ABSTRACT
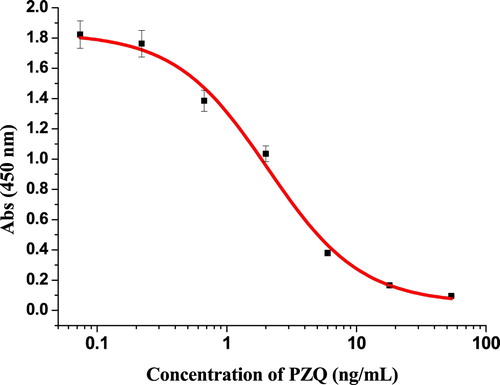
Praziquantel (PZQ) is a compound that usually used as insecticide, drug residue could be harm to human health. In this study, we have developed a simple, sensitive, and rapid lateral flow immunochromatographic assay (LFIA) for the detection of PZQ in fish (Lateolabrax Japonicus). We produced a sensitive mAb using enzyme-linked immunosorbent assay with half-maximal inhibitory concentration (2A11, IC50) of 2.06 ng/mL. The coating antigen (PZQ-HS-GA-OVA) and goat anti-mouse IgG antibody were sprayed onto a nitrocellulose membrane as the test and control lines, respectively. Under optimal conditions, the cut-off limits for PZQ detection on the test strips were determined to be 5 ng/mL in both 0.01 M phosphate buffered saline and fish samples, and results were obtained within 5 min. Our data suggests that this LFIA can be used for the sensitive, rapid, and specific on-site screening for PZQ in fish.
Introduction
Praziquantel (, PZQ) is a drug which belongs to the pyrazine and isoquinoline group of compounds. It is used as a treatment for a number of parasitic worm infections, particularly (recommended by the World Health Organization (WHO)) recommended for the treatment of schistosomiasis (Zhao et al., Citation2018). Since the 1980s PZQ has been used to prevent and treat tapeworm, Datylogyrus, Gyrodactylus, and other endo and ectoparasites found in aquaculture. It has high efficacy, needs only a short treatment regimen, and is quickly absorbed in aquatic animals. Furthermore, this compound can also be used as an insecticide (Blaylock & Bullard, Citation2014; He et al., Citation2017).
Drug residues with due to overuse of PZQ have been found to cause potential harm to human health, which can manifest as abdominal pain, vomiting, central nervous system disorders, fever, sweating, headache, and allergies (Bader et al., Citation2019; Hong, Citation2018; Zhang et al., Citation2017). Therefore, it is very important to monitor the drug residues in food and aquatic products using an easy and fast method. Many methods to detect PZQ have been described, including thin-layer chromatography (Pavlovic et al., Citation2019), high-performance liquid chromatography (Dey et al., Citation2017; Guerrero Garduno et al., Citation2016; Zrncic et al., Citation2014), and liquid chromatography-tandem mass spectrometry (Hui et al., Citation2019; Jian-Ping et al., Citation2019; Klausz et al., Citation2015; Ming-Ming et al., Citation2017). These methods are mainly used because they are highly sensitive, but the methods are time-consuming, expensive, and need professional technicians to run them (Li et al., Citation2020; Wang et al., Citation2020; Wu et al., Citation2020).
Recently, the determination of analytes using immunoassays has become popular, this approach is based on the interaction between antigen and antibody. The enzyme-linked immunosorbent assay (ELISA) has several advantages over other methods, including high specificity, high throughput of samples, it is inexpensive, and it is simple to use (Lin et al., Citation2018; Mukunzi et al., Citation2018; Zeng et al., Citation2018; Zhou et al., Citation2020). However, the lateral flow immunochromatographic assay (LFIA) is become increasingly popular for the detection of drug residues because of its rapidity (result within 5–10 min), simplicity, and ease of use. Unlike ELISAs, all reagents are sprayed onto the test strip with the LFIAs (Guo et al., Citation2019; Liu et al., Citation2019; Song et al., Citation2019; Xu et al., Citation2019). Rapid detection of PZQ using ELISAs and LFIAs in mackerel samples has been developed recently with a 50 ng/mL limit of detection (LOD) (Shen et al., Citation2019). In this study however, we aim to develop a more sensitive assay to detect PZQ by optimizing the strip test for use in the Japanese sea bass (Lateolabrax japonicus).
