ABSTRACT
As a severely harmful zoonotic disease, toxoplasmosis has complex clinical symptoms with atypical lesions, thus making it difficult for diagnosis in a timely manner. Therefore, developing an appropriate technique to diagnose and prevent toxoplasmosis has become a subject of many studies. In this study, a colloidal gold immunochromatographic test strips for the diagnosis of Toxoplasma gondii was established on the basis of the optimization of antigenic epitopes of GRA1 and GRA7 genes. Western blot and ELISA results showed that expressed rGRA exhibited specifically reaction with Toxoplasma gondii-positive mouse serum. This strip could be used to diagnose the toxoplasmosis of mouse. Moreover, compared with ELISA and PCR, GICA strip exhibited similar sensitivity in the diagnosis of Toxoplasma gondii in pigs. Here, we preliminarily established a diagnostic method for rapidly detecting toxoplasmosis, which may provide technical support for large-scale screening of Toxoplasma gondii.
Introduction
Toxoplasma gondii is a ubiquitous intracellular parasite that infects both humans and warm-blooded animals (Jabs, Citation1990). It can spread vertically through the placenta after infection of humans and animals, leading to foetal malformations, premature birth, and developmental disorders of the nervous system. Approximately 30% of human population worldwide is chronically infected with T. gondii (Moncada & Montoya, Citation2012). Human infections are primarily obtained by ingesting undercooked or raw meat containing viable tissue cysts, or by ingesting food or water contaminated with T. gondii oocysts (Dubey, Citation2008; Tenter et al., Citation2000).T. gondii has no strong host selectivity and can parasitize nucleated cells in many animals, causing serious threats to human and animal health (Tenter et al., Citation2000). Thus, it is urgent to develop a novel technology for the detection of Toxoplasma gondii.
Traditional approaches for the diagnosis of toxoplasmosis include etiological, immunological and imaging techniques. Diagnosis of toxoplasmosis has been improved by the emergence of molecular technologies to amplify parasite nucleic acids. Among these, polymerase chain reaction (PCR)-based molecular techniques have been useful for the genetic characterization of T. gondii (Liu et al.,Citation2015). However, specific instruments are required in most molecular diagnostic technologies which is not conductive to mass field screening. Meanwhile, the use of immunochromatographic test(ICT) based on recombinant antigens has gained popularity as it is fast, easy to use, economical, and can be used in the field (Huang et al., Citation2004; Terkawi et al., Citation2013). Nevertheless, considerable efforts still need to be made to improve ICT in the areas of manufacturing processes, sample volume requirements and production costs (Jiang et al., Citation2015).
Dense granule proteins GRAs are a class of immunogenic proteins secreted by Toxoplasma gondii organelles. Proteins in this family can be used as antigenic markers for Toxoplasma gondii (Carey et al., Citation2000; Terkawi et al., Citation2013). Previous researches suggested that GRA protein secretion continues throughout the Toxoplasma gondii infection process. GRA1 is one member of GRA family, which is known as calcium-binding protein. It plays an important role in the packaging of secreted products and can be used as a diagnostic antigen during the infection of acute or chronic Toxoplasma gondii (Cesbron-Delauw et al., Citation1989; Wang et al., Citation2014). GRA7 protein is another dense granule protein mainly localized in the host cell cytoplasm. During the Toxoplasma bradyzoite stage, GRA7 protein directly interacts with host immune system and induces a strong immune response both in early and advanced infection stages. Therefore, GRA7 has also become an ideal antigen molecule for the detection of toxoplasma-infected serum antibody (Vazini et al., Citation2018; Weeratunga et al., Citation2017). While GRA7 has been widely employed as antigen for ELISA, there is only one study that has reported its potential as antigen for immunochromatographic test (ICT) in pigs. As ICT is easy, rapid, and convenient to perform, and no special equipment is required, it is suitable for field application.
In this study, the antigenic epitopes of GRA1 and GRA7 were analysed and reconstructed for higher specific antigenicity. Recombinant GRA plasmid was constructed via subcloning GRA sequences into PET-32a(+) vector, following by the expression of fusion protein rGRA in Escherichia coli BL21(DE3). The immunogenicity of rGRA was analysed by western blot and ELISA. Subsequently, we conjugated recombinant SPG (rSPG) with colloidal gold to obtain rSPG-gold. By using rGRA and rSPG, we developed and evaluated the colloidal gold immunochromatographic assay (GICA) strip for the detection of Toxoplasma gondii.
