ABSTRACT
Diazinon is an organophosphate insecticide commonly used worldwide to chemically control different organisms considered as pests. In the past years, diazinon has been described to have immunotoxic effects in several species. Nile tilapia is a teleost fish with an ecological and economic importance, which is also one of the fishes with the highest consumption around the world. The aim of this study was to evaluate the production of neutrophil extracellular traps (NETs) from fish Nile tilapia (Oreochromis niloticus) exposed to diazinon (0.97, 1.95, and 3.91 mg/L) for 6 and 24 h. The results obtained indicate that the exposure to diazinon per se increases NETs production. Therefore, these results could be involved in the understanding of the inflammatory process induced by exposure to pesticides.
Introduction
Diazinon (DZN,O,O-DiethylO-[4-methyl-6-(propan-2-yl)pyrimidin-2yl]phosporothioat-e) is an organophosphate pesticide (OP) used as insecticide in agricultural, livestock, and domestic activities (CICOPLAFEST, Citation2004; EPA, Citation2006; Rasoulifard et al., Citation2015). This pesticide inhibits the activity of acetylcholinesterase enzyme (AChE), which leads to the accumulation of acetylcholine (ACh) neurotransmitter at axon terminals of organisms exposed to this type of insecticides. In consequence, there are toxic effects in the peripheral and central nervous systems (Al-Ghanim, Citation2012; El-Bouhy et al., Citation2016; Khoshbavar-Rostami et al., Citation2006). However, clear evidence in later years demonstrates that DZN also have immunotoxic effects in different organisms. In addition, it has been demonstrated that the acute exposure to DZN induces alterations in adaptive immunity parameters; still, the effects of DZN on innate immunity parameters are a few (Ahmadi et al., Citation2014; Banaee et al., Citation2008; Hajirezaee et al., Citation2019; Soltanian et al., Citation2018). Our research group previously reported that DZN reduces phagocytic ability and increases reactive oxygen species (ROS) production (Covantes-Rosales et al., Citation2019); however, the effects of DZN on the functionality of other types of innate cells, as neutrophils, have been scarcely explored. Neutrophils are innate immune cells relevant to the defense against pathogen agents, they are characterized by being the most abundant circulating cells and have several microbicide mechanisms against bacteria, fungi, and parasites (Rosales, Citation2018).
For a long time, neutrophils were believed to act against pathogen agents through ingestion and degradation (phagocytosis), the release of cytotoxic substances into the extracellular medium through degranulation, and the production of ROS and RNS (Brinkmann et al., Citation2004; Dale et al., Citation2008). Additionally, an extracellular defense mechanism, consisting in the release of NETs, has also been described (Medina, Citation2009). These traps are extracellular structures mostly constituted by nets of DNA and histones (H1, H2A, H2B, H3, and H4) and, to a lesser degree, microbicide proteins, such as elastase, cathepsin G, myeloperoxidase, lysozyme, and gelatinase (Palić et al., Citation2007; Papayannopoulos, Citation2018). The fibrous structure of NETs creates a physical barrier that prevents the dissemination of microorganisms and promotes the local concentration of antimicrobial peptides, which reduces damage in adjacent tissues and allows for the elimination of different pathogens (Brinkmann et al., Citation2004; Papayannopoulos, Citation2018). For over 10 years, our research group has used Nile tilapia (O. niloticus) as a model to evaluate the immunotoxic effects of OPs.
The Nile tilapia (O. niloticus) is a teleost fish with an ecological and economic importance around the world (distributed across most of the tropical and subtropical freshwater bodies). Also, commercially, O. niloticus belong to the group most harvested fish (769,936 tons) to worldwide. Therefore, it is high consumption among the population. It has been previously published that the acute exposure to DZN causes alterations in the proliferative ability of lymphocytes, phagocytosis index, and mechanisms related to intracellular signal transduction; additionally, it increases ROS production and leads to lymphocyte cell death (Covantes-Rosales et al., Citation2019; Díaz-Resendiz et al., Citation2019). However, there are no reports to date on the effect that DZN exposure can have on NETs production. Therefore, the aim of the present work was to evaluate the ability of Nile tilapia (O. niloticus) neutrophils to produce NETs when acutely exposed to sublethal concentrations of DZN.
