ABSTRACT
In developing countries, pesticides such as triazophos (TZP) are widely used in agriculture. However, the misuse TZP poses a threat to human health and the environment due to its high chemical and photochemical stability. In this study, we developed a lateral flow assay to determine TZP in cucumber. We produced anti-TZP monoclonal antibody with half inhibitory concentration of 2.013 ng/mL according to ELISA. Under optimum conditions, the lateral flow assay had TZP cut-off values of 100 ng/mL in 0.01 M PBS and 250 ng/mL in cucumber, and the results could be visualised with the naked eye in 5 min. our findings revealed that lateral flow assay is a rapid and sensitive method for detect TZP in cucumber.
Introduction
In the past two decades, the use of chemical pesticides has increased in an effort to control a large number and variety of insects, mites, and fungi. However, the misuse of pesticides has adverse effects on the quality and safety of agricultural and livestock products (e.g. fruits, vegetables, cereals, and milk). Pesticide residues can emerge in ground water, soil, sediment, and air (Huang et al., Citation2016; Pirsaheb et al., Citation2017; Xu et al., Citation2012).
Triazophos/ triazofos (TZP, C12H16N3O3PS; ) is an organophosphate pesticide used in acaricides, insecticides, and nematicides. Several Asian countries use TZP in vegetables, rice, tea, and fruits (Hong et al., Citation2019; Huan et al., Citation2016). However, the widespread of TZP presented a risk to human health. The toxicity of TZP had attracted TZP exposure can lead to sweating, blurred vision, headaches, dizziness, weakness, muscle spasms, coma, mental confusion, anorexia, and diarrhea (Holden et al., Citation2001; Wu et al., Citation2018; Yang et al., Citation2019; Zhang et al., Citation2017). Therefore, a sensitive and rapid detection of TZP residues in foods is necessary.
In general, chromatography and mass spectrometry such as gas chromatography, high performance liquid chromatography, and liquid chromatography-tandem mass spectrometry (Chen et al., Citation2016; Huang et al., Citation2019; Ling-Li et al., Citation2016; Rafique et al., Citation2016; Reinholds et al., Citation2016) have excellent sensitivity and reliability for TZP detection. However, these methods are expensive, time consuming, and require experienced technicians (Chao et al., Citation2020; Chen et al., Citation2019; Shen et al., Citation2019).
Immunoassays based on antibodies and antigens are potentially sensitive methods of TZP. The enzyme-linked immunosorbent assay (ELISA) has been used for TZP detection (Gui et al., Citation2006; Hong et al., Citation2018), which is highly specific and require minimum sample preparation, is effective high throughput applications; however, the assay is time consuming (Wu et al., Citation2020; Xu et al., Citation2020; Zhang et al., Citation2019). The lateral flow immunochromatography assay (LFA) is easy, fast (5–10 min), and sensitive (Kong et al., Citation2019; Li et al., Citation2020; Wang et al., Citation2020; Zeng et al., Citation2020). In this study, we developed the LFA for detect TZP in cucumber.
Materials and methods
TZP was obtained from J&K Scientific (Shanghai, China). Complete Freund’s adjuvant (FCA) and incomplete Freund’s adjuvant (FIA) were purchased from Sigma (St. Louis, MO, USA). Reagents for cell fusion were acquired from Sunshine Biotechnology Co., Ltd. (Nanjing, China). Bovine serum albumin (BSA) and keyhole-limpet hemocyanin (KLH) were supplied by Solarbio Science & Technology, Co, Ltd. (Beijing, China).
Nitrocellulose (NC) membrane was obtained from Whatman-Xinhua Filter Paper Co., Ltd. (Hangzhou, China). Glass fibre membrane (CB-SB08) used for the sample pad, polyvinylchloride (PVC) backing material and absorbance pad (SX18) were purchased from Goldbio Tech Co., Ltd. (Shanghai, China). Our laboratory developed the coating antigen (TZP-EDC-BSA) and specific monoclonal antibodies (mAb, 3G9). Ultrapure water (Milli-Q purification system, Millipore Co., Bedford, MA, USA) was used in the preparation of buffers. We used CM 4000 (Gene, Shanghai, China) as a strip cutting instrument.