Materials and methods
Materials
The standard Toxoplasma gondii negative and positive BALB/c mouse sera were stored in the laboratory. 150 porcine blood samples were collected from three anonymous companies(Company A, Company B, and Company C). The plasma was separated from blood cells and then stored in – 20°C instantly.
Synthesis of the Toxoplasma gondii GRA gene sequence
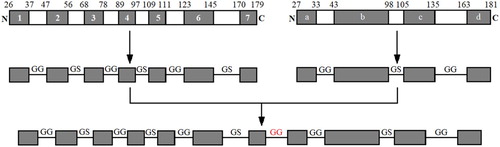
GRA1 and GRA7 antigenic epitopes for Toxoplasma gondii were analysed and the regions enriched with antigenic epitopes were linked by GG and GS codons. The rare codons of the sequences were replaced with E. coli-preferred codons subsequently. Finally, the sequences of GRA were obtained, TAA were inserted at its 3′ end and BamH1 and EcoR1 restriction sites were inserted at its 5′ and 3′ ends, respectively. The sequences of GRA were exhibited in .
Recombinant plasmid construction and expression
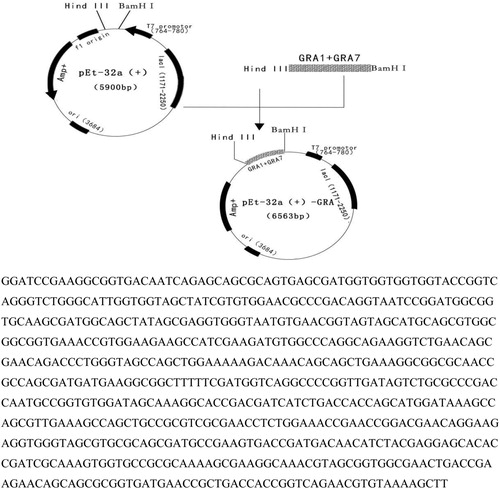
The resultant sequences were cloned into the pET-32a(+) vector (HuaGen Biotech, Shanghai, China) to generate recombinant plasmid pET-32a(+)-GRA. Then recombinant plasmid was transformed into host bacterium E. coli BL21 (DE3) (Transgen Biotech, Beijing, China). The transformants were cultured in solid LB medium containing ampicillin (50 μg/ml) for 10 h, then complete single colonies were transferred into liquid LB medium and shaken overnight at 37°C. After the identification of PCR, double enzyme digestion and sequencing, the fusion protein was expressed under the action of IPTG (Transgen Biotech, Beijing, China). Finally, rGRA was purified using Ni-NTA HisBind Resin (Novagen, Madison, WI, USA) following the manufacturer’s instructions.
Western blot assay
The purified recombinant protein was subjected to SDS-PAGE electrophoresis and transferred onto a nitrocellulose (NC) membrane (Millipore, MA, USA). After blocking with 5% non-fat milk powder for 2 h, Toxoplasma-positive BALB/c mouse serum (diluted with PBST at a ratio of 1:100) was added and incubated for 1.5 h at 37°C. Subsequently, the membranes were washed by PBST buffer for three times and incubated with HRP-conjugated goat anti-mouse IgG for another 1 h (Beijing Kangwei Century Biotech, Beijing, China). After three washes, the membrane was visualized using a HRP-DAB kit (Tiangen BioTech, Beijing, China).
Immunogenicity analysis of the recombinant protein by ELISA
Purified rGRA was diluted to 20, 10, 5, 2.5, and 1.25 μg/ml, and 100 μl of solution was added to each well and incubated at 4°C overnight. The wells were washed with PBST three times for 5 min, then blocked with 5% non-fat milk powder for 2 h. After washing, 100 μl toxoplasmosis-positive or – negative mouse sera diluted with PBST to a ratio of 1:50, 1:100, 1:200, 1:400, and 1:800 was added to each well, each dilution was performed in duplicate. The dilutions were incubated at 37°C for 1.5 h, followed by the addition of goat anti-mouse HRP-IgG (75 μl/well) for 1 h incubation at 37°C. After three time washes, 3,3’,5,5’-tetramethyl benzidine (TMB) (100 μl/well) was added to the plates and the reaction was stopped after 10 min using 2 M sulfuric acid (50 μl/well). The OD value of each well at a wavelength of 450 nm was detected using a microplate reader (Tecan, Mannedorf, Switzerland). To assess the results, P value represents the OD of positive serum, and N value represents the OD of negative serum. P/N ≥2.1 was considered to be positive, and P/N <2.1 was considered to be negative.