Materials and methods
Fishes
Male Nile tilapia fishes (273 ± 43 g and 20 ± 3 cm) were acquired from a local fish farm. The fish were kept under optimal conditions (26 ± 2°C, pH 8.0) for acclimation in 400-L tanks for 4 weeks. They were fed daily with Nutripec, Purina® (3% of their weight). All animal experiments were carried out in accordance with directive 2010/63/EU of the European parliament and of the council for animal experiments.
Before the bioassays, the fish were transferred to 30-L glass tanks (1 fish/tank) and were kept there for 24 h; temperature and ventilation were constant to reduce stress. The fish were not fed during this period to avoid prandial effects and faecal waste during the bioassay. After the acclimation period, the fish (n = 7) were exposed in vivo to 0.97, 1.95, and 3.91 mg/L of commercial DZN (25% active ingredient) for 6 or 24 h. The bioassays were static and without water replacement. The control group included organisms maintained under the same conditions but without pesticide.
Formation of NETS
NETs were assessed according to the methodology described by Hosseinzadeh et al. (Citation2012) Blood samples (3 mL) were collected by cardiac puncture and placed on Histopaque-1119 (3 mL) for centrifugation at 2500 rpm/40 min. Total leukocytes were collected, mixed with phosphate-buffered saline (PBS), and centrifuged at 3500 rpm/15 min. The cells were homogenized in PBS (1 mL) and layered on Histopaque-1077 (2 mL) for centrifugation at 2500 rpm/20 min. The lower ring (neutrophils) was recovered, mixed with PBS, and centrifuged at 3500 rpm/15 min. Cells were resuspended in PBS (1 mL) and viability was determined using 0.4% trypan blue. Neutrophils were transferred on coverslips (2.5 × 105 cells in 1 mL RPMI medium) and placed in 6-well plates for incubation at 37°C, 5% CO2 for 1 h. Cells were then fixed with 4% paraformaldehyde for 20 min, permeabilized with Triton X-100 (0.5%, 1 min) and washed with PBS. Adhered cells were incubated with anti-DNA/Histone H1 (Merck-Millipore MAB 3864) diluted in saline solution (1:5000) at room temperature for 1 h. Coverslips were washed with PBS, added Alexa Fluor® 488 conjugate (490/525 nm) (Ab172324, Abcam) diluted in PBS (1:1000), and incubated in the dark at room temperature for 1 h. Finally, the sample was incubated overnight with 500 µL 4', 6-diamidino-2-phenylindole (DAPI) (350/470 nm) (P36931, Molecular Probes) for DNA staining. Using a fluorescence microscope (OPTIKA B-1000FL-HBO), NETs were observed. Fluorescence images of 10 random fields were captured with a 20X lens. Images were processed with Image J (v 1.47) to identify the DAPI-stained area and compare different DZN treatments.
Determination of NETS
NETs were determined in neutrophils stimulated with phorbol 12-myristate 13-acetate (PMA, 200 ng/mL) for 3 h. The quantification of NETs was performed using the software IMAGEJ and measured the staining of DAPI outside of the cell. The stimuli were placed in the cell suspension before fixation with paraformaldehyde. Samples were processed afterwards as described in the previous paragraph. The statistical analysis was carried out with SigmaPlot® (v 10.0). Data were analysed with a one-way analysis of variance, followed by Dunn’s post-hoc test. Statistical significance was determined by p < .05.
