ABSTRACT
Arecoline, the dominant alkaloid existing in areca nuts, is an addictive substance and classified as a Group 2B potential human carcinogen. Currently, the detection of arecoline is mostly dependent on chromatography-based approaches, which are time-consuming and expensive. We used arecaidine as a hapten to produce a highly specific monoclonal antibody (mAb) against arecoline. An indirect competitive enzyme-linked immunosorbent assay (icELISA) was developed using the mAb-A5H12, to detect arecoline in traditional Chinese medicines and fresh areca nuts. The icELISA indicated that the half maximum inhibition concentration (IC50) for arecoline was 67.9 ng/mL, with a working range of 10.1–502.6 ng/mL and a limit of detection (LOD) of 3.6 ng/mL. High-performance liquid chromatographic (HPLC) confirmed the accuracy and the working range of icELISA, suggesting that the icELISA approach based on the arecoline specific antibody could be a widely applicable and easy operation method in detection of arecoline in foods and medicines.
Introduction
Arecoline is the predominant alkaloid in the areca (betel) nut, which is considered as the world’s fourth most common human psychoactive agent, after alcohol, nicotine, and caffeine (Papke et al., Citation2015). Arecoline has multiple effects on the central nervous system (including elation and anxiolysis) and is considered as an addictive substance (Heatubun et al., Citation2012; Volgin et al., Citation2019). However, arecoline has been proposed as a vital causative factor for oral submucous fibrosis (OSF) that is a pre-cancerous condition (Moutasim et al., Citation2011; Arakeri & Brennan, Citation2013; Prabhu et al., Citation2014; Yu et al., Citation2016). The IARC has listed betel nut as a Group 1 human carcinogen and classified arecoline as a Group 2B potential human carcinogen (WHO IARC, Citation1985; WHO IARC, Citation2004; IARC Monographs Vol Citation128 group, Citation2021). Multiple studies have demonstrated that arecoline is cytotoxic to human and animal cells (Wei et al., Citation2015; Liu et al., Citation2016), although arecoline does have extensive medical value and exists in some products such as traditional Chinese medicines (TCMs) (Liu et al., Citation2016). To reduce the negative effects of arecoline on humans, it is particularly important to strictly monitor the amount of arecoline in TCMs.
In 1888, arecoline was first isolated from plants by Jahns (Citation1888). Now, routine methods have been developed for the isolation and determination of arecoline, such as high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection (Huang & McLeish, Citation1989; Cox et al., Citation2004) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Pan et al., Citation2017). These methods are sensitive and accurate but time-consuming, and expensive equipment and highly trained operators are required (Lan et al., Citation2019a). Immunoassays are simple, cost-effective, selective, and sensitive, and they are commonly used for real-time and rapid determination of substances (Lan et al., Citation2019a). Owing to the similar structures of four related alkaloids (arecoline, arecaidine, guavacoline, and guavacine), an immunoassay has not yet been reported for the rapid monitoring of arecoline.
In the present study, we used arecaidine as a hapten and coupled it to keyhole limpet hemocyanin (KLH) as an immunogen. After mouse immunization and cell fusion, a hybridoma cell line secreting monoclonal antibody with high affinity and specificity against arecoline was selected. A monoclonal antibody-based icELISA was developed to measure arecoline in various TCMs and fresh areca nuts. To our knowledge, this is the first report describing an arecoline immunoassay using a specific monoclonal antibody.
