assays
Because milk is different from the PBS matrix, a series of concentrations of CRZ (0, 0.1, 0.25, 0.5, 1, and 2.5 ng/mL) was mixed into the blank milk sample for analysis by strips to verify the developed methods. Milk samples were obtained from a local market and analyzed by LC-MS to confirm the absence of CRZ. As shown in (b), when the CRZ concentration exceeded 2.5 ng/mL, no detection line was observed. Therefore, the sensitivity of the immunochromatographic assay for detecting CRZ in milk was confirmed to be the same as that for PBS. However, when the concentration reached 1 ng/mL, the colour of the test line was significantly less intense than that of the milk blank. Therefore, this detection method can be used to study residual amounts of CRZ in milk.
3.5. Detection of CRZ in porcine kidney samples through the strip method
Kidney samples were obtained from a local market and analyzed by LC-MS to confirm the absence of CRZ. Because these samples had stronger matrix interference than milk, CRZ (0, 0.25, 0.5, 1, 2.5, 5, and 10 ng/mL) was added to the kidney homogenate test cut-off value. After pretreatment, the results were obtained after 5 min, as shown in (c). When the CRZ concentration reached 2.5 ng/mL, the colour of the test line began to weaken. When the concentration reached 10 ng/mL, the test line became colourless. Therefore, the LOD was 2.5 ng/mL, and the cut-off value was 10 ng/mL. The CRZ content in pig kidney could therefore be determined from the test results. When the CRZ concentration was <2.5 ng/mL, a negative result was obtained. When the CRZ concentration was 2.5–10 ng/mL, the results were weakly positive, and when the CRZ concentration was >10 ng/mL, a positive result was obtained. However, compared with that for milk and PBS, the C line was lighter in colour and had a higher cut-off value, probably because the pretreatment of the kidney still resulted in the presence of complex compounds that affect the binding of the antibody to the antigen.
3.6. Analysis of CRZ in milk samples
In the recovery experiment, the accuracy of the CG assay was verified, and the concentration of CRZ in the milk sample was 0.1–5 ng/mL. The results are shown in . With ic-ELISA, the recovery of CRZ was 93.2% to 104.7%, and the coefficient of variation was 4.58% to 5.81%. With strip analysis, CRZ was able to be detected semi-quantitatively, and the results of CRZ detection in milk samples by ic-ELISA and CG band molecular analysis were consistent. Because all test results were able to be obtained within 10 min, this method was suitable for rapid detection and high-throughput on-site processing of milk samples.
Table 2. Recovery of CRZ in milk by ic-ELISA and strip assay (n = 3).
4. Conclusion
In this study, the introduction of a carboxyl group without destroying the original group resulted in high antigen exposure during the immunological recognition process; a new CRZ hapten was synthesized, and mAb 2F5, with high specificity and maximum sensitivity to CRZ was successfully produced from the hapten. In addition, in immunochromatographic analysis, banding results were produced in only 10 min and showed good stability and sensitivity in the analysis of milk samples and pig kidney samples. Therefore, the antibody can be used to detect CRZ in milk and porcine kidney samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aranas, A. T., Guidote, A. M., Haddad, P. R., & Quirino, J. P. (2011). Sweeping-micellar electrokinetic chromatography for the simultaneous analysis of tricyclic antidepressant and β-blocker drugs in wastewater. Talanta, 85(1), 86–90.
- Bock, C., & Stachel, C.S. (2013). Development and validation of a confirmatory method for the determination of tranquilisers and a β-blocker in porcine and bovine kidney by LC-MS/MS. Food Additives & Contaminants Part A Chemistry Analysis Control Exposure & Risk Assessment, 30(6), 1000–1011.
- Chen, Y., Kong, D., Liu, L., Song, S., Hua, K., & Xu, C. (2015). Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Analytical Methods, 9(4), 1–10.
- Cheng, L., Shen, J., Zhang, Q., Zhang, Y., & Zhang, S. (2015). Simultaneous determination of three Tranquillizers in lamb liver by ultra-performance liquid chromatography–tandem mass spectrometry. Food Analytical Methods, 8(7), 1876–1882.