Results
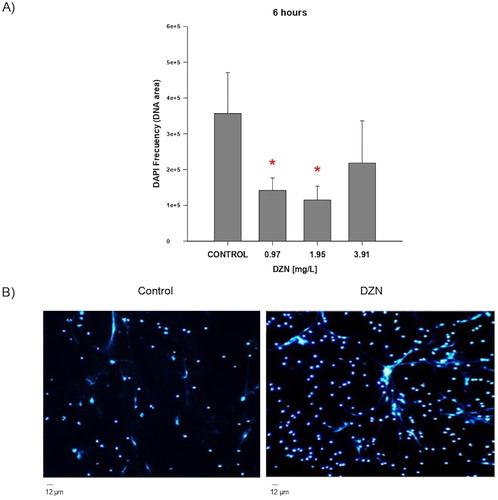
The results obtained indicate that the acute exposure to DZN in three concentrations (0.97, 1.95, and 3.91 mg/L) evaluated at 6 and 24 h significantly increases the formation of NETs in Nile tilapia (O. niloticus) as compared against basal NETs formation in control cells ((A,B)). However, when NETs formation was analysed in cells of fish exposed to DZN (0.97, 1.95, and 3.91 mg/L) for 6 h and incubated for 3 h with PMA (200 ng/mL) as mitogenic stimulus, results showed that neutrophils of control fish (not exposed to DZN) were significantly unable to respond to the stimulus by PMA ((A,B)).
Figure 1. NETs release in fish exposed to diazinon (DZN). (A) NETs production in controls and fish exposed in vivo to 0.97, 1.95, and 3.91 mg/L DZN for 6 and 24 h (n = 7). Data were analysed by one-way analysis of variance and Dunn’s test. *Statistically significant difference in NETs production in fish exposed with respect to control group (p < .05). Each bar represents ± SD; values were calculated after measuring ten randomly selected microscopic fields. (B) Representative image of NETs (stained with DAPI) from control neutrophils (not exposed to DZN) and exposed to 0.97 mg/L DZN for 24 h. Scale bar 12 µm.
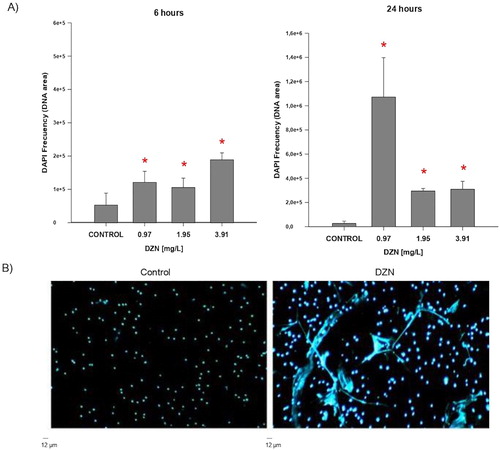
Figure 2. NETs release in fish exposed to diazinon (DZN) + PMA. (A) NETs production in controls and fish exposed in vivo to 0.97, 1.95, and 3.91 mg/L DZN for 6 h (n = 7). Before quantifying NETs, cells were incubated with PMA (200 ng/mL). Data were analysed through one-way ANOVA and Dunn’s test. *Statistically significant difference in NETs production in fish exposed with respect to control group (p < .05). Each bar represents the average ± SD. Values were calculated from the measurements in 10 randomly searched 10 microscopic fields. (B) Representative NETs image (stained with DAPI) of controls (not exposed to DZN) and neutrophils exposed to 0.97 mg/L DZN for 6 h. Before NETs quantification, cells were incubated with PMA (200 ng/mL) for 3 h. Scale bar, 12 µm.
Discussion
The Nile tilapia is relevant to worldwide due to the importance economic and aquaculture, the immunotoxic effect of pesticides affects the life of these fishes and probably has effects in the food for human consumption.
Organophosphate pesticides represent around 50% of the total pesticides used worldwide (Casida & Quistad, Citation2004) and, together with their metabolites, they have neurotoxic effects that inhibit the activity of enzyme AChE (Flaskos, Citation2012). Additionally, these compounds have been commonly related to immunosuppressive effects (Díaz-Resendiz et al., Citation2015; Galloway & Handy, Citation2003). In this context, there are reports relating OPs exposure to a high release of pro-inflammatory cytokines, which leads to the development of neurodegenerative and metabolic diseases (obesity and diabetes), as well as different types of cancer (Alavanja et al., Citation2004; Gangemi et al., Citation2016; Juntarawijit & Juntarawijit, Citation2018; Lerro et al., Citation2015; Rathish et al., Citation2016; Sánchez-Santed et al., Citation2016; Slotkin, Citation2011; Yan et al., Citation2016).