Materials and methods
Reagents
Arecoline and arecaidine were purchased from Shanghai Yuanye Bio-Technology Co. (Shanghai, China). Quick Antibody-Mouse 5 W immune adjuvant was obtained from Suzhou Biodragon Immunotechnologies Co. (Suzhou, China). Coating buffer (14.2 mM Na2CO3 and 35.8 mM NaHCO3, pH 9.6) and phosphate-buffered saline (PBS, containing 154 mM NaCl, 1.5 mM KH2PO4, and 8.3 mM Na2HPO4•12H2O, pH 7.5) were prepared. PBST was 0.1% (v/v) Tween-20 mixed in PBS, and PBSTG was 0.1% (w/v) gelatin dissolved in PBST. Ovalbumin (OVA), keyhole limpet hemocyanin (KLH), N-hydroxysuccinimide (NHS), N,N-dicyclohexylcarbodiimide (DCC), anhydrous N,N-dimethylformamide (DMF), 3,3’,5,5’-tetramethylbenzidine (TMB), dimethyl sulfoxide (DMSO), 8-azaguanine, hypoxanthine-aminopterin-thymidine (HAT) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal calf serum were obtained from Gibco BRL (Carlsbad, CA, USA). Goat anti-mouse IgG conjugated with horseradish peroxidase (IgG-HRP) was purchased from Jackson Immunoresearch Laboratories (West Grove, PA, USA).
BALB/c mice (6-8 weeks) with the health certificate were obtained from Guangdong Medical Laboratory Animal Center (Foshan, China). Sp2/0 cell lines were obtained from the Professor Baomin Wang’s laboratory at China Agricultural University (Beijing, China).
Hapten option and protein conjugate preparation
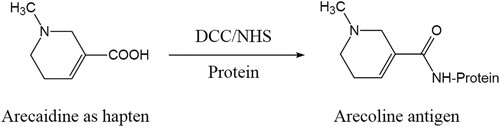
As a hapten, arecaidine was chosen to covalently conjugate to KLH and OVA, which used the NHS ester method. Here, 7 mg arecaidine was dissolved to 7 mg of NHS and 12 mg of DCC in 0.5 mL DMF. After constant agitation at room temperature (RT), the mixture was centrifuged, followed by gathering the supernatant. The solution that 20 mg of KLH dissolved in 3 mL coating buffer and 30 mg of OVA dissolved in 3 mL coating buffer was prepared. Next, 0.2 mL of supernatant was added as slow as possible to the prepared solution (). After stirring at RT over 12 h, the synthesized conjugates were dialyzed against 3 L of PBS at 4°C for 3 days. Besides, the dialysate was changed 3 times daily.
Determination of the coupling ratio of hapten to protein
The protein sample was dissolved in PBS and diluted to 1 mg/mL within PBS. A sinapinic acid (SA) matrix solution (20 mg/mL SA dissolved in 80% acetonitrile (ACN) containing 0.2% trifluoroacetic acid (TFA)) was evenly spread on the sample surface with a pipette, and after it dried, it was placed in a vacuum drying tank to dry further for 10 min. The glass slide was mounted with the matrix on the imaging target plate, and then it was analyzed by MALDI-TOF/TOF-MS under the same conditions (90% of laser, 5000 shots) with autoflex speed MALDI-TOF/TOF-MS from Bruker Daltonic.
Monoclonal antibody production
For mouse immunization and cell fusion, the experiments were carried out referred to the previous methods (Zhao et al., Citation2006). As an immunogen, 250 µL of arecaidine-KLH conjugate was fast and fully mixed with 250 µL of Quick Antibody-Mouse 5W immune adjuvant in a sterile environment. Five healthy BalB/c mice whose age was 6–7 weeks were immunized by injecting 100 µL of the above mixture to calf muscle of the hind legs for each mouse. Then a second injection was administered with the same method after 21 days. After 35 days of first immunizations, the blood from mice’s eye socket was drawn to identify the antibody activity by icELISA. The mouse that had the highest anti-arecoline activity was taken to perform the next step. Four days before cell fusion, without the above adjuvant, 100 µL solution containing 0.1 mg arecaidine-KLH conjugate was injected into the screened mouse's abdominal cavity. The collection of spleen cells was implemented followed by the fusion of the collected cell and Sp2/0 at a 10:1 ratio. After a week for the cell culture under HAT media screening, icELISA was implemented to screen out the positive hybridoma, followed by the limiting dilution method to clone. The monoclonal cell line, A5H12, exhibiting the highest titer and sensitivity, was utilized for the production of mAb-A5H12.
