ABSTRACT
Currently there is tremendous interest in the discovery of natural polysaccharide immunomodulators for treating immune-related diseases. Herein, the immunomodulatory activities of a polysaccharide fraction isolated from blackberry seeds (BSP-2) were examined in RAW264.7 cells. The results revealed that BSP-2 at 37.5–300 µg/mL could significantly enhance the phagocytic activity of RAW264.7 cells and their ability to release NO, TNF-α and IL-6 cytokines. BSP-2 could significantly up-regulate the mRNA expression of iNOS, TNF-α and IL-6, as well as the phosphorylation of p65, p38, ERK and JNK proteins. The mechanisms underlying the immunomodulatory actions of BSP-2 likely involve the activation of the NF-κB and MAPK signaling pathways and up-regulation of the phosphorylation of key proteins involved in these pathways. These findings suggest that BSP-2 could be applied as a polysaccharide immunotherapeutic adjuvant with the effective dose as low as 70 µg/mL (BSP-2 may be cytotoxic at levels > 600 μg/mL).
Highlights
Immunostimulatory potential of a blackberry seed polysaccharide (BSP-2) was examined.
BSP-2 at 37.5–300 µg/mL could enhance the phagocytic activity of RAW264.7 cells.
BSP-2 could significantly up-regulate the mRNA expression of iNOS, TNF-α and IL-6.
BSP-2 up-regulated the phosphorylation of p65, p38, ERK and JNK proteins.
Immunostimulatory BSP-2 acted via activating the NF-κB and MAPK signalling pathways.
1. Introduction
Blackberry (Rubus spp.) is a popular commercial fruit with a rich nutritional profile owing to its high content of nutrients and bioactive substances including amino acids, polysaccharides (including dietary fiber), flavonoids (including anthocyanins), vitamins (e.g. vitamins B, C and K) and minerals (including K, P, Ca, Mg, Cu, Mn, Zn and Fe). Consumption of blackberries therefore confers numerous health benefits, such as combating oxidative stress and offering anti-inflammatory and anti-cancer effects (Jakobsdottir et al., Citation2013; Wang et al., Citation2017). Previous in vitro and in vivo research conducted in our laboratory confirmed that blackberry fruit, especially the seeds of blackberry fruit, contained a wide range of bioactive compounds and exhibited strong antioxidant activity (Yin et al., Citation2013), liver-protecting properties (Zhang et al., Citation2014a), α-glucosidase-inhibitory effect (Yong, Citation2015), blood lipid-reducing function (Yin et al., Citation2014), antithrombotic capacity (Wang et al., Citation2017), and diabetes-combating effects (Zhang et al., Citation2014b). Like other seasonal “soft” fruits with short shelf lives, blackberry is consumed in the form of whole fresh fruit and in processed fruit products (like juice, jam and blackberry wine). As a result of handling and processing of fresh blackberry fruit, a large amount of secondary grade or rejected fresh fruit and waste by-products (such as seeds and pomace) are generated. Blackberry seeds, as the by-products of berry processing, were found particularly rich in bioactive compounds such as triterpenes, flavonoids and polysaccharides (Xie et al., Citation2017). Therefore, it is of both economic and environmental interest to maximise the utilisation of such seed wastes to produce health-promoting dietary products, thus providing motivation for the present study.
Amongst bioactive substances, polysaccharides are attracting growing attention on account of their diverse biological functions including immunomodulatory (Chen et al., Citation2017), anti-tumor (Zhao et al., Citation2010), anti-oxidative (Wu et al., Citation2012), and anti-virus (Karmakar et al., Citation2010) properties. Three polysaccharides isolated from blackberry wine demonstrated anti-inflammatory effects on lipopolysaccharides (LPS) -stimulated RAW264.7 macrophages, significantly reducing the production of nitric oxide (NO) and pro-inflammatory cytokines like tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) (Cordeiro Caillot et al., Citation2018). In our laboratory, four polysaccharides (called BSP-1a, BSP-1b, BSP-2 and BSP-3) were successfully isolated from blackberry seeds, separated by DEAE-52 column and purified by Sephadex G-100 column (Wang et al., Citation2017). Three of these four polysaccharides (BSP-1b, BSP-2 and BSP-3) exhibited significant anticoagulant activities and inhibitory effect on thrombus formation in blood stasis studies using a male rabbit model or male/female Sprague–Dawley rat models (Xie et al., Citation2017). Chemical analyses using gas chromatography (GC), high-performance gel permeation chromatography (HPSEC), fourier transform infrared (FT-IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy revealed that BSP-1b, BSP-2 and BSP-3 all contained α- and β-glycosidic linkages and four monosaccharides, but each polysaccharide had a different average molecular weight (178,500, 33,600 and 224,300 g/mol, respectively) and different molar ratios of monosaccharides (galactose, fructose: rhamnose: arabinose: xylose = 19.13: 2.27: 3.30: 5.48: 1.78 for BSP-1b; galactose, fructose: rhamnose: arabinose: xylose = 2.55: 1.95: 7.40: 17.98: 8.71 for BSP-2; galactose: fructose: rhamnose: arabinose = 0.58: 0.91: 3.41: 2.63 for BSP-3 (no xylose was detected for BSP-3)). This current study aimed to extend our prior published research on blackberry seed polysaccharides, by exploring the potential of BSP-2 (the bioactive polysaccharide fraction with the highest yield) as a polysaccharide immunotherapeutic adjuvant. This work supports current global research aimed at the discovery of natural polysaccharide immunomodulators.