Colloidal gold probe preparation
All glassware used were soaked in an acid tank for 24 h, rinsed with deionized water and oven-dried before use. In a 250 ml Erlenmeyer flask, 100 ml of deionized water and 1 mL of 1% chloroauric acid were boiled thoroughly, and then 1.5 ml of trisodium citrate was added slowly. After the colour of solution changed from light yellow to darker red, the solution was further boiled for another 15 min. Subsequently, the solution was cooled to room temperature and diluted to 100 ml with deionized water. The PH of colloidal gold solution was adjusted to an optimal value of 5.0 using 0.2% K2CO3. Next, 1.2 ml of 1 mg/ml rSPG was added into gold colloid solution dropwise. After vigorously stirred for 30 min, 10 ml of 10% PEG20000 was added to block the reaction of gold colloid, and then the mixture was stirred for another 30 min. Afterwards, the mixture was centrifuged at 2000 × g for 20 min, and the pellet was discarded. The solution was collected and centrifuged again at 10,000 r/min for 30 min, then the supernatant was discarded and the pellet was resuspended in 20 mL TBS. The prepared colloidal gold probe was uniformly dropped onto the glass fibre membranes (Jieyi Biotech, Shanghai, China) at a volume of 100 μl/cm and dried in vacuum using a freeze dryer (Thermo, Waltham, MA, USA).
Colloidal gold immunochromatographic test strip preparation
The nitrocellulose (NC) membrane was attached to plastic-backed support card (Jieyi Biotech, Shanghai, China). rGRA (1.5 or 0.75 mg/mL) and rSPG (0.5 mg/mL) were transferred onto the NC member using an XYZ Biostrip Dispenser (Bio-Dot, Irvine, CA, USA) at a volume of 1 μl/cm. Afterwards, the member was oven-dried overnight at 37°C. The colloidal gold conjugate pad, sample pad, and absorbent paper (Jieyi Biotech, Shanghai, China) were laminated and pasted onto plastic-backed support card, and a guillotine cutter (CM4000 Guillotine, Bio-Dot, Irvine, CA, USA) was used to cut the assembled scale board into strips measuring 3 × 60 mm. Assembly diagram of the strip was shown as below ().
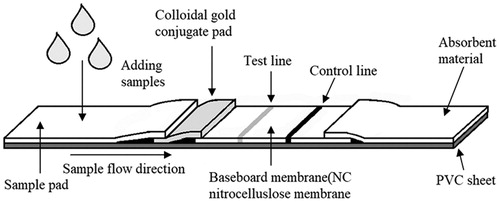
Figure 2. Schematic illustration of GICA strip. The serum is loaded onto the sample pad and the gold–rSPG conjugate is added onto the conjugate pad.rGRA is immobilized as the test line in the NC membrane. The rSPG is used as the control line. Following the application of a serum sample containing specific anti-Toxoplasma gondii IgG and non-specific IgG onto the NC membrane, the conjugated anti-Toxoplasma gondii IgG complex is captured by rGRA on the test line (T), resulting in a red band. The conjugated anti-Toxoplasma gondii IgG and nonspecific IgG are captured by the rSPG on the control line (C), resulting in another red band.
Determining the test strip detection results
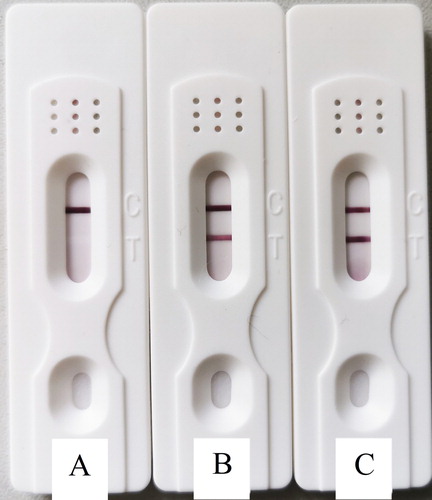
An appropriate amount of samples to be tested was dropped onto the sample pad of the test strip. After 5 min of incubation, the results were observed. If the T and C lines showed red colour, the result was considered to be positive. If the T line did not develop colour but the C line did, the sample was negative. If the C line did not develop colour, the test strip was invalid.