Materials and methods
Chemicals and materials
PZQ was obtained from J&K Scientific Ltd. (Shanghai, China). 3,3,5,5′-Tetramethylbenzidine, bovine serum albumin (BSA), and ovalbumin (OVA) were supplied from Sigma-Aldrich (St Louis, MO, USA). Gelatine, Freund’s complete adjuvant (FCA), and Freund’s incomplete adjuvant (FIA) were from Beijing Biodee Biotechnology Co., Ltd. (Beijing, China). The cell fusion reagents were acquired from Sunshine Biotechnology Co., Ltd. (Nanjing, China). All other chemicals and reagents were attained from the National Pharmaceutical Group Chemical Reagent Co, Ltd. (Shanghai, China).
Nitrocellulose (NC) was purchased from Whatman-Xinhua Filter Paper Co., Ltd. (Hangzhou, China), glass fibre membrane (CB-SB08), polyvinylchloride (PVC) backing material, and an absorbent pad (SX18) from Goldbio Tech Co., Ltd. (Shanghai, China). A Membrane Strip Reader GIC-HG (Wuxi Determine Bio-Tech Co., Ltd, Wuxi (China) was acquired for the detection of colour intensity on the test and control lines. Ultrapure water (Milli-Q purification system, Millipore Co., Ltd. (Bedford, MA, USA) was acquired for the preparation of all buffers and a strip cutting instrument was model HM3035 from Gold Bio (Shanghai, China).
Preparation of coating antigen and characterization of monoclonal anti-PZQ antibody
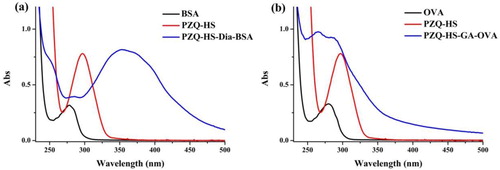
The hapten-PZQ (PZQ-HS) was synthesized by our laboratory (Shen et al., Citation2019). The hapten-PZQ was conjugated to OVA using the glutaraldehyde method. Briefly, 10 mg of hapten-PZQ was dissolved in N,N-dimethylformamide (DMF, 500 µL) and then mixed and stirred with 5% glutaraldehyde at 4°C in a dark chamber for 10 min (solution 1). Solution 2 was a dilution of OVA (10 mg) in carbonate buffer (2 mL), and then solution 1 was added dropwise to solution 2. After stirring for 1 h at 4°C, the PZQ-HS-OVA was dialysed against 0.01 M PBS for 3 d. PZQ-HS-OVA was used as coating antigen and characterized by UV/Vis spectroscopy ((b)).
Figure 2. Result of UV/vis spectrometry for, (a) PZQ-HS, BSA, and PZA-HS-Dia-BSA, and (b) PZQ-HS, OVA, and PZQ-HS-GA-OVA.
We produced the monoclonal antibody (mAb) from female BALB/c mice (6–8 weeks old). Immunizing mice using PZQ-HS-dia-BSA and the hapten-PZQ was coupled with BSA using the diazonium method. DMF (500 µL) was used to dissolve 10 mg of hapten-PZQ and the solution was mixed with 1 M HCl (50 µL). After 30 min of stirring at 4°C in a dark chamber, added 30% of NaNO2 (5.56 µL) at 4°C (solution 1). BSA was diluted using 0.05 M carbonate buffer (solution 2). Then added solution 1 into solution 2, and after stirring at 4°C for 6 h, the PZQ-HS-dia-BSA ((a)) was dialysed with PBS (0.01 M) for 3 d, and kept at −20°C for further use.