icELISA protocol
The icELISA method was performed by following the previously reported protocols with slight modifications (Mukunzi et al., Citation2018; Lan et al., Citation2019b). Firstly, as the coating antigen, 200 µL arecaidine-OVA diluted in coating buffer was added to each well of a microplate which has 96 wells, to coat the plate. The plate was placed in incubator (37°C) to be incubated for 3 h. Then, the plate was rinsed by using PBST for 3 times, followed by blocking with 10% bovine serum albumin (BSA) for 30 min in incubator (37°C). After washing 3 times with PBST, 100 µL of multiple concentrations of the standard or analytes which was diluted with PBSTG was pipetted into the corresponding wells of the plate, followed by pipetting 100 µL mAb-A5H12 diluted with PBSTG into each well. And then the plate was stranded in incubator (37°C) for 1 h. Each well was subsequently rinsed for 5 times. Then, 200 µL goat anti-mouse IgG-HRP diluted with PBSTG was pipetted into each well. After incubated for 30 min at 37°C, the plate was rinsed again, followed by 200 µL of TMB substrate solution was transferred to the corresponding wells until 10–20 min at room temperature. Finally, 100 µL 2 M H2SO4 was used to quench the colouration. The absorbance of the reaction solution which was at 450 nm was immediately recorded. The curve fitted to a logistic equation was established by the Origin 2019b software. The working range and the LOD was calculated as 10%–90% B/B0 and B0−3×SD (B0), respectively.
Furthermore, to understand the effects of the methanol in the sample diluent (PBSTG), 5%, 10%, and 20% methanol were each added to PBSTG solutions. Then, the IC50 values were used to evaluate the effects of methanol on the icELISA standard curve.
Cross-reactivity (CR)
The CRs with arecoline and other analogs were carried out to identify the specificity of the mAb-A5H12 against arecoline. Performing icELISA, the IC50 values for above compounds were determined by building standard curves in Origin 2019b software. Through the equation given below, the CRs values were obtained. CR (%) = [IC50 (arecoline)/IC50 (compound)] × 100.
Sample fortification and recovery
Samples of areca leaves, areca nut pericarp, and areca nut endosperm were ground to a powder. Next, 200 mg of each TCM, 50 mg pericarp or endosperm, and 200 mg leaves were weighed and transferred to 2-mL centrifuge tubes. Samples were homogenized with 1.5 mL 50% methanol. A different volume of standard arecoline solutions (1 mg/mL) was added to the samples for a recovery test, followed by ultrasonic shaking for 30 min. Afterward, samples were centrifuged at 12,000 rpm for 10 min, and the precipitates were discarded. For the icELISA test, the supernatant was diluted with PBSTG in a certain dilution ratio. The supernatants of leaves and areca nut pericarp w diluted 1000 times and the supernatant of areca nut endosperm was diluted 6000 times. The supernatants of Kai Xiong Shun Qi Pills, Shu Gan Jian Wei Pills, Bing Lang Si Xiao Pills, Wei Su Particles, Jin Sang Li Yan Capsules were diluted 500, 500, 750, 300, 100 times, respectively. The precision and accuracy of icELISA were evaluated by the coefficient of variation (CV) and recovery, respectively. CV (%) = SD / mean × 100.
HPLC validation
We quantified the arecoline concentration in various TCMs and fresh tissues from areca trees via the developed icELISA, and HPLC was used as the confirmatory method. The samples included five types of TCMs, areca leaves, areca nut pericarp, and areca nut endosperm. Then, 200 mg of each TCM, 50 mg pericarp or endosperm and 200 mg leaves were weighed and then transferred to 2-mL centrifuge tube. The sample pretreatment method was the same as that described in above section.