The immune system is an extremely complex and important physiological system, with phagocytes acting as the first line of immunological defense against invading substances and infections (Janeway & Medzhitov, Citation2002; Uthaisangsook et al., Citation2002). Macrophages, as the phagocytic cells critical for innate and adaptive immune responses, regulate the production of various cytokines to exert immunomodulatory effects (such as tumour necrosis factor-α (TNF-α), interleukin 6 (IL-6) and interleukin-1β (IL-1β)) and control the release of inflammatory molecules involved in immune responses (such as NO) (Birk et al., Citation2001). RAW264.7 cells are a monocyte/macrophage like cell linage and are frequently used as a model of macrophages for in vitro evaluation of immune regulatory activity, as these cells are capable of responding to stimuli in vitro, release NO and immune-related cytokines (Gu et al., Citation2017; Simas-Tosin et al., Citation2012). Lipopolysaccharides (LPS) are pathogenic endotoxins that have been widely been used in the development of inflammatory models owing its ability to induce an acute inflammatory response and excessive release of inflammatory mediators (Sweet & Hume, Citation1996). In this study, the immunomodulatory potential of BSP-2, a kind of purified polysaccharide with the highest yield (Wang et al., Citation2017) was examined in RAW264.7 cells with and without LPS stimulation. Since the nuclear factor κB (NF-κB) and mitogen-activated protein kinases (MAPKs) are important intracellular and interactive signalling molecules in response to stimuli (such as cell stress) and for immunity enhancement (Carter et al., Citation1999; Fengyang et al., Citation2012; Shao et al., Citation2013), the effectiveness of BSP-2 in activating NF-κB and MAPKs was the focus of this study. The changes induced by BSP-2 in RAW 264.7 cell proliferation as well as the contents of NO, TNF-α, IL6 and IL-1β were monitored and discussed.
2. Materials and methods
2.1. Chemicals and reagents
BSP-2 (total carbohydrate 93.76%, total protein 4.06%, uronic acid 8.91%) was produced by our laboratory (National R & D Center for Edible Fungus Processing Technology, Henan University) from blackberry seeds according to a reported method (Wang et al., Citation2017). Dulbecco’s modified Eagle’s medium (DMEM) and neutral red were purchased from Solarbio (Beijing, China). Fetal bovine serum (FBS) was purchased from Gibco (Grand Island, NY, USA). A Nitric oxide kit was procured from Nanjing Jancheng Bioengineering Institute (Nanjing, China). TNF-α and IL-6 ELISA kits were purchased from Mr Ng Nanjing Biological (Nanjing, China). LPS PDTC, PD98059, SP600125 and SB203580 were purchased from Sigma (St. Louis, MO, USA). Primers iNOS, TNF-α and IL-6 were purchased from Thermo Fisher Scientific (Shanghai, China). PrimeScriptTMRT reagent kit along with gDNA Eraser kit, TB GreenTM Premix Ex TaqTM II (Tli RNadeH Plus) and Bulk kit were purchased from TaKaRa (Shiga, Japan). Antibodies to NF-kB p65, phospho-NF-kB p65, p38 MAPK, phospho-p38 MAPK, phospho-p38 MAPK, SAPK/JNK, phospho-SAPK/JNK, p44/p22 MAPK and phospho-p44/p22 MAPK were sourced from Cell Signalling (Beverly, MA, USA).