Diazinon is OP widely used in agriculture; however, it has been reported that DZN can reduce the number of leukocytes and relative weight of lymphoid organs, such as spleen, thymus, and lymph nodes (Handy et al., Citation2002). Regarding innate immune parameters, it has been reported that the exposure to DZN induces a significant reduction in the phagocytic ability but increases ROS production in this type of cells (Covantes-Rosales et al., Citation2019). This is likely related to the results obtained in this investigation since, as demonstrated, the exposure to DZN per se significantly induces NETs production; this phenomenon could trigger inflammatory processes. Studies in chlorpyrifos (OP) and others compounds (avermectin, atrazine, and cadmium) in carp neutrophils cause the release of ROS and activation of pro-apoptotic proteins; however, the production of NETs are in inhibited (Jiaxin et al., Citation2020; Wang et al., Citation2019; Zhang et al., Citation2019; Zheng et al., Citation2020). This evidence is in contradiction with our results; nevertheless, the exposure to selenium in carp neutrophils increased the formation of NETs similar to our data. This could be due to the generation of different metabolites by each of the compounds or their participation in different signalling pathways (mTOR, ERK).
The overactivation of neutrophils and NETs production by exposure to DZN could be key factors to the inflammatory processes that characterize these diseases. Interestingly, in our work neutrophils from fish exposed to DZN do not increase NETs production in the presence of PMA, as occurs in the control group not exposed to DZN. These can suggest that exposure to DZN cause anergy or senescence. Similar results were previously reported when evaluating the intracellular calcium flux (Díaz-Resendiz et al., Citation2015; Girón-Pérez et al., Citation2007).
The acute exposure to DZN clearly causes immunotoxicity (immunosuppression or exacerbation of immune response). The molecular mechanisms involved in this type of phenomena remain unclear, although several mechanisms have been proposed given that pesticides exert direct immunotoxicity; it has also been hypothesized that the immunotoxic effect is induced by OPs secondary metabolites, as dialkylphosphates Alluwaimi & Hussein, Citation2007; Medina-Buelvas et al., Citation2019). The alteration of the leukocyte cholinergic system could be another OPs immunotoxicity mechanism. Our research group has demonstrated that the exposure to DZN increases the concentration of ACh neurotransmitter, decreases AChE activity, and reduces nAChR and mAChR levels in mononuclear cells (Toledo-Ibarra et al., Citation2016).
The toxic effect of DZN on neutrophils could be the result of nAChR and mAChR overstimulation. There is clear evidence that this type of cells is able to respond to stimulus through these receptors. For instance, the use of agonists as ACh or nicotine modifies chemotactic activity, induces degranulation, and alters ROS production and cytokine (IL-8) synthesis (Covantes-Rosales et al., Citation2019). In fact, Hosseinzadeh et al. (Citation2016) and Lee et al. (Citation2017) have confirmed the role of nAChR in NETs release; the treatment with nicotine (10 µM), nAChR agonist, blocks Akt deactivation and induces NETs formation, while nAChRα7 block NETs release (Hosseinzadeh et al., Citation2016). This represents a similar scenario to that of DZN where an excess of agonist ACh and the overstimulation of AChR are induced by the exposure to the pesticide. In turn, these could induce an increase in the NETs levels here reported; these structures could potentially damage fish tissues inducing the inflammatory process (Farrera & Fadeel, Citation2013). A previously reported study demonstrates that the treatment with nicotine (500–1500 µg/mL) and several stimuli (C5a, leukotriene-ß4, IL-8, and fMLP) modifies the chemotactic ability and reduces the phagocytic ability of neutrophils (Seow et al., Citation1994). Still, PMA is the most commonly used stimulus to induce NETs; it is a non-physiological compound that imitates diacylglycerol and is a potent physiological activator of neutrophils (Appelberg, Citation2007).