Preparation and characterization of monoclonal anti-TZP antibody
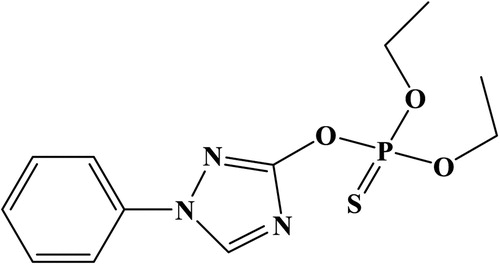
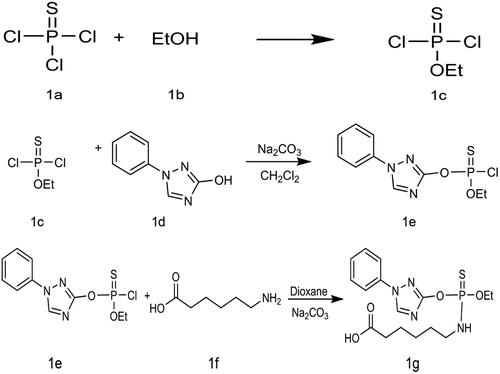
TZP-hapten was synthesised in our lab in . First measure 15 mL of thiophosphoryl chloride (1a) was stirred vigorously at −5–0°C for 2 h, slowly added to 30 mL of absolute ethanol (1b). The mixture was shaken 2–3 times in ice water to remove ethanol and HCl. After the organic layer was allowed to dry over anhydrous sodium sulfate, a colourless liquid was obtained (1c).
Weight 483 g of 3-hydroxy-1-phenyl-1,2,4-triazole (1d), added 5 mL of dicloromethane and sodium carbonate under cool condition (0°C), then dropwise 1c (1.07 g) with stirring, monitored with thin layer chromatography (TLC), then extracted with dichloromethane after the reaction was completed. The flash column chromatograph gave a purified product (1e).
To the purified product (1e, 303 mg, 1 mmol), we added 5 mL of dioxane and stirred at 0°C. Subsequently, we added 2 mL of aqueous Na2CO3 (424 mg, 4 mmol) and 1f (157 mg, 1.2 mmol) dropwise under constant stirring. While monitoring using TLC, we adjusted the pH value, and extracted with dichloromethane, column chromatography to obtain the final product (1 g). 1H NMR (600 MHz, CDCl 3) δ 8.35 (s, 1H), 7.69–7.58 (m, 2H), 7.48 (t, J = 8.0 Hz, 2H), 7.39 (d, J = 7.4 Hz, 1H), 4.30 (dd, J = 9.4, 7.1 Hz, 2H), 3.53 (s, 1H), 3.16 (s, 2H), 2.34 (t, J = 7.4 Hz, 2H), 1.69–1.62 (m, 2H), 1.61–1.54 (m, 2H), 1.40 (t, J = 7.1 Hz, 5H).
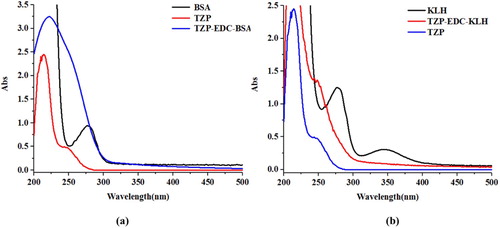
Hapten TZP was conjugated with BSA and KLH. Hapten TZP (3 mg) was dissolved in N,N-dimethylformamide (300 µL; solution A). N-hydroxysuccinimide (2.6 mg) was dissolved in MES buffer solution (100 µL; solution B). 1-(3-Dimethylaminopropyl)-3-etylcarbodiimide hydrochloride (4.3 mg) was dissolved into MES buffer (100 µL; solution C). Solution B was mixed with solution A at room temperature. After 10 min, solution C was added, and the reaction was stirred at room temperature for 6 h. KLH and BSA were diluted with 0.01 M carbonate buffer (4 mg/mL). The activation solution was added slowly, drop by drop, and the reaction was stirred at room temperature. After 8–10 h, the solution was dialysed against PBS solution (0.01 M), and the dialysate was changed every 4 h for 3 d. The conjugate identified by using UV spectrophotometry ().
Figure 3. UV spectra characterisation for; (a) BSA, TZP, and TZP-EDC-BSA, and (b) KLH, TZP, and TZP-EDC-KLH.
Female BALB/c mice (6–8 weeks of age) were used to produce anti-TZP mAb. The mice were immunised with TZP-EDC-KLH in FCA (100 µg), and in FIA (50 µg) every three weeks (2–5 times). The mouse with the highest titre was sacrificed. Sp 2/0 murine myeloma cells were fused with splenocytes. For mAb production, we injected the best hybridoma cells into the mice (Suryoprabowo et al., Citation2014). The resulting ascites were purified and mAb were stored at −20°C.