FCA was used for the first immunization (100 µg), and in the next three weeks followed by FIA for subsequent intensive injection (50 µg, 2–5 times). The antibodies in the blood of immunized mice were detected using ELISA method. The mice with the best titre were sacrificed and the spleen cells were fused with murine myeloma cells of Sp 2/0, continue with screening the hybridomas with an icELISA. The best hybridoma cells were amplified and injected into mice to produce the mAb. Ascites was collected and using caprylic acid-ammonium sulphate precipitation method to purified the mAb (Chen et al., Citation2014), and then stored mAb at −20°C.
Development of the LFIA
Preparation of gold nanoparticles (GNPs)
A solution of HAuCl4 (10 mM; 12.5 mL) mixed with filtered ultrapure water (487.5 mL), then boiled and constantly stirred for a further 15 min. 10% sodium citrate tribasic dehydrate (1 mL) (w/v) was added and then stirred (approximately 30 min) and the mixture became a red-wine colour. Then the solution was cooled to room temperature and prior to antibody labelling, the GNPs were stored at 4°C.
The half-maximal inhibitory concentration (2A11, IC50) of the purified anti-PZQ mAb using ELISAs method was 2.06 ng/mL (), which was used to prepare mAb labelled by colloidal gold (CG). Firstly, we added 0.1 M of K2CO3 (80 µL) into the CG solution (10 mL), then added 0.4 mL of purified mAb (adjusted to a final concentration of 0.2 mg/mL with 0.02 M of borate buffer) into the CG, and the mixture was allowed to stand at room temperature for 35 min. At room temperature, 1 mL of 10% (w/v) BSA was used to block free CG. After 2 h of blocking step, the mixture was centrifuged for 45 min at 8000 rpm and 4°C. After centrifugation, removed the supernatant and then added the 1 mL of resuspension buffer (0.02 M Tris-HCl, 0.1% Tween-20, 0.1% PEG, 1% PVP, 5% sucrose, 4% trehalose, 2% sorbitol, 1% mannitol. 0.04% NaN3, and 0.2% BSA), and the CG-labelled mAb was stored at 4°C.
Strip performance
The sensitivity of this assay was determined by testing PZQ standards, which were used at the following concentrations: 0, 0.1, 0.25, 0.5, 1, 2.5, and 5 ng/mL in 0.01 M PBS (pH 7.4). The strip performed as firstly mixed the GNP-labelled mAb (50 µL) with sample extract (100 µL), reacted for 5 min, and then added the strip test onto the mixture. After 5 min, the high concentration of PZQ produced a visible colour reaction, and its intensity was different from that of 0 ng/mL of PZQ represented the LOD. Six replicates were analysed for each concentration on the same day and the LOD seen was defines for each concentration with the naked eye.
Determination of PZQ in Lateolabrax japonicus
Fish (Lateolabrax japonicus) were purchased from a local market (Wuxi, China). Fish tissue mass was minced and kept at −20°C. Approximately 2 g fish tissue was mixed with ethyl acetate (4 mL) in 50 mL centrifuge tube and vortex (10 min), then centrifuge at 8000 rpm for 10 min at 4°C. The resulting clear liquid was dried under nitrogen (40°C). After dried completely, the residue was dissolved with PBS plus 0.5% of Tween-20 (10 mL).
PZQ with different standards concentration were spiked into fish samples with final concentration was 0, 0.1, 0.25, 0.5, 1, 2.5, and 5 ng/mL. Each concentration was repeated six times.