2.2. Cell culture and cell viability assay
RAW264.7 macrophages were obtained from Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, China). The cells were grown in the DMEM medium containing 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin before incubation at 37°C in 24h under a humidified atmosphere of 5% CO2. The viability of the cells was determined by the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay. The cells were inoculated at 1×104cells/well into the wells of the 96-well cell culture plate, before a 24-h treatment at 37°C with BSP-2 at different concentrations (37.5-600 µg/mL) for the experimental group or with only culture medium only for the blank control group. Upon addition of 10 µL of the MTT solution to each well, the mitochondrial succinate dehydrogenase in the living cells was reduced from yellow to water-insoluble blue-purple crystals with an absorption maximum wavelength at 490 nm. The absorbance was measured using a microplate reader (Thermo Multiskan Go).
2.3. Phagocytic activity
The RAW264.7 cells were inoculated in a 96-well cell culture plate (at a density of 1×106 cells/mL) and treated with BSP-2 (at a fixed concentration) and LPS (1 µg/mL) for 24 h. A aliquot (0.075%) of neutral red solution was added to each well and allowed to stand for 1 h at 37 oC, before the cell lysis solution containing 1% acetic acid-anhydrous alcohol (1:1, v:v) was added to each well at indoor temperature. The absorbance was measured at 540 nm.
2.4. NO assay
The NO kit was used to measure the levels of NO in the supernatants of the cell cultures. RAW264.7 cells were seeded in 24-well plates (at a density of 1×106 cells/mL) and treated with BSP-2 (at a fixed concentration) and LPS (1 µg/mL) for 24 h at 37 oC. The resulting supernatants were collected and the levels of NO determined by Nitric oxide kit according to the nitric acid reduction method.
2.5. Enzyme-linked immune sorbent assay (ELISA)
RAW264.7 cells were inoculated in the wells of a 6-well cell culture plate (at a density of 2×106 cells/ well) and treated with BSP-2 (at a fixed concentration) and LPS (1 µg/mL) for 30 min 37 oC. The levels of TNF-α and IL-6 in RAW264.7 cells were measured using ELISA assay kits according to the manufacturer’s instructions.
2.6. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Real-time PCR was used to determine the expression of iNOS, TNF-α and IL-6 (Wang et al., Citation2019). In brief, total RNA was isolated and reversed further to cDNA with PrimeScriptTM RT reagent kit and gDNA Eraser kit following the manufacturer’s guidelines. The expression of mRNAs was presented as 2−ΔΔct. The primers for iNOS, TNF-α, IL-6 and GAPDH are listed in .
Table 1. Primers for real-time RT-PCR.
2.7. Western blot
The cytoplasmic and nuclear proteins were extracted following the nuclear protein kit’s instructions, before analysis of the protein concentration by the BCA method. The proteins (30 µg) in 5×loading buffer were separated by 8% SDS-PAGE before being electroblotted onto polyvinylidene fluoride (PVDF) membranes from Beijing Solarbio Science & Technology Co., Ltd. (BeiJing, China). The membranes were blocked with 5% skim milk powder in PBS buffer at pH 7.2-7.4 before the in-situ hybridisation with the phosphorylated and non-phosphorylated antibodies (NF-kBp65, ERK, JNK, p38) overnight at 4 oC. The reactive bands were incubated for 2h at indoor temperature with an appropriate amount of horseradish peroxidase (HRP)-conjugated secondary antibody via the enhanced chemiluminescence (ECL) Plus hypersensitive luminescence solution method. The grayscale values of the bands were quantitatively analyzed by Image J software.
The RAW264.7 cells were inoculated in the wells of the 6-well plates (at a density of 1×106 cells/mL) and stimulated with different concentrations of BSP-2 for 30 min. The positive control group was treated with LPS only (1 μg/mL). The expression of phosphorylated p65, p38 MAPK, p44/p22 MAPK, SPAK/JNK and their respective total proteins were detected using the above-described method of western blot.
2.8. Statistical analysis
All experiments were repeated independently at least three times and analyzed statistically by SPSS 19.0 software. Data were expressed as mean ± standard deviation. Statistical significance was calculated by the one-way ANOVA analysis, with the comparisons between two groups performed using the T-test.
3. Results
3.1. Effects of BSP-2 on cells viability and phagocytic activity
BSP-2 at a concentration lower than 300 μg/mL promoted the proliferation of RAW264.7 cells (P<0.001) (A). Inhibition on the cell viability was observed at a BSP-2 concentration of 600 μg/mL, indicating cytotoxicity at this level. Therefore, the BSP-2 concentration range of 37.5–300 µg/mL was chosen for further investigations with BSP-2.