For HPLC determination, the supernatant of fresh tissues from areca trees was diluted with 50% methanol in a certain proportion. Each sample was tested three times, and the supernatant of TCMs was directly tested. A Prominence HPLC system (e2695, Waters, USA) with a diode array detector (2998, Waters, USA) was used. A cation-exchange HPLC column (300 SCX, 250 mm × 4.6 mm × 5 µm, Agilent, USA) was used. The proportions of acetonitrile and the mobile phase were 30% and 70%, respectively, with a flow rate of 1.0 mL/min. The mobile phase consisted of 0.2% phosphate buffer, whose pH was adjusted to 3.6–3.8 with concentrated ammonia. The wavelength of detection was set as 215 nm. Subsequently, the solutions of real samples were diluted with PBSTG to different suitable concentrations for icELISA analysis. The correlation between the two methods was analyzed, and the consistency of the results reflects the reliability of the developed icELISA.
Results and discussion
Conjugation of hapten and identification of conjugates
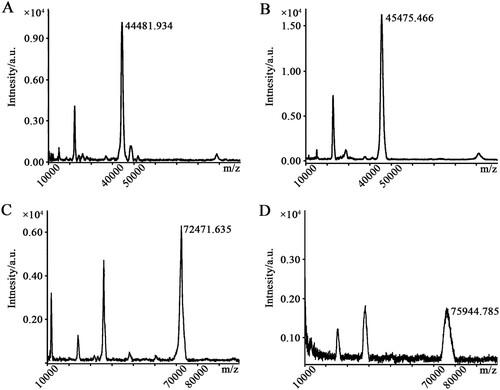
Because position 3 of the tetrahydropyridine ring of arecoline is occupied by a methoxycarbonyl group, we cannot conjugate arecoline as a hapten directly to a protein carrier. Therefore, we used compound arecaidine, which provides a carboxylic acid group, to couple with protein carriers (OVA and KLH) to synthesize a complete antigen. The number of arecaidine conjugated to the protein carriers can be determined via MALDI-TOF/TOF MS analysis (Nuntawong et al., Citation2021). The previous work showed that a density of 8–30 haptens per protein carrier would lead to a good response (Erlanger, Citation1980; Singh et al., Citation2004). The results of MALDI-TOF/TOF-MS showed that the m/z values of the coating antigen and OVA were 45,475.466 and 44,481.934, and the m/z values of the immunogen and KLH were 75,944.785 and 72,471.635, respectively (). Additionally, the coupling ratios of arecaidine-OVA and arecaidine-KLH were 8 and 28, respectively, suggesting that the conjugation to the protein carrier was successful.
Characterization of the mAb
After cell fusion, confirmation of the hybridoma cells was performed by icELISA with acecoline, to identify the hybridoma cells secreting the specific mAb. The results demonstrated that the hybridoma cell line A5H12 exhibited the greatest sensitivity and specificity could be used to produce ascites. Additionally, mAb-A5H12 was determined as the type of IgG1 which carried a light kappa chain.
The CRs of arecoline and other related analogs were tested to determine the specificity of mAb-A5H12. As shown in , the half-maximum inhibition concentration (IC50) was 67.9 ng/mL for arecoline, and the CR was 100%. No CRs with other analogs were detected, such as nicotinic acid, trigonelline, salicylic acid, benzoic acid, picolinic acid, or nicotinamide. Even if arecaidine, guvacoline, or guvacine shared the main molecular structures with arecoline, there were also no CRs between mAbs and them.
Table 1. Cross reactivity of arecoline and related alkaloids to the mAb-A5H12 in the icELISA.