Results and discussion
Antigen characterization
The conjugation of the hapten-PZQ with BSA was performed using the diazonium method and OVA using the glutaraldehyde method, and the reactions were confirmed by UV/vis spectrometry at a wavelength of 250–500 nm. confirms that the hapten-PZQ (PZQ-HS) has a characteristic absorbance at 300 nm, whereas the carried proteins (BSA and OVA) showed absorbance at 280 nm. The peak absorbance of PZQ-HS-Dia-BSA changed significantly to close to 280 nm and a broad peak was seen near 350–400 nm. Whereas the peak absorbance for PZQ-HS-GA-OVA at 280 nm was wider and higher than that of the carrier protein, which indicated that the hapten-protein conjugates were successfully synthesized.
Principle and optimization of the LFIA
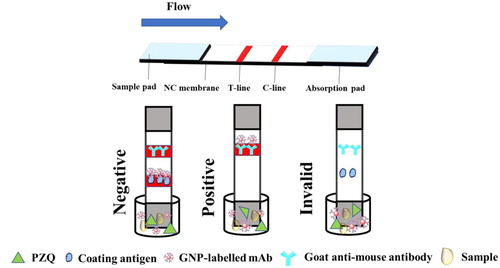
Three parts of the strip test are shown in . The first part is a sample pad for running the sample, the second part is the NC membrane that is sprayed with the coating antigen (PZQ-HS-GA-OVA, test line) and goat anti-mouse IgG antibody as control line, and the last part is the absorbent pad, to prevent liquid leakage. The principle of the LFIA, involves firstly adding the mAb labelled with GNP (50 µL) into 150 µL of sample solution. After mixing, the sample is reacted for 5 min, and then the test strip is added. The results can be seen within 5 min, after the solution flows completely to the absorbent pad. Therefore, in successful test, the control line will always have red band.
If the LFIA is negative results, the immobilized PZQ-HS-GA-OVA conjugate captures a limited amount of CG-labelled mAb, and a clear red band on control and test lines appears. However, if the sample is positive for PZQ, the GNP-labelled mAb reacts with the PZQ to produce a lighter test line, or a test line with no colour. If no colour appears on test and control lines, the test procedure has failed and the strip should be repeated.
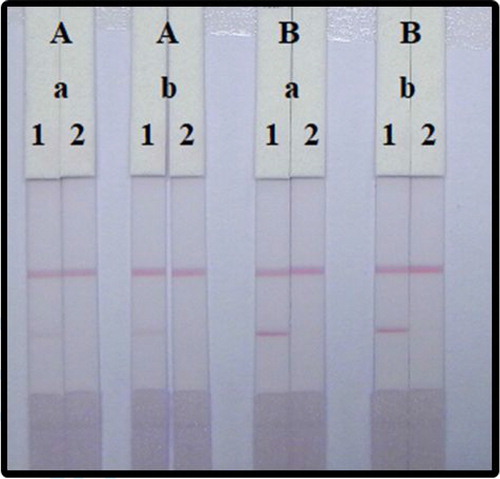
Both mAbs attached to GNPs and coated antigen concentrations can affect the sensitivity of detection. We compare two different amounts of mAb with GNP (8 and 10 µg/mL) and reacted with negative and positive (10 ng/mL) samples. As shown in , the colour intensity between 8 and 10 µg/mL had slight differences. According to the colour intensity of the two lines, the best concentration of mAb with the GNPs was 10 µg/mL. There was also different colour intensity in 0.3 and 0.8 mg/mL of coated antigen, and we found that the concentration of antigen with 0.3 mg/mL has weaker colour intensity than 0.8 mg/mL. Therefore, the optimal to detect PZQ in 0.01 M PBS was using 0.8 mg/mL coated antigen and 10 µg/mL for the GNP-labelled mAb.
Figure 5. Optimization of LFIA with 0.01 M PBS (pH 7.4). The concentration of coating antigen: (A) 0.3 mg/mL and (B) 0.8 mg/mL. The dosage of mAb that conjugate with GNP; (a) 10 µg/mL, and (b) 8 µg/mL. Standard concentration; (1) 0 ng/mL, and (2) 10 ng/mL.