Results
The sequence of rGRA and the construction of recombinant plasmid
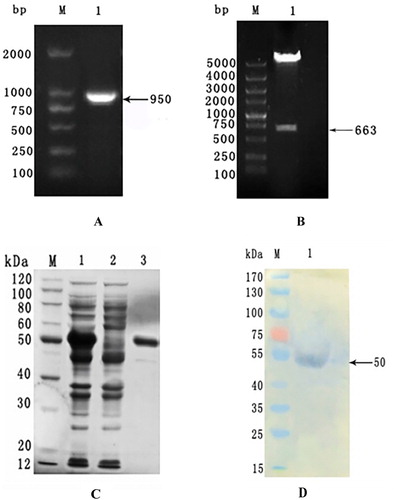
The prokaryotic expression plasmid PET-32a(+)-C3-rGRA was constructed by PCR and double enzyme digestion(). After analysing the sequence of the correct recombinant plasmid, the result illustrated that the constructed prokaryotic expression plasmid had the target genes which was approximately 663 bp.
Expression, purification and identification of rGRA
The PCR assay was used to detect bacteria solution with primers T7, and showed that the target gene was approximately 950-bp in length (containing 663-bp GRA and part of the vector sequence) (A). 663-bp GRA was separated from the recombinant plasmid after double-restriction enzyme digestion, which was consistent with our expectation (B). The soluble His-tagged rGRA with an expected size of 50 kDa was purified by affinity chromatography and verified by SDS-PAGE (C). Western blot assay was performed to identify the rGRA using Toxoplasma gondii-positive mouse serum (D).
Figure 4. rGRA expression, purification and identification. Expression, purification and identification of rGRA. A: PCR identification of bacteria solution. M: DNA marker. 1: Amplified GRA gene fragment. B: Double-enzyme digestion identification M: DNA marker. 1: Recombinant plasmid digested with restriction enzymes. C: Purification of rGRA. M: Protein marker, 1: Supernatant of the expression product; 2: Precipitate of the expression product; 3: Purified rGRA. D: Western-blot analysis of rGRA. M: Protein marker 1: Purified rGRA recognized with Toxoplasma-positive serum.
Antigenicity analysis of the recombinant rGRA protein
ELISA analysis was employed to identify the antigenicity of rGRA by diluting to different multiple, and the results showed that diluted rGRA could recognize with Toxoplasma-positive mouse serum at a dilution of 1:800 (), suggesting that the rGRA exhibited high antigenicity and had the potential to function as an antigen to distinguish Toxoplasma negative and positive serum.
Table 1. Results of ELISA assay on the detection threshold of rGRA.
Mouse serum detection using colloidal gold immuno-chromatographic test strips
When 1.5 mg/ml of recombinant rGRA protein was used for marking, it did not react with the negative mouse sera, and the result was negative (A). When 0.75 mg/ml of recombinant rGRA protein was used for marking, it did not react noticeably with the 1:10-diluted positive mouse serum, and the result was weakly positive (B). When 1.5 g/ml recombinant rGRA protein was used for marking, it reacted strongly with the 1:10-diluted positive mouse serum, and the result was strongly positive (C). This indicated that the optimal detection result was achieved when the recombinant protein marking concentration was at 1.5 mg/ml, and the sample dilution was 1:10.
Evaluating pig serum detection by the GICA method
Prepared GICA test strips were used to test serum samples collected from different pig farms, and results showed that the sample detection rates for the three pig farms were 26%, 24% and 30%, which is similar consistent with the results of ELISA and PCR (). These results suggest that this Toxoplasma gondii GICA test strip has a higher detection rate and can thus be used to rapidly diagnose Toxoplasma gondii.
Table 2. Comparison of GICA, ELISA and PCR assays.
Discussion
As a severely harmful zoonotic disease, toxoplasmosis has complex clinical symptoms with a typical lesions, thus making it difficult for diagnosis in a timely manner. Since treatment only relies on chemical drugs due to no commercial vaccines are available (Innes, Citation2010), developing an appropriate technique to diagnose and prevent toxoplasmosis has become a subject of many studies. Diagnosis of toxoplasmosis by demonstration of T. gondii is usually difficult. Bioassays using mice or cats are not suitable for rapid detection or field application, due to their price, sensitivity and the length of time to reach the results (Wang et al., Citation2014). Serological tests are still considered useful for evaluation of the infection in humans and animals. IHA, MAT, LAT, IFAT, ICT and ELISA have been used to detect T. gondii infection in dogs (Cedillo-Pelaez et al., Citation2012; Li & Liu et al., Citation2012; Li & Zhong et al., Citation2012; Tsai et al., Citation2008; Wanha et al., Citation2005). However, the sensitivity and specificity of these tests may vary between sampling methods, or reference populations, even when the same tests are used in different species. IHA has poor sensitivity and concordance when compared with ICT and ELISA, precluding their use for detection of T. gondii infection in dogs (Meireles et al., Citation2004).