Development of LFA
Preparation of colloidal gold particles
HAuCl4 (10 mM; 12.5 mL) mixed with filtered ultrapure water (487.5 mL) was boiled and stirred for 15 min. Sodium citrate tribasic dehydrate (1 mL; 10%) (w/v) was added and stirred for 30 min, when the mixture turned red-wine colour. The solution was allowed to cool at room temperature, and the gold nanoparticles (GNPs) were stored at 4°C.
According to ELISA, the half-maximal inhibitory concentration (IC50, 3G9) of purified anti-TZP mAbs was 2.013 ng/mL. To produce colloidal gold (CG)-labelled mAb, we added 0.1 M of K2CO3 (80 µL) to the CG solution (10 mL) followed by purified mAbs (0.4 mL, adjusted to 0.2 mg/mL with 0.02 M borate buffer). The mixture was allowed to stand at room temperature for 45 min. BSA (1 mL, 10% (w/v)) was used to block free CG for 2 h. The mixture was centrifuged at 8,000 rpm for 45 min at 4°C. After centrifugation, the supernatant was discarded. We added the resuspension buffer (1 mL, 0.02 M Tris-HCl, 0.1% Tween-20, 0.1% PEG, 1% PVP, 5% sucrose, 4% trehalose, 2% sorbitol, 1% mannitol. 0.04% NaN3, and 0.2% BSA), and the CG-labeled mAbs were stored at 4°C.
Strip performance
The sensitivity of the assay was evaluated by analysing a TZP standards at different concentrations (0, 2.5, 5, 10, 25, 50, and 100 ng/mL) in 0.01 M PBS (pH 7.4). The GNP-labelled mAbs (50 µL) were allowed to react with the sample (150 µL) for 5 min, and subsequently the strip test was added into mixture. After 5 min, the high concentration of TZP produced a visible colour reaction, and its intensity was different from that of 0 ng/mL of TZP represented the LOD. Six replicates were analysed for each concentration on the same day, and LOD was assessed for each concentration with the naked eye.
Detection of TZP in cucumber
Cucumbers were purchased at a local market (Wuxi, China) and crushed (40 g). the resulting cucumber juice was spiked with TZP (final concentrations: 0, 10, 25, 50, 100, and 250 ng/mL). The experiment was repeated six times.
Results and discussion
Principle of LFA
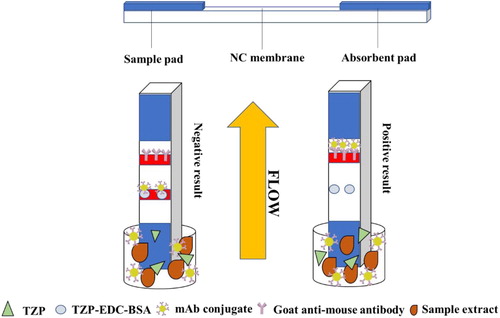
The LFA strip has three components: sample pad, NC membrane, which includes the control and test lines (TZP-EDC-BSA), and absorbent pad (). The sample reaches the control line. In a properly assembled strip, the control line turns red. In TZP-positive samples, TZP competes with the coating antigen (TZP-EDC-BSA), and free TZP binds to mAbs. In TZP-negative samples, mAbs will TZP-EDC-BSA, resulting in red test and control lines.
Optimisation of strip test
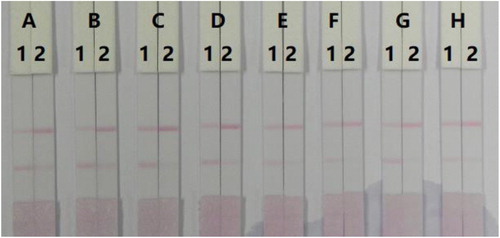
The LFA components must be hydrophilic. Even though the NC membrane is hydrophobic, the addition of reagents can render it hydrophilic. Reagents or surfactants are one of the important ingredients and may affect in developing the assay. In this study, we used resuspension buffer with 5% of polyvinylpyrrolidone (PVP), polyethylene glycol (PEG), BSA, sucrose, Tween-20, Brij-30, Triton X-100, or Rhodasurf® On-870. shows that resuspension buffer with 5% of Brij-30 generated a stable colour with 50 ng/mL TZP.