In this study, we examined the series of PZQ standards for the sensitivity of the strip. Compared the negative control (no PZQ added) with the amount sample that added PZQ, both had significant difference in the colour intensity. In , the higher concentration of PZQ changed the colour signal into weak from very deep colour at 0 ng/mL, and finally no colour appears at 5 ng/mL PZQ. As shown in , the results show that this mAb specific into PZQ.
Figure 6. LFIA of detection PZQ in 0.01 M PBS (7.4). PZQ concentration: 1 = 0 ng/mL, 2 = 0.1 ng/mL, 3 = 0.25 ng/mL, 4 = 0.5 ng/mL, 5 = 1 ng/mL, 6 = 2.5 ng/mL, and 7 = 5 ng/mL.
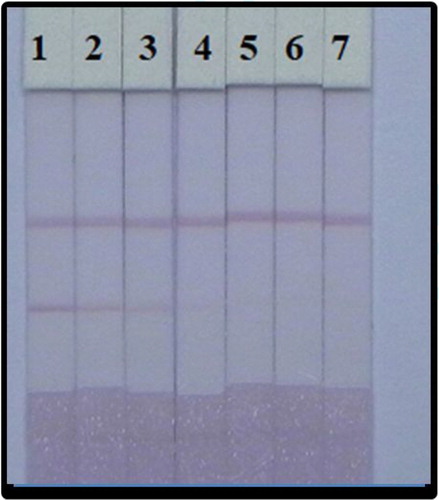
Table 1. Cross-reaction results for the assay selectivity using ELISAs.
Determination of PZQ in Lateolabrax japonicus
To verify the validity of this method, the muscle samples of Lateolabrax japonicus collected in Wuxi, China local market were tested and no PZQ residue was detected in samples. The detection of drug residues in food is very important because it has potential impact on human health. Therefore, screening needs fast and accurate methods. However, fish tissues contain a lot of protein and fat, which need to be extracted before screening using LFIA. This sample preparation is a very important step in the analysis method of matrix effect evaluation.
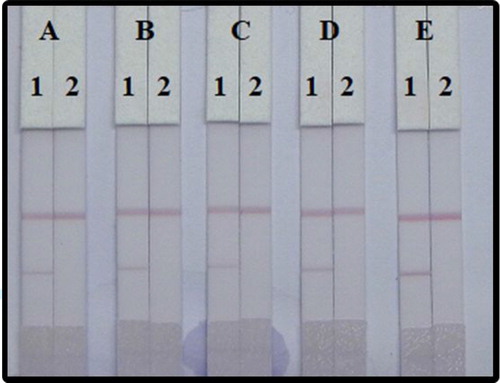
To optimize the assay, the choice of surfactant reagent is very important, as it can have a major influence on the assay results. We added the resuspension buffer with four different surfactants including polyvinylpyrrolidone (PVP), polyethylene glycol (PEG), BSA, and Rhodasurf® On-870. As shown , the addition of Rhodasurf® On-870 to the basic buffer gave the best results and was therefore, used for subsequent experiments.
Figure 7. Optimization LFIA spiked in fish. 5 different kinds of reagents; (A) basic buffer, (B) PVP, (C) PEG, (D) BSA, and (E) Rhodasurf® On-870. Standard concentration; (1) 0 µg/mL, and (2) 10 ng/mL.