As a rapid diagnostic technique, colloidal gold immunochromatographic assay (GICA) is widely used to monitor various diseases and diagnose parasitic diseases. Xu et al. used soluble egg protein from blood flukes as the antigen to develop the colloidal gold immunochromatographic test strip for detecting serum schistosomes. This diagnostic method has a simple operation, with better sensitivity and specificity (Xu et al., Citation2017). Srivastava et al. established GICA detection technology using the rK39 antigen of leishmania and the colloidal gold-labelled protein A, and results showed that the sensitivity and specificity were 97.6% and 92.6%, respectively (Srivastava et al., Citation2011). Wang et al. developed a rapid immunochromatographic strip procedure that can detect circulating antigens in the blood of animals during the acute stage of toxoplasmosis (Wang et al.,Citation2011).
Above studies also further confirmed the importance of the GICA strip in rapidly detecting parasitic diseases.
GRAs are a group of proteins secreted by electron dense granules in Toxoplasma gondii after granules enter the parasitophorous vacuole. GRA1 involves in the process of tachyzoite invasion by regulating signal transduction and mediating cellular immune responses (Gong et al., Citation2016; Li et al., Citation2010). As a candidate vaccine for Toxoplasma gondii, GRA7 also has a higher application value as a diagnostic antigen (Kotresha et al., Citation2012). Wang et al. used GRA1 and GRA7 recombinant proteins expressed in the prokaryotic system as diagnostic antigens to establish an ELISA detection method for Toxoplasma gondii, and results showed that the recombinant proteins were more sensitive and specific and could be used as potential markers for serological diagnosis of Toxoplasma gondii in canines (Wang et al., Citation2014). Sun et al. further validated the important roles of GRA1 and GRA7 recombinant proteins as a diagnose marker of toxoplasmosis in chickens (Sun et al., Citation2015). The antigenic epitope is an important functional segment of the target protein. GRA1 and GRA7 antigenicity is also primarily manifested by their antigenic epitopes. Therefore, to improve the serological detection of Toxoplasma gondii, in this study, we analysed the antigenic epitopes of GRA1 and GRA7 genes. The signal peptides and C-terminal hydrophobic sequences were excluded and B-cell epitopes for protein expression was selected to reconstruct the recombinant GRA. This recombinant GRA was then expressed in E. coli, and its antigenicity was analysed by western blot and ELISA. The results showed that the rGRA was highly expressed as a soluble protein in the E. coli expression system with better antigenicity.
To establish the GICA detection technique for Toxoplasma gondii, we used the trisodium citrate reduction method to prepare colloidal gold and label the Streptococcal recombinant protein, rSPG. The recombinant rGRA was used as the GICA test strip test lines, and the rSPG was used as the quality control line to develop the GICA Toxoplasma gondii diagnostic test strip. Positive and negative mouse sera were used for GICA assay, and results showed that the test strip labelled with rGRA could recognize with the positive, but not the negative mouse serum. Detection results for swine sera from different pig farms showed that the detection rate of Toxoplasma gondii by the GICA method was more consistent with the results of ELISA and PCR.