Optimization of icELISA
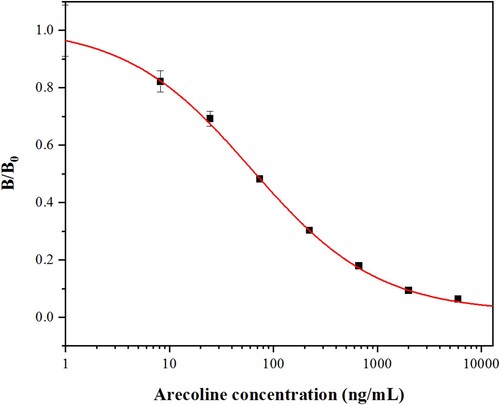
After optimizing the icELISA parameters, the optimal concentration combination was 250 ng/mL of coating antigen (arecaidine-OVA) and a 1:40,000 ratio of the mAb-A5H12 dilution. The standard inhibition curve for arecoline in icELISA was established by icELISA under optimum conditions (). The IC50 was 67.9 ng/mL with a working range of 10.1–502.6 ng/mL (20%−80% inhibition rate), and the LOD was 3.6 ng/mL (10% inhibition rate).
Figure 3. Standard inhibition curve for arecoline by icELISA. Each point represents the average data for three determinations ± standard deviations. B0 and B denote the absorbance at 450 nm in the absence and presence of arecoline, respectively. The R2 value is 0.99,917.
Because methanol was used as a solvent in this study to extract arecoline, the effects of the methanol content in PBSTG were tested (Jiang et al., Citation2011). The effects of methanol were estimated by establishing several standard inhibition curves under different conditions. As shown in , the IC50 values were calculated. With the increase in methanol concentration from 5% to 20%, the IC50 values gradually increased from 74.7 ng/mL to 113.4 ng/mL.
Table 2. The effects of methanol content in sample diluent in icELISA.
In the icELISA protocol we used in this study, the samples were extracted in 50% methanol, and then diluted in PBSTG by at least 100 times. Therefore, the residual methanol was 0.5% in the tested solution. We observed that 5% additional methanol increased the IC50 to 74.7 ng/mL, which suggested that even methanol can inhibit the binding of antibodies to arecoline, although there was an acceptable concentration of residual methanol in the PBSTG solution.
Fortification and Validation of the icELISA method
To test the accuracy and precision of icELISA, a recovery test using the icELISA method was performed (Li et al., Citation2021). Each sample was processed and analyzed three times. As shown in , the recovery rates for icELISA ranged from 98.42% to 116.02%. The CVs ranged from 0.05% to 14.04%. In the previous study, Fu et al. reported that the recovery rates of immunoassays for cadmium and ciprofloxacin ranged from 89.03 to 95.81% and 73.80%-123.35%, respectively (Fu et al., Citation2021; Wang et al., Citation2021). So the recoveries of the present icELISA and CVs meet the requirement of immunoassay detection. These results showed that the established icELISA has satisfactory accuracy and reproducibility.
Table 3. The recoveries of arecoline determined by icELISA in spiked areca nut leaves, pericarp and endosperm samples (n = 3).
Detection of arecoline in TCMs and fresh areca nuts
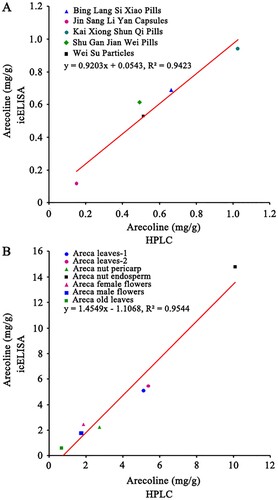
We quantified the arecoline concentration of five TCMs purchased from pharmacies and fresh tissue samples collected from areca trees via the icELISA established by specific monoclonal antibody (mAb-A5H12). HPLC was applied to validate the icELISA results. Semen Arecae is listed as an ingredient in all five of the TCMs that we analyzed, and each TCM is used to cure different symptoms (Supplementary Table S1). The results obtained by icELISA indicated that these medicines contained arecoline from 0.12 to 0.94 mg/g (). These results were similar to the results obtained by HPLC, suggesting that the icELISA has high accuracy and is reliable for medical applications. The detection and quantification of arecolines in fresh plant tissues can support this conclusion ().