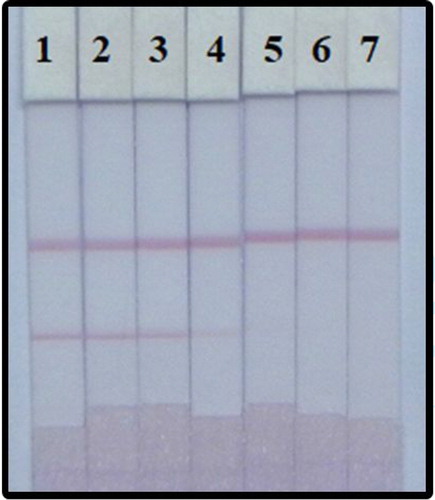
We examined the performance of the LFIA in determination of PZQ in fish samples. The fish samples were spiked with a PZQ standard with final concentration was 0, 0.1, 0.25, 0.5, 1, 2.5, and 5 ng/mL. As result seen in , the optimal conditions to detect PZQ in fish are using: coated antigen (0.8 mg/mL), 10 µg/mL of the mAb with GNPs, and Rhodasurf® On-870 as the surfactant in the matrix. Under these conditions results using our developed LFIA can be seen within 5 min and the cut-off concentration of PZQ in fish using the naked eye is 5 ng/mL.
Figure 8. Determination of PZQ spiked in fish. After optimization (1–7) represents concentrations of 0, 0.1, 0.25, 0.5, 1, 2.5, and 5 ng/mL.
There have been several previous studies to determine PZQ using diverse methods; for example, the use of RP-HPLC in bulk powder with an LOD of 0.56 µg/mL (Hashem et al., Citation2017); in bovine muscle using HPLC with a quantification limit of 0.02 mg/kg and a detection limit of 0.01 mg/kg (Sun & Bu, Citation2012), and in edible bovine tissues using liquid chromatography-tandem mass spectrometry with an LOD of 1 mug/kg and at a minimum limit of quantification of 2.5 mug/kg. In conclusion, the chromatographic assays remain the most common high sensitivity assay for the determination of analytes. However, the advantages of our developed, LFIA for the detection of PZQ residues include its simplicity, sensitivity, and rapid detection time (within 5 min).
Conclusions
We established a rapid and sensitive assay to detect PZQ in fish sample with a sensitive coating antigen (PZQ-HS-GA-OVA) and anti-PZQ mAb (2A11, IC50 2.06 ng/mL) using LFIA. After optimal conditions, to detect PZQ dilutes in 0.01 M PBS the cut-off was found to be 5 ng/mL. After optimization using 0.8 mg/mL coated antigen, 10 µg/mL of mAb with GNP, and Rhodasurf® On-870 as surfactant, the cut-off value of PZQ in fish samples was 5 ng/mL. All results can be obtained within 5 min.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bader, C., Starling, D. E., Jones, D. E., & Brewer, M. T. (2019). Use of praziquantel to control platyhelminth parasites of fish. Journal of Veterinary Pharmacology and Therapeutics, 42, 139–153. https://doi.org/10.1111/jvp.12735
- Blaylock, R. B., & Bullard, S. A. (2014). Counter-insurgents of the blue revolution? Parasites and diseases affecting aquaculture and science. Journal of Parasitology, 100(6), 743–755. https://doi.org/10.1645/14-605.1
- Chen, X., Xu, L., Ma, W., Liu, L., Kuang, H., Wang, L., & Xu, C. (2014). General immunoassay for pyrethroids based on a monoclonal antibody. Food and Agricultural Immunology, 25(3), 341–349. https://doi.org/10.1080/09540105.2013.794328
- Dey, S., Ghosh, M., Rangra, N. K., Kant, K., Shah, S. R., Pradhan, P. K., & Singh, S. (2017). High-performance liquid chromatography determination of praziquantel in rat plasma; application to pharmacokinetic studies. Indian Journal of Pharmaceutical Sciences, 79, 885–892. https://doi.org/10.4172/pharmaceutical-sciences.1000304