Consent for publication
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Acknowledgments
The authors wish to thank the Shanghai Veterinary Research Institute for facilitating the implementation of this study. ZW, JL and RX performed the experiments. ZW wrote the manuscript. YS and RJ completed the statistics part. ZW and ZC designed the study and critically. YS revised the manuscript. ZW, JL, RX, YS, RJ provided technical input on the experiments. All the authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Carey, K. L., Donahue, C. G., & Ward, G. E. (2000). Identification and molecular characterization of GRA8, a novel, proline-rich, dense granule protein of Toxoplasma gondii. Molecular and Biochemical Parasitology, 105(1), 25–37. https://doi.org/10.1016/s0166-6851(99)00160-7
- Cedillo-Pelaez, C., Diaz-Figueroa, I. D., Jimenez-Seres, M. I., Sanchez-Hernandez, G., & Correa, D. (2012). Frequency of antibodies to Toxoplasma gondii in stray dogs of Oaxaca, Mexico. Journal of Parasitology, 98(4), 871–872. https://doi.org/10.1645/GE-3095.1
- Cesbron-Delauw, M. F., Guy, B., Torpier, G., Pierce, R. J., Lenzen, G., Cesbron, J. Y., Charif H., Lepage P., Darcy F., & Lecocq J. P. (1989). Molecular characterization of a 23-kilodalton major antigen secreted by Toxoplasma gondii. Proceedings of the National Academy of Sciences, 86(19), 7537–7541. https://doi.org/10.1073/pnas.86.19.7537
- Dubey, J. P. (2008). The history of Toxoplasma gondii–the first 100 years. Journal of Eukaryotic Microbiology, 55(6), 467–475. https://doi.org/10.1111/j.1550-7408.2008.00345.x
- Gong, P., Cao, L., Guo, Y., Dong, H., Yuan, S., Yao, X., Ren, W., Yao, L., Xu, Z., Sun, Q., & Zhang, X. (2016). Toxoplasma gondii: Protective immunity induced by a DNA vaccine expressing GRA1 and MIC3 against toxoplasmosis in BALB/c mice. Experimental Parasitology, 166, 131–136. https://doi.org/10.1016/j.exppara.2016.04.003
- Huang, X., Xuan, X., Hirata, H., Yokoyama, N., Xu, L., Suzuki, N., & Igarashi, I. (2004). Rapid immunochromatographic test using recombinant SAG2 for detection of antibodies against Toxoplasma gondii in cats. Journal of Clinical Microbiology, 42(1), 351–353. https://doi.org/10.1128/jcm.42.1.351-353.2004
- Innes, E. A. (2010). A brief history and overview of Toxoplasma gondii. Zoonoses and Public Health, 57(1), 1–7. https://doi.org/10.1111/j.1863-2378.2009.01276.x
- Jabs, D. A. (1990). Ocular toxoplasmosis. International Ophthalmology Clinics, 30(4), 264–270. https://doi.org/10.1097/00004397-199030040-00009
- Jiang, W., Liu, Y., Chen, Y., Yang, Q., Chun, P., Yao, K., Han, X., Wang, S., Yu, S., Liu, Y., & Wang, Q. (2015). A novel dynamic flow immunochromatographic test (DFICT) using gold nanoparticles for the serological detection of Toxoplasma gondii infection in dogs and cats. Biosensors and Bioelectronics, 72, 133–139. https://doi.org/10.1016/j.bios.2015.04.035
- Kotresha, D., Poonam, D., Muhammad Hafiznur, Y., Saadatnia, G., Nurulhasanah, O., Sabariah, O., & Rahmah, N. (2012). Recombinant proteins from new constructs of SAG1 and GRA7 sequences and their usefulness to detect acute toxoplasmosis. Tropical Biomedicine, 29(1), 129–137. https://www.ncbi.nlm.nih.gov/pubmed/22543613/
- Li, Y., Liu, Q., Li, S., Wei, F., Jin, H., & Yang, M. (2012). Seroprevalence of Toxoplasma gondii infection in dogs in Jiangsu Province, eastern China. Journal of Parasitology, 98(4), 878–879. https://doi.org/10.1645/GE-3098.1
- Li, B., Oledzka, G., McFarlane, R. G., Spellerberg, M. B., Smith, S. M., Gelder, F. B., Kur, J., & Stankiewicz, M. (2010). Immunological response of sheep to injections of plasmids encoding Toxoplasma gondii SAG1 and ROP1 genes. Parasite Immunology, 32(9-10), 671–683. https://doi.org/10.1111/j.1365-3024.2010.01228.x
- Li, B., Zhong, N., Peng, W., Shang, L., Jin, H., & Liu, Q. (2012). Seroprevalence of Toxoplasma gondii infection in dogs in Sichuan Province, southwestern China. Journal of Parasitology, 98(1), 209–210. https://doi.org/10.1645/GE-2942.1