Figure 1. Effects of BSP-2 on cell viability and phagocytosis of RAW264.7 cells detected by the MTT assay. *P<0.05, **P<0.01, ***P<0.001 vs. the control group. Data are expressed as “mean ± standard deviation” of three independent experiments. LPS refers to lipopolysaccharide.
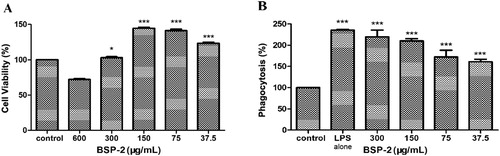
The phagocytic activity of RAW264.7 cells was examined by the neutral red method. B shows that LPS and BSP-2 could promote the phagocytosis of RAW264.7 cells at different concentrations (P<0.001) compared with the control group. The results indicate that BSP-2 could significantly increase the phagocytic activity of RAW264.7 macrophages.
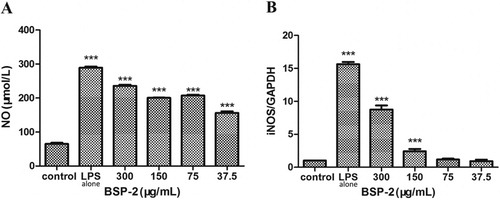
3.2. Effects of BSP-2 on NO production and iNOS mRNA expression
As shown in A, the level of NO in RAW264.7 cells increased markedly upon the stimulation with LPS (1 µg/mL) (P<0.001) compared with the control group. The presence of BSP-2 decreased the level of NO generated. In B, the stimulation with LPS alone up-regulated the expression of iNOS mRNA remarkably. The presence of BSP-2 significantly increased the level of iNOS mRNA, with the extent of such suppression being highest at low BSP-2 concentrations (effectiveness of suppression: 300 μg/mL < 150 μg/mL < 75 ≅ 37.5 μg/mL) (P< 0.001). Therefore, BSP-2 could effectively regulate the secretion of NO in RAW264.7 cells whilst minimising LPS-induced up-regulation of iNOS gene expression.
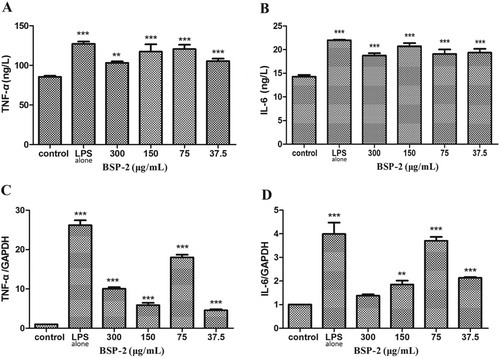
3.3. Effects of BSP-2 on the expression of TNF-α, IL-6 and their mRNA in RAW264.7 cells
As the shows, the stimulation of LPS alone promoted the expression of TNF-α and IL-6, and up-regulated the mRNA levels of TNF-α and IL-6. Treatments with BSP-2 in the concentration range 37.5–300 μg/mL only slightly suppressed the LPS-induced increase in the levels of TNF-α and IL-6, but was able to reduce the LPS-induced up-regulation of TNF-α and IL-6 mRNA (a BSP-2 concentration of 75 μg/mL was the least effective) (P<0.001). BSP-2 concentrations of 37.5 and 150 μg/mL exerted the greatest and second greatest suppression, respectively, on LPS-induced up-regulation of TNF-α mRNA expression, whereas, BSP-2 at 300 μg/mL imparted the greatest suppression on the LPS-induced up-regulation of IL-6 mRNA expression (with no significant difference between the concentrations of 37.5 and 150 μg/mL). Accordingly BSP-2 demonstrated an immune-modulating ability towards the expression of TNF-α and IL-6 mRNA, with the effectiveness varying with both the BSP-2 concentration and the type of cytokine.
3.4. Effects of BSP-2 on the NF-κb and MAPK signalling pathways in RAW264.7 cells
A (bar chart) shows that BSP-2 could significantly enhance protein expression (especially at 75 μg/mL), compared to the control (untreated cells). BSP-2 treatment led to a smaller enhancement of p65 and ERK expression, greater enhancement of p38 expression, and comparable enhancement of JNK expression, compared with the treatment with LPS alone.