- Guerrero Garduno, O., Gonzalez-Esquivel, D. F., Escalante-Membrillo, C., Fernandez, A., Susana Rojas-Tome, I., Jung Cook, H., & Castro, N. (2016). Comparison of a high-performance liquid chromatography method for quantification of carbamazepine with chemiluminescent microparticle immunoassay. Biomedical Chromatography, 30(6), 933–937. https://doi.org/10.1002/bmc.3631
- Guo, L., Liu, L., Cui, G., Ma, S., Wu, X., & Kuang, H. (2019). Gold immunochromatographic assay for kitasamycin and josamycin residues screening in milk and egg samples. Food and Agricultural Immunology, 30(1), 1189–1201. https://doi.org/10.1080/09540105.2019.1677567
- Hashem, H., Ibrahim, A. E., & Elhenawee, M. (2017). A rapid stability indicating LC-method for determination of praziquantel in presence of its pharmacopoeial impurities. Arabian Journal of Chemistry, 10, S35–S41. https://doi.org/10.1016/j.arabjc.2012.07.005
- He, L., Gao, F., Li, E., Lee, J. T., Bian, L., & Armstrong, D. W. (2017). Chromatographic separation of racemic praziquantel and its residual determination in perch by LC-MS/MS. Talanta, 174, 380–386. https://doi.org/10.1016/j.talanta.2017.05.026
- Hong, S.-T. (2018). Albendazole and praziquantel: Review and safety monitoring in Korea. Infection and Chemotherapy, 50(1), 1–10. https://doi.org/10.3947/ic.2018.50.1.1
- Hui, R., Gao, Y., Liu, J., Liu, H., Tang, C., & Feng, B. (2019). Determination of an isonicotinylhydrazide derivative, a novel positive inotropic compound, in mouse plasma by LC-MS/MS and its application to a pharmacokinetics study. Journal of Pharmaceutical and Biomedical Analysis, 165, 12–17. https://doi.org/10.1016/j.jpba.2018.10.034
- Jian-Ping, W., Jing, Z., Song-Song, G., Feng, Y., Juan, P., & Shi-Xin, H. (2019). Determination of praziquantel in edible bovine tissues by dispersive solid phase extraction combined with liquid chromatography-tandem mass spectrometry. Journal of Food Safety and Quality, 10, 3379–3389.
- Klausz, G., Keller, E., Sara, Z., Szekely-Koermoeczy, P., Laczay, P., Ary, K., Sotonyi, P., & Rona, K. (2015). Simultaneous determination of praziquantel, pyrantel embonate, febantel and its active metabolites, oxfendazole and fenbendazole, in dog plasma by liquid chromatography/mass spectrometry. Biomedical Chromatography, 29(12), 1859–1865. https://doi.org/10.1002/bmc.3507
- Li, S., Wu, X., Kuang, H., & Liu, L. (2020). Development of an ic-ELISA and an immunochromatographic strip assay for the detection of aconitine. Food and Agricultural Immunology, 31(1), 243–254. https://doi.org/10.1080/09540105.2020.1714555
- Lin, L., Jiang, W., Xu, L., Liu, L., Song, S., & Kuang, H. (2018). Development of IC-ELISA and immunochromatographic strip assay for the detection of flunixin meglumine in milk. Food and Agricultural Immunology, 29(1), 193–203. https://doi.org/10.1080/09540105.2017.1364710
- Liu, Z., Liu, L., Cui, G., Wu, X., & Kuang, H. (2019). Development of an immunochromatographic strip assay based on a monoclonal antibody for detection of cimaterol. Food and Agricultural Immunology, 30(1), 1162–1173. https://doi.org/10.1080/09540105.2019.1674787
- Ming-Ming, S., Lu, Y., Ying-Gang, Z., & Ying, J. (2017). Simultaneous determination of praziquantel residues in feed and bovine serum by liquid chromatography-tandem mass spectrometry. Journal of Food Safety and Quality, 8, 2263–2266.