- Liu, Q., Wang, Z. D., Huang, S. Y., & Zhu, X. Q. (2015). Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites & Vectors, 8(1), 292. https://doi.org/10.1186/s13071-015-0902-6
- Meireles, L. R., Galisteo, A. J., Jr., Pompeu, E., & Andrade, H. F., Jr. (2004). Toxoplasma gondii spreading in an urban area evaluated by seroprevalence in free-living cats and dogs. Tropical Medicine and International Health, 9(8), 876–881. https://doi.org/10.1111/j.1365-3156.2004.01280.x
- Moncada, P. A., & Montoya, J. G. (2012). Toxoplasmosis in the fetus and newborn: An update on prevalence, diagnosis and treatment. Expert Review of Anti-Infective Therapy, 10(7), 815–828. https://doi.org/10.1586/eri.12.58
- Srivastava, P., Dayama, A., Mehrotra, S., & Sundar, S. (2011). Diagnosis of visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene, 105(1), 1–6. https://doi.org/10.1016/j.trstmh.2010.09.006
- Sun, X., Wang, Z., Li, J., Wei, F., & Liu, Q. (2015). Evaluation of an indirect ELISA using recombinant granule antigen GRA1, GRA7 and soluble antigens for serodiagnosis of Toxoplasma gondii infection in chickens. Research in Veterinary Science, 100, 161–164. https://doi.org/10.1016/j.rvsc.2015.04.011
- Tenter, A. M., Heckeroth, A. R., & Weiss, L. M. (2000). Toxoplasma gondii: From animals to humans. International Journal for Parasitology, 30(12-13), 1217–1258. https://doi.org/10.1016/s0020-7519(00)00124-7
- Terkawi, M. A., Kameyama, K., Rasul, N. H., Xuan, X., & Nishikawa, Y. (2013). Development of an immunochromatographic assay based on dense granule protein 7 for serological detection of Toxoplasma gondii infection. Clinical and Vaccine Immunology, 20(4), 596–601. https://doi.org/10.1128/CVI.00747-12
- Tsai, Y. J., Chung, W. C., Fei, A. C., Hong, C. L., Tsai, Y. Y., Peng, S., & Wu, Y. L. (2008). Prevalence of Toxoplasma gondii antibodies in stray dogs in Taipei, Taiwan. Journal of Parasitology, 94(6), 1437. https://doi.org/10.1645/GE-1445.1
- Vazini, H., Ghafarifar, F., Sharifi, Z., & Dalimi, A. (2018). Evaluation of immune responses Induced by GRA7 and ROP2 genes by DNA vaccine Cocktails Against acute toxoplasmosis in BALB/c mice. Avicenna Journal of Medical Biotechnology, 10(1), 2–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5742649/
- Wang, Z., Ge, W., Huang, S. Y., Li, J., Zhu, X. Q., & Liu, Q. (2014). Evaluation of recombinant granule antigens GRA1 and GRA7 for serodiagnosis of Toxoplasma gondii infection in dogs. BMC Veterinary Research, 10(1), 158. https://doi.org/10.1186/1746-6148-10-158
- Wang, Y. H., Li, X. R., Wang, G. X., Yin, H., Cai, X. P., Fu, B. Q., & Zhang, D. L. (2011). Development of an immunochromatographic strip for the rapid detection of Toxoplasma gondii circulating antigens. Parasitology International, 60(1), 105–107. https://doi.org/10.1016/j.parint.2010.11.002
- Wanha, K., Edelhofer, R., Gabler-Eduardo, C., & Prosl, H. (2005). Prevalence of antibodies against Neospora caninum and Toxoplasma gondii in dogs and foxes in Austria. Veterinary Parasitology, 128(3-4), 189–193. https://doi.org/10.1016/j.vetpar.2004.11.027
- Weeratunga, P., Herath, T. U. B., Kim, T. H., Lee, H. C., Kim, J. H., Lee, B. H., Lee, E.-S., Chathuranga, K., Chathuranga, W. A. G., Yang, C.-S., Ma, J. Y., & Lee, J. S. (2017). Dense granule protein-7 (GRA-7) of Toxoplasma gondii inhibits viral replication in vitro and in vivo. Journal of Microbiology, 55(11), 909–917. https://doi.org/10.1007/s12275-017-7392-5
- Xu, R., Feng, J., Hong, Y., Lv, C., Zhao, D., Lin, J., Lu, K., Li, H., Liu, J., Cao, X., Wang, T., Zai, J., Wang, Z., Jia, B., Han, Q., & Zhu, C. (2017). A novel colloidal gold immunochromatography assay strip for the diagnosis of schistosomiasis japonica in domestic animals. Infectious Diseases of Poverty, 6(1), 84. doi: 10.1186/s40249-017-0297-z