Figure 4. Effects of BSP-2 on the expression of proteins (p65, p38, ERK and JNK) associated with the NF-κB and MAPK signaling pathways (bar graph). (A)RAW 264.7 cells were stimulated with 37.5, 75, 150 and 300 µg/ml of BSP-2 for the indicated time and (B) RAW 264.7 cells were pretreated with PDTC (30 μM), PD98059 (30 μM), SP600125 (3 μM), SB203580 (10 μM) for 30 min. “Relative Fold” equals to the ratio of specific proteins expression of the cells in each group to that in the blank group. The inserted image shows the expression of p38 MAPK, ERKl/2 and SPAK/JNK proteins with and without phosphorylation. Data represent mean ± SD of three independent experiments. Results are compared with the control, **P < 0.01, ***P < 0.001. The abbrevations p-NF-κB p65, p-p38, p-ERK and p-JNK refer to phosphorylated-NF-κB p65, phosphorylated-p38, phosphorylated-ERK and phosphorylated-JNK, respectively.
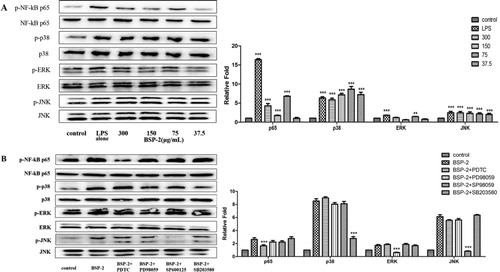
As shown by the image in A, the expression of phosphorylated p38 MAPK, ERKl/2 and SPAK/JNK proteins was significantly higher than that in the control group (P < 0.001). Moreover, either BSP-2 (especially at 70–300 μg/mL) or LPS (1 μg/mL) could significantly up-regulate the expression of phosphorylated p65 in the nucleus, resulting in a significant increase in the ratio of p-p65/p65. Meanwhile, for verifying the signalling pathways of BSP-2 in another perspective, pretreatments with four inhibitors including PDTC (30 μM), PD98059 (30 μM), SP600125 (3 μM) and SB203580 (10 μM) was conducted separately in four groups for 30 min, and the result shown in the B revealed that pretreatments with PDTC (NF-κB inhibitor), SP600125 (SAPK/JNK inhibitor), PD98059 (ERK inhibitor) and SB203580 (p38 inhibitor) could significantly reduce the expression level of phosphorylated proteins. Thus, BSP-2 could enhance the expression of NF-κB in the nucleus (rather than in the cytoplasm). BSP-2 likely up-regulated the level of phosphorylation of the key proteins related to the NF-κB and MAPKs pathways in RAW264.7 cells.
4. Discussion
Macrophages possess immunomodulatory capacity, and participate in promoting tissue repair and regeneration as well as silencing the inflammatory response. NO, produced mostly by nitric oxide synthase (NOS), is an important endogenous molecule for the immune function and antitumor activity of macrophages (such as killing pathogenic microorganisms) (Chen et al., Citation2009; Liang et al., Citation2008). The downstream NF-κB, as an important transcription factor, is capable of inducing the expression of iNOS gene. Once macrophages are activated, they exert a variety of biological functions through innate immune responses, such as phagocytosis, chemotaxis and destruction of target microorganisms to remove foreign substances, and can activate iNOS to release NO. In this study, the effects of BSP-2 on cell viability and phagocytosis of RAW264.7 cells, as well as the production of NO and cytokine, mRNA expression and NF-kB/MAPK signalling pathways in RAW264.7 cells, were investigated with LPS (1 μg/mL) as a positive control. The results of the neutral red test showed that BSP-2 could significantly (P < 0.001) enhance the phagocytic activity of RAW264.7 cells and also promote the release of NO in RAW264.7 cells (). The RT–PCR results revealed that the expression of iNOS mRNA for the BSP-2 group was significantly higher than that of the control group, indicating that BSP-2 could promote the secretion of NO by up-regulating the expression of iNOS gene in RAW264.7 cells to regulate the immune activity of the cells.
The cytokines secreted by RAW264.7 cells were mainly the characteristic cytokines of the M1/M2 cell inflammatory response, with TNF-α and IL-6 being the iconic cytokines of M1 and M2 macrophages, respectively. TNF-α plays an important role in the host defense mechanism, including the stimulation of the expression of various immunomodulatory mediators and inflammatory mediators (Baugh & Bucala, Citation2001). IL-6 is a major contributor to the host defense and multifunctional cytokine that influences inflammation, immune or auto-immune response, and hematopoiesis (Tanaka et al., Citation2016). As shown in , RAW264.7 cells spontaneously secrete cytokines in the resting state, and such secretion in RAW264.7 cells was significantly enhanced by LPS stimulation. BSP-2 could also significantly increase the levels of TNF-α and IL-6 secreted in RAW264.7 cells. The results of RT–PCR further revealed that the mRNA expression of TNF-α and IL-6 was significantly increased through BSP-2 treatment, especially when BSP-2 was at a concentration of 75 µg/mL. Therefore, BSP-2 could stimulate the differentiation and expression of Ml-type and M2-type cells to induce a Th1/Th2 balanced immune response (Xiao-Xi et al., Citation2008).