The release of NETs induced by PMA is mediated by Akt activation; however, it has also been described that Akt inhibition induces apoptosis. Then, Akt can modulate the induction of apoptosis or NETosis (Hosseinzadeh et al., Citation2016) state that the structure and kinetics of NETs production are different when induced by nicotine (cholinergic agonist) or PMA. In this context, the present study observed that the treatment with DZN (which causes excess of ACh) and exposure to PMA decreased the ability to release NETs in neutrophils exposed against controls (without pesticide). In addition, our research group has previously demonstrated that the acute exposure to sublethal DZN doses induces apoptosis, senescence, and alterations in signal transduction mechanisms (MAPK activity and calcium flux) in leukocytes of Nile tilapia (Díaz-Resendiz et al., Citation2019) and increases ROS levels in granulocytes (Covantes-Rosales et al., Citation2019).
This is in agreement with the findings by Hosseinzadeh et al. (Citation2016) who demonstrated the involvement of mitochondrial ROS in NETs formation, a phenomenon related to calcium flux. This study provides clear evidence of an imbalance in the NETs response of organisms exposed to DZN. It must be considered that NETs play a key role in controlling extracellular antigens. However, producing an excess of NETs basally (without antigenic stimulus) can induce exacerbated inflammation and, in consequence, organic insufficiency and even death if the regulatory mechanisms fail (Kenny et al., Citation2017; Liu et al., Citation2017). Therefore, this investigation provides a new overview of how the exposure to DZN can induce inflammatory diseases through the excessive release of NETs in neutrophils of Nile tilapia and this causes negative effects in the health of these fishes.
Acknowledgments
Covantes-Rosales C. E. was a PhD student from the “Doctorado en Ciencias Biológico- Agropecuarias” graduate program at the Universidad Autónoma de Nayarit (México). Toledo-Ibarra G. A. is a doctoral student from “Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM)”. The first and second authors received a grant from CONACyT-México [425359 and 509635, respectively].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmadi, K., Mirvaghefei, A. R., Banaee, M., & Vosoghei, A. R. (2014). Effects of long-term diazinon exposure on some immunological and haematological parameters in rainbow trout Oncorhynchus mykiss (Walbaum, 1792). Toxicology and Environmental Health Sciences, 6(1), 1–7. https://doi.org/10.1007/s13530-014-0181-1
- Al-Ghanim, K. A. (2012). Acute toxicity and effects of sub-lethal malathion exposure on biochemical and haematological parameters of Oreochromis niloticus. Scientific Research Essays, 7(16), 1674–1680. https://doi.org/10.5897/SRE12.039
- Alavanja, M. C., Hoppin, J. A., & Kamel, F. (2004). Health effects of chronic pesticide exposure: Cancer and neurotoxicity. Annual Review of Public Health, 1(25), 155–197. https://doi.org/10.1146/annurev.publhealth.25.101802.123020
- Alluwaimi, A. M., & Hussein, Y. (2007). Diazinon immunotoxicity in mice: Modulation of cytokines level and their gene expression. Toxicology, 236(1–2), 123–131. https://doi.org/10.1016/j.tox.2007.04.004
- Appelberg, R. (2007). Neutrophils and intracellular pathogens: Beyond phagocytosis and killing. Trends in Microbiology, 15(2), 87–92. https://doi.org/10.1016/j.tim.2006.11.009
- Banaee, M., Mirvagefei, A. R., Rafei, G. R., & Majazi Amiri, B. (2008). Effect of sub- lethal diazinon concentrations on blood plasma biochemistry. International Journal of Environmental Research, 2(2), 189–198. https://doi.org/10.22059/IJER.2010.193