- Cooper, J., Delahaut, P., Fodey, T. L., & Elliott, C. T. (2004). Development of a rapid screening test for veterinary sedatives and the beta-blocker carazolol in porcine kidney by ELISA. The Analyst, 129(2), 169–174.
- Dubois, M. (2004). Multiresidue method for the detection of tranquillisers, xylazine, and a beta-blocker in animal production by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 1054(1), 373–378.
- Fan, S., Hong, M., Zhao, Y., Chen, H., & Wu, Y. (2013). Simultaneous detection of residues of 25 β2-agonists and 23 β-blockers in animal foods by high-performance liquid chromatography coupled with linear Ion Trap mass spectrometry. Journal of Agricultural & Food Chemistry, 60(8), 1898–1905.
- He, L., Wang, J., Zhang, G., Liu, R., & Fang, B. (2012). Simultaneous determination of tranquilizers and carazolol residues in swine tissues by liquid chromatography-tandem mass spectrometry. Analytical Letters, 45(11), 1377–1389.
- Hui, X. U., Zhang, H. W., Wang, F. M., Zhang, X. M., & Wang, P. F. (2014). Determination of 9 β-blockers residues in dairy products by liquid chromatography-tandem mass spectrometry. Journal of Food Safety & Quality, 5(12), 3884–3890.
- Isanga, J., Tochi, B. N., Mukunzi, D., Chen, Y., Liu, L., Kuang, H., & Xu, C. (2016). Development of a specific monoclonal antibody assay and a rapid testing strip for the detection of apramycin residues in food samples. Food and Agricultural Immunology, 28(1), 49–66. doi: 10.1080/09540105.2016.1202211
- Jiangeng, H., Luqin, S., Zhaoze, F., Lei, H., Jun, Q., & Gao, L. (2011). In vitro metabolic stability and metabolite profiling of TJ0711 hydrochloride, a newly developed vasodilatory β-blocker, using a liquid chromatography-tandem mass spectrometry method. Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences, 879(30), 3386–3392.
- Le, T., Yi, S.-H., Zhao, Z.-W., & Wei, W. (2011). Rapid and sensitive enzyme-linked immunosorbent assay and immunochromatographic assay for the detection of chlortetracycline residues in edible animal tissues. Food Additives & Contaminants, 28(11), 1516–1523.
- Lei, X., Xu, L., Song, S., Liu, L., & Hua, K. (2017). Development of an ultrasensitive ic-ELISA and immunochromatographic strip assay for the simultaneous detection of florfenicol and thiamphenicol in eggs. Food & Agricultural Immunology, 29(1), 254–266.
- Li, J., Zhi, A., Jia, G., Hui, N., & Ai, L. (2017). Development of an ic-ELISA and immunochromatographic strip for detection of sparfloxacin in honey. Food & Agricultural Immunology, 29(1), 147–158.
- Mercader, J. V., López-Moreno, R., Esteve-Turrillas, F. A., Agulló, C., Abad-Somovilla, A., & Abad-Fuentes, A. (2014). Sensitive monoclonal antibody-based immunoassays for kresoxim-methyl analysis in QuEChERS-based food extracts. Journal of Agricultural and Food Chemistry, 13(62), 2816–2821.
- Mitrowska, K., Posyniak, A., & Zmudzki, J. (2009). Rapid method for the determination of tranquilizers and a beta-blocker in porcine and bovine kidney by liquid chromatography with tandem mass spectrometry. Analytica Chimica Acta, 637(1), 185–192.
- Nocentini, A., Ceruso, M., Bua, S., Lomelino, C. L., Andring, J. T., McKenna, R., … Supuran, C. T. (2018). Discovery of β-adrenergic receptors blocker-carbonic anhydrase inhibitor hybrids for multitargeted antiglaucoma therapy. Journal of Medicinal Chemistry, 61(12), 5380–5394. doi: 10.1021/acs.jmedchem.8b00625
- Qiu, Y. (2006). Determination of four benzodiazepine residues in pork using multiwalled carbon nanotube solid-phase extraction and gas chromatography-mass spectrometry. Journal of Chromatography A, 1136(1), 99–105.