Figure 5. Result of optimisation 8 kinds of surfactant in 0.01 M PBS. (A) PVP, (B) PEG, (C) BSA, (D) Sucrose, (E) Tween-20, (F) Brij-30, (G) Triton X-100, and (H) Rhodasurf® On-870. (1) 0 ng/mL, and (2) 50 ng/mL.
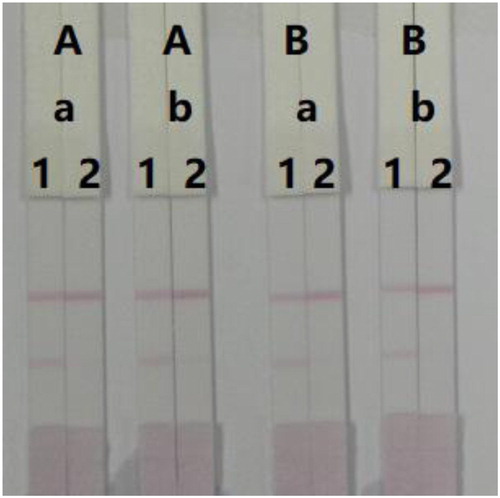
Additionally, we optimised the concentration of coating antigen and mAb. We evaluated 0.05 and 0.1 mg/mL coating antigen, and 8 and 10 µg/mL mAb (). There was a slightly difference in colour intensity from TZP-negative samples between 0.05 and 0.1 mg/mL coating antigen. However, we obtained colour from TZP-positive samples (100 ng/mL) with 0.1 mg/mL coating antigen. Even though there was no significant different in colour intensity between 8 and 10 µg/mL mAb, the optimum concentration of mAb in GNP was 8 µg/mL because intense colours developed in both control and test lines when we tested TZP-negative and TZP-positive samples.
Figure 6. Optimisation of LFA to detect TZP in 0.01 M PBS. Concentration of coating antigen (A) 0.1 mg/mL, and (B) 0.05 mg/mL. The amount of mAb added in GNP (a) 8 µg/mL, and (b) 10 µg/mL. (1) 0 ng/mL and (2) 100 ng/mL.
Therefore, the optimum coating antigen and mAb concentrations were 0.1 mg/mL and 8 µg/mL, respectively, for the detection of TZP in 0.01 M PBS (pH 7.0). The sensitivity of LFA in the detection of TZP in 0.01 M PBS (pH 7.0) was evaluated. shows that the colour intensity changed from strong (negative sample) to weak (25 ng/mL) and completely disappeared at 100 ng/mL TZP.
Detection of TZP in cucumber
TZP in cucumber samples was determined using LFA. Cucumber juice was spiked with a TZP standard. The colour disappeared completely at 250 ng/mL (). The results were visualised by the naked eye in 5 min.
Figure 8. Result of colloidal gold immunochromatographic assay to detect TZP spiked in cucumber. 1–6 represents concentrations of 0, 10, 25, 50, 100, and 250 ng/mL.

Immunoassays based on antibodies and antigens including ELISA are quite popular because of their high sensitivity and accuracy. In cabbage and apple samples, ELISA had a IC50 of 0.0001 µg TZP/L (Hong et al., Citation2018). (Gui et al., Citation2006) ELISA had an LOD 0.11 ng TZP/mL in PBS buffer. ELISA had an IC50 of 0.21 ng/mL in water and soil samples (Liang et al., Citation2007). Even though ELISA offers several advantages, it is time consuming and laborious. In this study, we developed a fast and sensitive TZP detection method.