Table 4. Arecoline concentrations in traditional Chinese medicines tested by icELISA and HPLC (n = 3, the receipts of these medicines are shown in supplementary ).
Table 5. Arecoline concentrations of fresh areca nuts tested by icELISA and HPLC (n = 3).
We further analyzed the correlation between icELISA and HPLC based on the results of the analysis of TCMs and fresh plant tissues (). The linear regression equations were y = 0.9203x + 0.0543 and y = 1.4549x−1.1068, respectively. The correlation coefficients were all greater than 0.94 (R2 > 0.94), which indicated that the developed icELISA was reliable and can be used for detection of real samples. Since arecoline has been added to foods, energy drink and liquid of e- cigarette in markets, we believe this detection method could have wide applications.
Figure 4. Validation between the results of icELISA and HPLC for (A) five TCMs and (B) fresh tissues from areca trees.
There are over 600 million of populations chewing areca nuts and 10%-20% among them prefer to chew betel quid (an areca nut enveloped in a betel leaf together with slaked lime), which alerts us that the number of users exposed to arecoline is shocking (Arora & Squier, Citation2019). The toxic effects in the long exposure of arecoline have been increasing investigated, especially OSF, oral cancers and genotoxicity (Kumpawat et al., Citation2003; Tsai et al., Citation2008; Liu et al., Citation2016). It is necessary to detect the arecoline content in foods and set a safe dose standard of arecoline for human (Liu et al., Citation2016). To monitor the medicinal value of areca nuts and improve the quality standard and controllability of TCMs, it is vital to detect the content of arecoline (Lu et al., Citation2018).
In previous studies, the determination of the arecoline has been performed by other methods. For example, the good linear range of ultra-high performance liquid chromatography (UPLC) for arecoline in areca nut extract was between 13–663 µg/mL (Kong et al., Citation2018). The LOD of microfluidic chip with contactless conductivity detection for arecoline in areca was 2 µg/mL (Yang et al., Citation2015) and the detection range with good linearity by photometry was 19.5–112.1 µg/mL (Gao & Gao, Citation2003). In our study, the IC50 value of icELISA was 67.9 ng/mL with the LOD of 3.6 ng/mL, indicating that the established icELISA has higher sensitivity. Moreover, good correlation between icELISA and HPLC detection in fresh plant tissues and TCMs suggests that icELISA method can be applied to the determination of arecoline to monitor the arecoline content in the actual samples.
Conclusion
In the present study, arecaidine was used as a hapten and coupled with the carrier protein OVA and KLH as a coating antigen and immunogen, respectively. A highly sensitive and specific monoclonal antibody (mAb-A5H12) for arecoline was subsequently produced. The icELISA determined that the IC50 value was 67.9 ng/mL with a working range of 10.1–502.6 ng/mL, and the LOD was 3.6 ng/mL. There was no cross-reactivity between mAb-A5H12 and other arecoline analogs. The recovery rates of icELISA indicated that the icELISA system is reliable. The results of icELISA correlated well with real samples analyzed by HPLC. Therefore, the developed icELISA is an efficient and sensitive tool for detecting arecoline in TCMs and fresh areca nuts and can be applied to other food and medicine products.