- Sun, C., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of a highly sensitive ELISA and immunochromatographic strip to detect pentachlorophenol. Food and Agricultural Immunology, 27(5), 689–699. doi: 10.1080/09540105.2016.1148668
- Tan, X., Yang, J., Yang, Q., & Li, Q. (2017). A highly sensitive resonance Rayleigh scattering and colorimetric assay for the recognition of propranolol in β-adrenergic blocker. Luminescence, 32(7), 1221–1226.
- Wang, L., Zeng, Z., Xufeng, W., Yang, J., Chen, Z., & He, L. (2013). Multi-residue analysis of 9 β-agonists in animal muscles by LC-MS/MS based on a new polymer cartridge for sample cleanup. Journal of Separation Science, 36(11), 1843–1852.
- Wang, Y., Wu, Q., Cheng, M., & Cai, C. (2011). Determination of β-blockers in pharmaceutical and human urine by capillary electrophoresis with electrochemiluminescence detection and studies on the pharmacokinetics. Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences, 879(13), 871–877.
- Wang, Z., Xie, Z., Gang, C., Liu, L., Song, S., Hua, K., & Xu, C. (2017). Development of an indirect competitive enzyme-linked immunosorbent assay and immunochromatographic assay for hydrocortisone residues in milk. Food & Agricultural Immunology, 28(3), 476–488.
- Wang, Z., Zheng, Q., Guo, L., Suryoprabowo, S., Liu, L., & Kuang, H. (2017). Preparation of an anti-dexamethasone monoclonal antibody and its use in development of a colloidal gold immunoassay. Food and Agricultural Immunology, 28(6), 958–968. doi: 10.1080/09540105.2017.1320360
- Xie, Z., Kong, D., Liu, L., Song, S., & Hua, K. (2017). Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the simultaneous detection of avermectin and ivermectin. Food & Agricultural Immunology, 28(3), 439–451.
- Xing, C., Hao, C., Liu, L., Xu, C., & Hua, K. (2014). A highly sensitive enzyme-linked immunosorbent assay for copper(II) determination in drinking water. Food & Agricultural Immunology, 25(3), 432–442.
- Xu, Y., Huang, Z. B., He, Q. H., Deng, S. Z., Li, L. S., & Li, Y. P. (2010). Development of an immunochromatographic strip test for the rapid detection of deoxynivalenol in wheat and maize. Food Chemistry, 119(2), 834–839.
- Yan, H., Liu, L., Xu, N., Hua, K., & Xu, C. (2015). Development of an immunoassay for carbendazim based on a class-selective monoclonal antibody. Food & Agricultural Immunology, 26(5), 659–670.
- Yun-Jie, F. U., Liu, Z. G., & Yu-Xiang, W. U. (2010). Development of a one-step immunochromatographic strip test for rapid detection of chlortetracycline residue in food. Food Science, 31(2), 191–194.
- Zhang, J., Shao, B., Yin, J., Wu, Y., & Duan, H. (2009). Simultaneous detection of residues of β-adrenergic receptor blockers and sedatives in animal tissues by high-performance liquid chromatography/tandem mass spectrometry. Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences, 877(20), 1915–1922.
- Zheng, M.-M., Zhang, M.-Y., & Feng, Y.-Q. (2009). Polymer monolith microextraction online coupled to hydrophilic interaction chromatography/mass spectrometry for analysis of β2 -agonist in human urine. Journal of Separation Science, 32(11), 1965–1974.
- Zhou, M., Peng, J., He, R., He, Y., Zhang, J., & Li, A. (2015). High performance liquid chromatography coupled with resonance Rayleigh scattering for the detection of three fluoroquinolones and mechanism study. Spectrochimica Acta Part A Molecular & Biomolecular Spectroscopy, 136(136PB), 1181–1187.