Conclusions
LFA is a fast and sensitive method for the detection of TZP in cucumber. Under optimum conditions, LFA had TZP cut-off limits of 100 ng/mL in 0.01 M PBS and 250 ng/mL in cucumber. The results could be visualised with the naked eye in 5 min. These results represent that LFA is a quick and sensitive assay to detect TZP in 0.01 M PBS and cucumber.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chao, M., Liu, L., Song, S., Wu, X., & Kuang, H. (2020). Development of a gold nanoparticle-based strip assay for detection of clopidol in the chicken. Food and Agricultural Immunology, 31(1), 489–500. https://doi.org/10.1080/09540105.2020.1737655
- Chen, J., Zhou, G., Deng, Y., Cheng, H., Shen, J., Gao, Y., & Peng, G. (2016). Ultrapreconcentration and determination of organophosphorus pesticides in water by solid-phase extraction combined with dispersive liquid-liquid microextraction and high-performance liquid chromatography. Journal of Separation Science, 39(2), 272–278. https://doi.org/10.1002/jssc.201501007
- Chen, Y., Liu, L., Cui, G., Wu, X., Kuang, H., & Xu, C. (2019). Development of an immunochromatographic strip for the detection of rosiglitazone in functional foods based on monoclonal antibodies. Analytical Methods, 11(38), 4910–4916. https://doi.org/10.1039/C9AY01534E
- Gui, W. J., Jin, R. Y., Chen, Z. L., Cheng, J. L., & Zhu, G. N. (2006). Hapten synthesis for enzyme-linked immunoassay of the insecticide triazophos. Analytical Biochemistry, 357(1), 9–14. https://doi.org/10.1016/j.ab.2006.07.023
- Holden, A. J., Chen, L., & Shaw, I. C. (2001). Thermal stability of organophosphorus pesticide triazophos and its relevance in the assessment of risk to the consumer of triazophos residues in food. Journal of Agricultural and Food Chemistry, 49(1), 103–106. https://doi.org/10.1021/jf0002589
- Hong, S., She, Y., Cao, X., Wang, M., He, Y., Zheng, L., Wang, S., Abd El-Aty, A. M., Hacimuftuoglu, A., Yan, M., & Wang, J. (2019). A novel CdSe/ZnS quantum dots fluorescence assay based on molecularly imprinted sensitive membranes for determination of triazophos residues in cabbage and apple. Frontiers in Chemistry, 7. https://doi.org/10.3389/fchem.2019.00130
- Hong, S., She, Y., Cao, X., Wang, M., Zhang, C., Zheng, L., Wang, S., Ma, X., Shao, H., Jin, M., & Jin, F. (2018). Biomimetic enzyme-linked immunoassay based on a molecularly imprinted 96-well plate for the determination of triazophos residues in real samples. Rsc Advances, 8(37), 20549–20556. https://doi.org/10.1039/C8RA03531H
- Huan, Z., Xu, Z., Luo, J., & Xie, D. (2016). Monitoring and exposure assessment of pesticide residues in cowpea (Vigna unguiculata L. Walp) from five provinces of southern China. Regulatory Toxicology and Pharmacology, 81, 260–267. https://doi.org/10.1016/j.yrtph.2016.09.012
- Huang, J., Hou, C., Lei, J., Huo, D., Luo, X., & Dong, L. (2016). A novel device based on a fluorescent cross-responsive sensor array for detecting pesticide residue. Measurement Science and Technology, 27. https://doi.org/10.1088/0957-0233/27/11/115104
- Huang, X.-C., Ma, J.-K., Feng, R.-X., & Wei, S.-L. (2019). Simultaneous determination of five organophosphorus pesticide residues in different food samples by solid-phase microextraction fibers coupled with high-performance liquid chromatography. Journal of the Science of Food and Agriculture, 99(15), 6998–7007. https://doi.org/10.1002/jsfa.9990
- Kong, D., Wu, X., Li, Y., Liu, L., Song, S., Zheng, Q., Kuang, H., & Xu, C. (2019). Ultrasensitive and eco-friendly immunoassays based monoclonal antibody for detection of deoxynivalenol in cereal and feed samples. Food Chemistry, 270, 130–137. https://doi.org/10.1016/j.foodchem.2018.07.075
- Li, Y., Liu, L., Kuang, H., & Xu, C. (2020). Preparing monoclonal antibodies and developing immunochromatographic strips for paraquat determination in water. Food Chemistry, 311. https://doi.org/10.1016/j.foodchem.2019.125897
- Liang, C., Jin, R., Gui, W., & Zhu, G. (2007). Enzyme-linked immunosorbent assay based on a monoclonal antibody for the detection of the insecticide triazophos: Assay optimization and application to environmental samples. Environmental Science & Technology, 41(19), 6783–6788. https://doi.org/10.1021/es070828m
- Ling-Li, Z., Li, H., Shao-Rong, L., Ling-An, G., Jian-Fei, M., Xi, L., & Xian-Lin, Y. (2016). Determination of 6 pesticide residues in tea by ultra performance liquid chromatography-tandem mass spectrometry. Journal of Food Safety and Quality, 7, 2081–2086.