NF-κB is a transcriptional activator consisting of p65 and p50, and plays an important role in regulating the expression of iNOS, COX-2 and proinflammatory cytokines (TNF-α, IL-1 β and IL-6) (Imanifooladi et al., Citation2010). Previous studies have shown that the phosphorylation of IκBα (the main inhibitor of NF-κB) could be enhanced by plant polysaccharides, causing rapid degradation of IκB protease (Ghosh & Hayden, Citation2008; Karin & Ben-Neriah, Citation2000). Moreover, MAPKs are comprised of p38, ERK and JNK subtypes, with the activation of the p38 MAPK pathway being essential to immune response-related functions in macrophages and transcriptional regulation of iNOS and COX-2 (Ashwell, Citation2006). In addition, NF-κB and p38 MAPK pathways interact and work collaboratively (Craig et al., Citation2000). MAPK is located upstream of NF-κB (Vanden Berghe et al., Citation1998), with some specific MAPK inhibitors being capable of blocking the activation of NF-κB (Caivano, Citation1998). The verification of the nuclear transfer of NF-κB can thus help to confirm whether or not NF-κB was activated. In this study, the level of p65 protein in the nucleus was determined by the western blot method, with the obtained results presented in . BSP-2 significantly up-regulated the expression of phosphorylated p65 protein in the nucleus, which was in agreement with the detected secretion and gene expression of cytokines (). Therefore, BSP-2 could promote the production of proinflammatory factors through activating the NF-κB signalling pathway. As shown in , the phosphorylation of p38, ERK1/2, JNK was significantly increased in RAW264.7 macrophages after stimulation by BSP-2, suggesting that BSP-2 could also activate the signalling pathway of MAPK. Similarly, the protein phosphorylation level was significantly decreased after stimulation with pathway inhibitors. Results demonstrate that the immunomodulatory effect of BSP-2 was associated closely with the enhancement of MAPK phosphorylation.
5. Conclusion
A polysaccharide fraction isolated from blackberry seeds (BSP-2) demonstrated an immunomodulatory effect of RAW264.7 macrophages. BSP-2 could enhance the phagocytosis of RAW264.7 cells and the mRNA expression of cytokines, and regulate the secretion of NO in RAW264.7 cells whilst alleviating Lipopolysaccharide-induced up-regulation of iNOS gene expression. In addition, BSP-2 could also up-regulate the expression of key proteins in the NF-κB and MAPK signalling pathways, indicating that the immunomodulatory ability of BSP-2 was realised through activating the NF-κB and MAPK signalling pathways.
Acknowledgments
This work was supported by Department of Science and Technology, Henan Province (192102110112 and 192102110214).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ashwell, J. D. (2006). The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nature Reviews Immunology, 6(7), 532–540. https://doi.org/10.1038/nri1865
- Baugh, J. A., & Bucala, R. (2001). Mechanisms for modulating TNF alpha in immune and inflammatory disease. Current Opinion in Drug Discovery & Development, 4(5), 635–650. https://pubmed.ncbi.nlm.nih.gov/12825458/
- Birk, R. W., Gratchev, A., Hakiy, N., Politz, O., Schledzewski, K., Guillot, P., Orfanos, C. E., & Goerdt, S. (2001). Alternative activation of antigen-presenting cells: Concepts and clinical relevance. Der Hautarzt, 52(3), 193–200. https://doi.org/10.1007/s001050051289
- Caivano, M. (1998). Role of MAP kinase cascades in inducing arginine transporters and nitric oxide synthetase in RAW264 macrophages. FEBS Letters, 429(3), 249–253. https://doi.org/10.1016/S0014-5793(98)00578-X