- Brinkmann, V., Reichard, U., Goosmann, C., Fauler, B., Uhlemann, Y., Weiss, D. S., & Zychlinsky, A. (2004). Neutrophil extracellular traps kill bacteria. Science, 303(5663), 1532–1535. https://doi.org/10.1126/science.1092385
- Casida, J. E., & Quistad, G. B. (2004). Organophosphate toxicology: Safety aspects of non-acetylcholinesterase secondary targets. Chemical Research in Toxicology, 17(8), 983–998. https://doi.org/10.1021/tx0499259
- CICOPLAFEST. (2004). Comisión intersecretarial para el control del proceso y uso de plaguicidas, fertilizantes y sustancias toxicas – Secretaria de salud. Retrieved February 1, 2020, from http://www.siicex.gob.mx/portalSiicex/SICETECA/Acuerdos/Regulaciones/SSA/cicoplafest.htm
- Covantes-Rosales, C. E., Toledo-Ibarra, G. A., Diaz-Resendiz, K. J. G., Ventura-Ramón, G. H., Pavón, L., & Girón-Pérez, M. I. (2019). Modulation of the extraneuronal cholinergic system on main innate response leukocytes. Journal of Neuroimmunology, 327(3), 22–35. https://doi.org/10.1016/j.jneuroim.2019.01.008
- Dale, D. C., Boxer, L., & Liles, W. C. (2008). The phagocytes: Neutrophils and monocytes. Blood, The Journal of the American Society of Hematology, 112(4), 935–945. http://doi.org/10.1182/blood-2007-12-077917
- Díaz-Resendiz, K. J. G., Ortiz-Lazareno, P. C., Covantes-Rosales, C. E., Trujillo-Lepe, A. M., Toledo-Ibarra, G. A., Ventura-Ramón, G. H., & Girón- Pérez, M. I. (2019). Effect of diazinon, an organophosphate pesticide, on signal transduction and death induction in mononuclear cells of Nile tilapia fish (Oreochromis niloticus). Fish & Shellfish Immunology, 89(3), 12–17. https://doi.org/10.1016/j.fsi.2019.03.036
- Díaz-Resendiz, K. J. G., Toledo-Ibarra, G. A., & Girón-Pérez, M. I. (2015). Modulation of immune response by organophosphorus pesticides: Fishes as a potential model in immunotoxicology. Journal of Immunology Research, 21(3), 8–36. https://doi.org/10.1155/2015/213836
- El-Bouhy, Z., El-Nobi, G., Reda, R. M., & Ibrahim, R. (2016). Effect of insecticide “chlorpyrifos” on immune response of Oreochromis niloticus. Zagazig Veterinary Journal, 44(3), 196–204. https://doi.org/10.21608/zvjz.2016.7872
- EPA, U.S. Environmental Protection Agency. (2006). Pesticides industry sales and usage: (2006–2007) Market Estimates. Environmental Protection Agency. Retrieved February 1, 2020, from http://www.panna.org/sites/default/files/EPA%20market_estimates2007.pdf, 2011.
- Farrera, C., & Fadeel, B. (2013). Macrophage clearance of neutrophil extracellular traps is a silent process. The Journal of Immunology, 191(5), 2647–2656. https://doi.org/10.4049/jimmunol.1300436
- Flaskos, J. (2012). The developmental neurotoxicity of organophosphorus insecticides: A direct role for the oxon metabolites. Toxicology Letters, 209(1), 86–93. https://doi.org/10.1016/j.toxlet.2011.11.026
- Galloway, T., & Handy, R. (2003). Immunotoxicity of organophosphorous pesticides. Ecotoxicology, 12(1-4), 345–363. https://doi.org/10.1023/A:1022579416322
- Gangemi, S., Gofita, E., Costa, C., Teodoro, M., Briguglio, G., Nikitovic, D., & Fenga, C. (2016). Occupational and environmental exposure to pesticides and cytokine pathways in chronic diseases. International Journal of Molecular Medicine, 38(4), 1012–1020. https://doi.org/10.3892/ijmm.2016.2728