- Pirsaheb, M., Fattahi, N., Rahimi, R., Sharafi, K., & Ghaffari, H. R. (2017). Evaluation of abamectin, diazinon and chlorpyrifos pesticide residues in apple product of Mahabad region gardens: Iran in 2014. Food Chemistry, 231, 148–155. https://doi.org/10.1016/j.foodchem.2017.03.120
- Rafique, N., Tariq, S. R., & Ahmed, D. (2016). Monitoring and distribution patterns of pesticide residues in soil from cotton/wheat fields of Pakistan. Environmental Monitoring and Assessment, 188. https://doi.org/10.1007/s10661-016-5668-6
- Reinholds, I., Pugajeva, I., & Bartkevics, V. (2016). A reliable screening of mycotoxins and pesticide residues in paprika using ultra-high performance liquid chromatography coupled to high resolution Orbitrap mass spectrometry. Food Control, 60, 683–689. https://doi.org/10.1016/j.foodcont.2015.09.008
- Shen, X., Liu, L., Xu, L., Ma, W., Wu, X., Cui, G., & Kuang, H. (2019). Rapid detection of praziquantel using monoclonal antibody-based ic-ELISA and immunochromatographic strips. Food and Agricultural Immunology, 30(1), 913–923. https://doi.org/10.1080/09540105.2019.1641068
- Suryoprabowo, S., Liu, L. Q., Peng, J., Kuang, H., & Xu, C. L. (2014). Development of a broad specific monoclonal antibody for fluoroquinolone analysis. Food Analytical Methods, 7(10), 2163–2168. https://doi.org/10.1007/s12161-014-9863-1
- Wang, Z., Wu, X., Liu, L., Xu, L., Kuang, H., & Xu, C. (2020). Rapid and sensitive detection of diclazuril in chicken samples using a gold nanoparticle-based lateral-flow strip. Food Chemistry, 312. https://doi.org/10.1016/j.foodchem.2019.126116
- Wu, S., Li, X., Liu, X., Yang, G., An, X., Wang, Q., & Wang, Y. (2018). Joint toxic effects of triazophos and imidacloprid on zebrafish (Danio rerio). Environmental Pollution, 235, 470–481. https://doi.org/10.1016/j.envpol.2017.12.120
- Wu, X., Suryoprabowo, S., Kuang, H., & Liu, L. (2020). Detection of aminophylline in serum using an immunochromatographic strip test. Food and Agricultural Immunology, 31(1), 33–44. https://doi.org/10.1080/09540105.2019.1691508
- Xu, X., Liu, L., Wu, X., Kuang, H., & Xu, C. (2020). Ultrasensitive immunochromatographic strips for fast screening of the nicarbazin marker in chicken breast and liver samples based on monoclonal antibodies. Analytical Methods, 12(16), 2143–2151. https://doi.org/10.1039/D0AY00414F
- Xu, Z.-L., Deng, H., Lei, H.-T., Jiang, Y.-M., Campbell, K., Shen, Y.-D., Yang, J.-Y., Wang, H., & Sun, Y.-M. (2012). Development of a broad-specificity monoclonal antibody-based Immunoaffinity chromatography cleanup for organophosphorus pesticide determination in environmental samples. Journal of Agricultural and Food Chemistry, 60(23), 5847–5852. https://doi.org/10.1021/jf300896z
- Yang, F.-W., Li, Y.-X., Ren, F.-Z., Wang, R., & Pang, G.-F. (2019). Toxicity, residue, degradation and detection methods of the insecticide triazophos. Environmental Chemistry Letters, 17(4), 1769–1785. https://doi.org/10.1007/s10311-019-00910-z
- Zeng, L., Wu, X., Liu, L., Xu, L., Kuang, H., & Xu, C. (2020). Production of a monoclonal antibody for the detection of vitamin B-1 and its use in an indirect enzyme-linked immunosorbent assay and immunochromatographic strip. Journal of Materials Chemistry B, 8(9), 1935–1943. https://doi.org/10.1039/C9TB02839K
- Zhang, L., Sun, W., Zhang, Z., Chen, H., Jia, X., & Cai, W. (2017). Gender-specific metabolic responses in gonad of mussel Perna viridis to triazophos. Marine Pollution Bulletin, 123(1-2), 39–46. https://doi.org/10.1016/j.marpolbul.2017.09.032
- Zhang, X., Liu, L., Cui, G., Song, S., Kuang, H., & Xu, C. (2019). Preparation of an anti-isoprocarb monoclonal antibody and its application in developing an immunochromatographic strip assay. Biomedical Chromatography, 33. https://doi.org/10.1002/bmc.4660