- Carter, A. B., Knudtson, K. L., Monick, M. M., & Hunninghake, G. W. (1999). The p38 mitogen-activated protein Kinase Is required for NF-κB-dependent gene expression: The role of Tata-Binding Protein (TBP). Journal of Biological Chemistry, 274(43), 30858–30863. https://doi.org/10.1074/jbc.274.43.30858
- Chen, J., Gu, X., Chen, J., Luo, Y., Wang, M., Yang, H., Guo, X., & Zhu, X. (2017). Immunomodulatory effects of Glycyrrhiza uralensis polysaccharide in glycinin-induced allergic mouse model. Food and Agricultural Immunology, 28(2), 179–188. https://doi.org/10.1080/09540105.2016.1251393
- Chen, Y. Q., Nie, S. P., Huang, D. F., Han, C., & Xie, M. Y. (2009). Effect of polysaccharide isolated from the seed of Plantago asiatica L. on the nitric oxide production by RAW264.7 cells. Chinese Pharmacological Bulletin, 25(8), 1119–1120. http://doi.org/10.3321/j.issn:1001-1978.2009.08.035
- Cordeiro Caillot, A. R., de Lacerda Bezerra, I., Palhares, L. C. G. F., Santana-Filho, A. P., Chavante, S. F., & Sassaki, G. L. (2018). Structural characterization of blackberry wine polysaccharides and immunomodulatory effects on LPS-activated RAW 264.7 macrophages. Food Chemistry, 257, 143–149. https://doi.org/10.1016/j.foodchem.2018.02.122
- Craig, R., Larkin, A., Mingo, A. M., Thuerauf, D. J., Andrews, C., McDonough, P. M., & Glembotski, C. C. (2000). P38 MAPK and NF-κB collaborate to induce interleukin-6 gene expression and release: Evidence for a cytoprotective Autocrine signaling pathway in a cardiac myocyte model system. Journal of Biological Chemistry, 275(31), 23814–23824. https://doi.org/10.1074/jbc.M909695199
- Fengyang, L., Yunhe, F., Bo, L., Zhicheng, L., Depeng, L., Dejie, L., Wen, Z., Yongguo, C., Naisheng, Z., Xichen, Z., & Zhengtao, Y. (2012). Stevioside suppressed inflammatory cytokine secretion by down regulation of NF-κB and MAPK signaling pathways in LPS-stimulated RAW264.7 cells. Inflammation, 35(5), 1669–1675. https://doi.org/10.1007/s10753-012-9483-0
- Ghosh, S., & Hayden, M. S. (2008). New regulators of NF-κB in inflammation. Nature Reviews Immunology, 8(11), 837–848. https://doi.org/10.1038/nri2423
- Gu, Q., Yang, H., & Shi, Q. (2017). Macrophages and bone inflammation. Journal of Orthopaedic Translation, 10, 86–93. https://doi.org/10.1016/j.jot.2017.05.002
- Imanifooladi, A. A., Yazdani, S., & Nourani, M. R. (2010). The role of nuclear factor-κB in inflammatory lung disease. Inflammation & Allergy - Drug Targets, 9(3), 197–205. https://doi.org/10.2174/187152810792231904
- Jakobsdottir, G., Blanco, N., Xu, J., Ahrné, S., Molin, G., Sterner, O., & Nyman, M. (2013). Formation of short-chain fatty acids, excretion of anthocyanins, and microbial diversity in rats fed blackcurrants, blackberries, and raspberries. Journal of Nutrition and Metabolism, 2013, 202534. https://doi.org/10.1155/2013/202534
- Janeway, C. A., & Medzhitov, R. (2002). Innate immune recognition [J]. Annual Review of Immunology, 20(1), 197–216. https://doi.org/10.1146/annurev.immunol.20.083001.084359
- Karin, M., & Ben-Neriah, Y. (2000). Phosphorylation meets Ubiquitination: The control of NF-κB activity. Annual Review of Immunology, 18(18), 621–663. https://doi.org/10.1146/annurev.immunol.18.1.621
- Karmakar, P., Pujol, C. A., Damonte, E. B., Ghosh, T., & Ray, B. (2010). Polysaccharides from Padina tetrastromatica: Structural features, chemical modification and antiviral activity. Carbohydrate Polymers, 80(2), 513–520. https://doi.org/10.1016/j.carbpol.2009.12.014
- Liang, Z. D., Zeng, Y. Y., & Huang, X. Y. (2008). The effect of Apigenin on proliferation and NO secretion and phagocytosis of RAW264.7 cells. Journal of Jinan University (Natural Science Edition), 29(1), 95–98. http://doi.org/10.3969/j.issn.1000-9965.2008.01.022