- Girón-Pérez, M. I., Santerre, A., Gonzalez-Jaime, F., Casas-Solis, J., Hernández-Coronado, M., Peregrina-Sandoval, J., & Zaitseva, G. (2007). Immunotoxicity and hepatic function evaluation in Nile tilapia (Oreochromis niloticus) exposed to diazinon. Fish & Shellfish Immunology, 23(4), 760–769. https://doi.org/10.1016/j.fsi.2007.02.004
- Hajirezaee, S., Rafieepour, A., Shafiei, S., & Rahimi, R. (2019). Immunostimulating effects of Ginkgo biloba extract against toxicity induced by organophosphate pesticide, diazinon in rainbow trout, Oncorhynchus mykiss: Innate immunity components and immune-related genes. Environmental Science and Pollution Research, 26(9), 8798–8807. https://doi.org/10.1007/s11356-019-04327-7
- Handy, R. D., Abd-El Samei, H. A., Bayomy, M. F. F., Mahran, A. M., Abdeen, A. M., & El-Elaimy, E. A. (2002). Chronic diazinon exposure: Pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse. Toxicology, 172(1), 13–34. https://doi.org/10.1016/S0300-483X(01)00575-3
- Hosseinzadeh, A., Messer, P. K., & Urban, C. F. (2012). Stable redox- cycling nitroxide tempol inhibits NET formation. Frontiers in Immunology, 1(3), 3–91. http://doi.org/10.1189/jlb.3AB0815-379RR
- Hosseinzadeh, A., Thompson, P. R., Segal, B. H., & Urban, C. F. (2016). Nicotine induces neutrophil extracellular traps. Journal of Leukocyte Biology, 100(5), 1105–1112. https://doi.org/10.1189/jlb.3AB0815-379RR
- Jiaxin, S., Shengchen, W., Yirong, C., Shuting, W., & Shu, L. (2020). Cadmium exposure induces apoptosis, inflammation and immunosuppression through CYPs activation and antioxidant dysfunction in common carp neutrophils. Fish & Shellfish Immunology, 99, 284–290. https://doi.org/10.1016/j.fsi.2020.02.015
- Juntarawijit, C., & Juntarawijit, Y. (2018). Association between diabetes and pesticides: A case-control study among Thai farmers. Environmental Health and Preventive Medicine, 23(1), 3–24. https://doi.org/10.1186/s12199-018-0692-5
- Kenny, E. F., Herzig, A., Krüger, R., Muth, A., Mondal, S., Thompson, P. R., & Zychlinsky, A. (2017). Diverse stimuli engage different neutrophil extracellular trap pathways. Elife, 6(3), 24–43. http://doi.org/10.7554/eLife.24437
- Khoshbavar-Rostami, H. A., Soltani, M., & Hassan, H. M. D. (2006). Immune response of great sturgeon (Huso huso) subjected to long-term exposure to sublethal concentration of the organophosphate, diazinon. Aquaculture, 256(1–4), 88–94. https://doi.org/10.1016/j.aquaculture.2006.02.041
- Lee, J., Luria, A., Rhodes, C., Raghu, H., Lingampalli, N., Sharpe, O., & Sokolove, J. (2017). Nicotine drives neutrophil extracellular traps formation and accelerates collagen-induced arthritis. Rheumatology, 56(4), 644–653. http.//doi.org/10.1093/rheumatology/kew449
- Lerro, C. C., Koutros, S., Andreotti, G., Friesen, M. C., Alavanj a, M. C., Blair, A., & Zhang, Y. (2015). Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the agricultural health study. Occupational and Environmental Medicine, 72(10), 736–744. https://doi.org/10.1136/oemed-2014-102798
- Liu, T., Wang, F. P., Wang, G., & Mao, H. (2017). Role of neutrophil extracellular traps in asthma and chronic obstructive pulmonary disease. Chinese Medical Journal, 130(6), 730–743. https://doi.org/10.4103/0366-6999.201608
- Medina-Buelvas, D. M., Estrada-Muñiz, E., Rodríguez-Sosa, M., Shibayama, M., & Vega, L. (2019). Increased heart fibrosis and acute infection in a murine Chagas disease model associated with organophosphorus pesticide metabolite exposure. Scientific Reports, 9(1), 1–12. https://doi.org/10.1038/s41598-018-37186-2
- Medina, E. (2009). Neutrophil extracellular traps: A strategic tactic to defeat pathogens with potential consequences for the host. Journal of Innate Immunity, 1(3), 176–180. https://doi.org/10.1159/000203699