- Mukunzi, D., Suryoprabowo, S., Song, S., Liu, L., & Kuang, H. (2018). Development of an indirect enzyme-linked immunosorbent assay and lateral-flow test strips for pefloxacin and its analogues in chicken muscle samples. Food and Agricultural Immunology, 29(1), 484–497. https://doi.org/10.1080/09540105.2017.1406460
- Pavlovic, D. M., Kraljevic, T. G., Pavic, R., & Mrda, J. (2019). Determination of anthelmintic pharmaceuticals in wastewater by solid-phase extraction and thin-layer chromatography. JPC - Journal of Planar Chromatography - Modern TLC, 32(5), 421–429. https://doi.org/10.1556/1006.2019.32.5.10
- Shen, X., Liu, L., Xu, L., Ma, W., Wu, X., Cui, G., & Kuang, H. (2019). Rapid detection of praziquantel using monoclonal antibody-based ic-ELISA and immunochromatographic strips. Food and Agricultural Immunology, 30(1), 913–923. https://doi.org/10.1080/09540105.2019.1641068
- Song, S., Suryoprabowo, S., Liu, L., Kuang, H., Xu, L., Ma, W., & Wu, X. (2019). Development of monoclonal antibody-based colloidal gold immunochromatographic assay for analysis of halofuginone in milk. Food and Agricultural Immunology, 30(1), 112–122. https://doi.org/10.1080/09540105.2018.1550058
- Sun, Y., & Bu, S.-J. (2012). Simple, cheap and effective high-performance liquid chromatographic method for determination of praziquantel in bovine muscle. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 899, 160–162. https://doi.org/10.1016/j.jchromb.2012.05.004
- Wang, Z., Wu, X., Liu, L., Xu, L., Kuang, H., & Xu, C. (2020). Rapid and sensitive detection of diclazuril in chicken samples using a gold nanoparticle-based lateral-flow strip. Food Chemistry, 312, 126116. https://doi.org/10.1016/j.foodchem.2019.126116
- Wu, X., Suryoprabowo, S., Kuang, H., & Liu, L. (2020). Detection of aminophylline in serum using an immunochromatographic strip test. Food and Agricultural Immunology, 31(1), 33–44. https://doi.org/10.1080/09540105.2019.1691508
- Xu, X., Liu, L., Cui, G., Wu, X., & Kuang, H. (2019). Development of an immunochromatography assay for salinomycin and methyl salinomycin in honey. Food and Agricultural Immunology, 30(1), 995–1006. https://doi.org/10.1080/09540105.2019.1649370
- Zeng, L., Jiang, W., Liu, L., Song, S., & Kuang, H. (2018). Development of ic-ELISA and lateral-flow immunochromatographic strip for detection of vitamin B-2 in an energy drink and vitamin tablets. Food and Agricultural Immunology, 29(1), 121–132. https://doi.org/10.1080/09540105.2017.1360257
- Zhang, D., Wang, H., Ji, J., Nie, L., & Sun, D. (2017). A quantification method for determination of racemate praziquantel and R-enantiomer in rat plasma for comparison of their pharmacokinetics. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 1048, 64–69. https://doi.org/10.1016/j.jchromb.2017.02.013
- Zhao, M., Meng, R., Lu, Y., Hu, L., Sun, N., Nie, L., & Wang, H. (2018). A useful quantitative model for determination of enantiomeric composition of racemate praziquantel by ultraviolet spectroscopy combined with partial least squares and its application to praziquantel tablets. Journal of Innovative Optical Health Sciences, 11(03), 1850011. https://doi.org/10.1142/S1793545818500116.
- Zhou, S., Kuang, H., & Liu, L. (2020). Development of an ic-ELISA and colloidal gold strip for the detection of the beta-blocker carazolol. Food and Agricultural Immunology, 31(1), 217–230. https://doi.org/10.1080/09540105.2019.1710113
- Zrncic, M., Gros, M., Babic, S., Kastelan-Macan, M., Barcelo, D., & Petrovic, M. (2014). Analysis of anthelmintics in surface water by ultra high performance liquid chromatography coupled to quadrupole linear ion trap tandem mass spectrometry. Chemosphere, 99, 224–232. https://doi.org/10.1016/j.chemosphere.2013.10.091