- Shao, J., Li, Y., Wang, Z., Xiao, M., Yin, P., Lu, Y., Qian, X., Xu, Y., & Liu, J. (2013). 7b, a novel naphthalimide derivative, exhibited anti-inflammatory effects via targeted-inhibiting TAK1 following down-regulation of ERK1/2- and p38 MAPK-mediated activation of NF-κB in LPS-stimulated RAW264.7 macrophages [J]. International Immunopharmacology, 17(2), 216–228. https://doi.org/10.1016/j.intimp.2013.06.008
- Simas-Tosin, F. F., Abud, A. P. R., De Oliveira, C. C., Gorin, P. A. J., Sassaki, G. L., Bucchi, D. F., & Iacomini, M. (2012). Polysaccharides from peach pulp: Structure and effects on mouse peritoneal macrophages. Food Chemistry, 134(4), 2257–2260. https://doi.org/10.1016/j.foodchem.2012.04.041
- Sweet, M. J., & Hume, D. A. (1996). Endotoxin signal transduction in macrophages. Journal of Leukocyte Biology, 60(1), 8–26. https://doi.org/10.1002/jlb.60.1.8
- Tanaka, T., Narazaki, M., Masuda, K., & Kishimoto, T. (2016). Regulation of IL-6 in immunity and diseases. In X. Ma (Ed.), Regulation of cytokine gene expression in immunity and diseases (Vol. 941, pp. 79–88). Springer Netherlands. http://doi.org/10.1007/978-94-024-0921-5_4
- Uthaisangsook, S., Day, N. K., Bahna, S. L., Good, R. A., & Haraguchi, S. (2002). Innate immunity and its role against infections. Annals of Allergy, Asthma and Immunology, 88(3), 253–265. https://doi.org/10.1016/S1081-1206(10)62005-4
- Vanden Berghe, W., Plaisance, S., Boone, E., De Bosscher, K., Schmitz, M. L., Fiers, W., & Haegeman, G. (1998). P38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. Journal of Biological Chemistry, 273(6), 3285–3290. https://doi.org/10.1074/jbc.273.6.3285
- Wang, J., Lian, P., Yu, Q., Wei, J., & Kang, W. (2017). Antithrombotic mechanism of polysaccharides in blackberry (Rubus spp.) seeds. Food & Nutrition Research, 61(1), 1379862. https://doi.org/10.1080/16546628.2017.1379862
- Wang, J., Wang, H., Zhang, H., Liu, Z., Ma, C., & Kang, W. (2019). Immunomodulation of ADPs-1a and ADPs-3a on RAW264.7 cells through NF-κB/MAPK signaling pathway. International Journal of Biological Macromolecules, 132, 1024–1030. https://doi.org/10.1016/j.ijbiomac.2019.04.031
- Wu, W., Zhu, Y., Zhang, L., Yang, R., & Zhou, Y. (2012). Extraction, preliminary structural characterization, and antioxidant activities of polysaccharides from Salvia miltiorrhiza Bunge. Carbohydrate Polymers, 87(2), 1348–1353. https://doi.org/10.1016/j.carbpol.2011.09.024
- Xiao-Xi, L., Ning, G., & Xue-Tao, C. (2008). Tumor-associated macrophage promoting growth and metastasis of tumor: An update. Chinese Journal of Cancer Biotherapy, 15(1), 78–81. https://doi.org/10.3872/j.issn.1007-385X.2008.01.017
- Xie, P., Zhang, Y., Wang, X., Wei, J., & Kang, W. (2017). Antithrombotic effect and mechanism of Rubus spp. Blackberry. Food & Function, 8(5), 2000–2012. https://doi.org/10.1039/C6FO01717G
- Yin, Z. H., Wang, J. J., & Gu, H. P. (2013). Antioxidan activity of the fruits of blackberry (Shawnee) in vitro. Nat Prod Res Dev, 25(4), 530–532. https://doi.org/10.3969/j.issn.1001-6880.2013.04.021
- Yin, Z. H., Zhang, W., Feng, F. J., & Kang, W. Y. (2014). Study on effective extracts of Rubus spp seed for reducing blood lipid. Chin J Exp Tradit Med Form, 20(24), 194–198. https://doi.org/10.13422/j.cnki.syfjx.2014240194
- Yong, Z. (2015). Study on the bioactivity constituents of Myristica fragrans Houtt. And Rubus spp. Backberry Seeds.
- Zhang, W., Yin, Z. H., Feng, J. F., & Kang, W. Y. (2014a). Effective extracts of blackberry seed for protection of acute liver injury. Food Science and Technology, 12, 94–97. https://doi.org/10.13684/j.cnki.spkj.2014.12.020
- Zhang, W., Yin, Z. H., & Kang, W. Y. (2014b). Effect of blackberry seed on Alloxan induced diabetes in mice. Sci Technol Food Ind, 19, 351–354. http://doi.org/10.13386/j.issn1002-0306.2014.19.068
- Zhao, L., Dong, Y., Chen, G., & Hu, Q. (2010). Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydrate Polymers, 80(3), 783–789. https://doi.org/10.1016/j.carbpol.2009.12.029