- Palić, D., Andreasen, C. B., Ostojić, J., Tell, R. M., & Roth, J. A. (2007). Zebrafish (Danio rerio) whole kidney assays to measure neutrophil extracellular trap release and degranulation of primary granules. Journal of Immunological Methods, 319(1-2), 87–97. https://doi.org/10.1016/j.jim.2006.11.003
- Papayannopoulos, V. (2018). Neutrophil extracellular traps in immunity and disease. Nature Reviews Immunology, 18(2), 134–138. https://doi.org/10.1038/nri.2017.105
- Rasoulifard, M. H., Akrami, M., & Eskandarian, M. R. (2015). Degradation of organophosphorus pesticide diazinon using activated persulfate: Optimization of operational parameters and comparative study by Taguchi's method. Journal of the Taiwan Institute of Chemical Engineers, 57(17), 77–90. https://doi.org/10.1016/j.jtice.2015.05.014
- Rathish, D., Agampodi, S. B., Jayasumana, M. A. C. S., & Siribaddana, S. H. (2016). From organophosphate poisoning to diabetes mellitus: The incretin effect. Medical Hypotheses, 91(1), 53–55. https://doi.org/10.1016/j.mehy.2016.04.002
- Rosales, C. (2018). Neutrophil: A cell with many roles in inflammation or several cell types? Frontiers in Physiology, 9(7), 113–113. https://doi.org/10.3389/fphys.2018.00113
- Sánchez-Santed, F., Colomina, M. T., & Hernández, E. H. (2016). Organophosphate pesticide exposure and neurodegeneration. Cortex, 74(3), 417–426. https://doi.org/10.1016/j.cortex.2015.10.003
- Seow, W. K., Thong, Y. H., Nelson, R. D., MacFarlane, G. D., & Herzberg, M. C. (1994). Nicotine-induced release of elastase and eicosanoids by human neutrophils. Inflammation, 18(2), 119–127. https://doi.org/10.1007/BF01534553
- Slotkin, T. A. (2011). Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reproductive Toxicology, 31(3), 297–301. https://doi.org/10.1016/j.reprotox.2010.07.012
- Soltanian, S., Fallahi, R., & Fereidouni, M. S. (2018). Effects of diazinon on some innate resistance parameters in the Caspian pond turtle (Mauremys caspica caspica). Bulgarian Journal of Veterinary Medicine, 21(2), 47–55. https://doi.org/10.15547/bjvm.1038
- Toledo-Ibarra, G. A., Díaz-Resendiz, K. J. G., Pavón-Romero, L., Rojas- García, A. E., Medina-Díaz, I. M., & Girón-Pérez, M. I. (2016). Effects of diazinon on the lymphocytic cholinergic system of Nile tilapia fish (Oreochromis niloticus). Veterinary Immunology and Immunopathology, 17(6), 58–63. https://doi.org/10.1016/j.vetimm.2016.05.010
- Wang, S., Zhang, Q., Zheng, S., Chen, M., Zhao, F., & Xu, S. (2019). Atrazine exposure triggers common carp neutrophil apoptosis via the CYP450s/ROS pathway. Fish & Shellfish Immunology, 84, 551–557. https://doi.org/10.1016/j.fsi.2018.10.029
- Yan, D., Zhang, Y., Liu, L., & Yan, H. (2016). Pesticide exposure and risk of Alzheimer’s disease: A systematic review and meta-analysis. Scientific Reports, 6(1), 1–9. https://doi.org/10.1038/s41598-016-0001-8
- Zhang, Q., Wang, S., Zheng, S., Zhang, Z., & Xu, S. (2019). Chlorpyrifos suppresses neutrophil extracellular traps in carp by promoting necroptosis and inhibiting respiratory burst caused by the PKC/MAPK pathway. Oxidative Medicine and Cellular Longevity, 1(1), 63–89. https://doi.org/10.1155/2019/1763589
- Zheng, S., Wang, S., Zhang, Q., Zhang, Z., & Xu, S. (2020). Avermectin inhibits neutrophil extracellular traps release by activating PTEN demethylation to negatively regulate the PI3K-ERK pathway and reducing respiratory burst in carp. Journal of Hazardous Materials, 389(1), 21–85. https://doi.org/10.1016/j.jhazmat.2019.121885