Author contributions
Background information, immunoassay experiments, statistical analysis, and writing - original draft, Y.W. and H.M.; HPLC, statistical analysis, J.W.; monoclonal antibody production, statistical analysis, experiment designing, and manuscript writing, H.Z.; experiment designing, statistical analysis, and revising and editing manuscript. Y.W. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arakeri, G., & Brennan, P. A. (2013). Oral submucous fibrosis: An overview of the aetiology, pathogenesis, classification, and principles of management. British Journal of Oral and Maxillofacial Surgery, 51(7), 587–593. https://doi.org/10.1016/j.bjoms.2012.08.014
- Arora, S., & Squier, C. (2019). Areca nut trade, globalisation and its health impact: Perspectives from India and South-East Asia. Perspectives in Public Health, 139(1), 44–48. https://doi.org/10.1177/1757913918785398
- Cox, S., Piatkov, I., Vickers, E. R., & Ma, G. (2004). High-performance liquid chromatographic determination of arecoline in human saliva. Journal of Chromatography A, 1032(1-2), 93–95. https://doi.org/10.1016/j.chroma.2003.11.076
- Erlanger, B. F. (1980). The preparation of antigenic hapten-carrier conjugates: A survey. Methods in Enzymology, 70, 85–104. https://doi.org/10.1016/S0076-6879(80)70043-5
- Fu, X., Chen, E., Ma, B., Xu, Y., Hao, P., Zhang, M., Ye, Z., Yu, X., Li, C., & Ji, Q. (2021). Establishment of an indirect competitive enzyme-linked immunosorbent method for the detection of heavy metal cadmium in food packaging materials. Foods (basel, Switzerland), 10(2), 413. https://doi.org/10.3390/foods10020413
- Gao, M., & Gao, S. (2003). Determination of arecoline in areca by photometry. Chemical Analysis and Meterage, 12(1), 28–30. https://ss.cqvip.com/Qikan/Article/Detail?id=7385226
- Heatubun, C. D., Dransfield, J., Flynn, T., Tjitrosoedirdjo, S. S., Mogea, J. P., & Baker, W. J. (2012). A monograph of the betel nut palms (Areca: Arecaceae) of East Malesia. Botanical Journal of the Linnean Society, 168(2), 147–173. https://doi.org/10.1111/j.1095-8339.2011.01199.x
- Huang, J., & McLeish, M. J. (1989). High-performance liquid chromatographic determination of the alkaloids in betel nut. Journal of Chromatography A, 475(2), 447–450. https://doi.org/10.1016/S0021-9673(01)89702-8
- IARC Monographs Vol 128 group. (2021). Carcinogenicity of acrolein, crotonaldehyde, and arecoline. Lancet Oncology, 22(1), 19–20. https://doi.org/10.1016/S1470-2045(20)30727-0
- Jahns, E. (1888). Ueber die alkaloïde der arecanuss. European Journal of Inorganic Chemistry, 21(2), 3404–3409.
- Jiang, J., Zhang, H., Fan, G., Ma, J., Wang, Z., & Wang, J. (2011). Preparation of monoclonal antibody based indirect competitive ELISA for detecting 19-nortestosterone residue. Chinese Science Bulletin, 56(25), 2698–2705. https://doi.org/10.1007/s11434-011-4604-y
- Kong, L., Yang, S., Yang, P., He, Y., & Qiu, C. (2018). Determination of arecoline from areca nut extract by ultra-high performance liquid chromatography (UPLC). Journal of Anhui Agricultural Sciences, 46(8), 177–178. https://doi.org/10.13989/j.cnki.0517-6611.2018.08.054
- Kumpawat, K., Deb, S., Ray, S., & Chatterjee, A. (2003). Genotoxic effect of raw betel-nut extract in relation to endogenous glutathione levels and its mechanism of action in mammalian cells. Mutation Research, 538(1-2), 1–12. https://doi.org/10.1016/S1383-5718(03)00048-2
- Lan, J., Wang, M., Ding, S., Fan, Y., Diao, X., Li, Q. X., & Zhao, H. (2019a). Simultaneous detection of carbofuran and 3-hydroxy-carbofuran in vegetables and fruits by broad-specific monoclonal antibody-based ELISA. Food and Agricultural Immunology, 30(1), 1085–1096. https://doi.org/10.1080/09540105.2019.1664997
- Lan, J., Zhao, H., Jin, X., Guan, H., Song, Y., Fan, Y., Diao, X., Wang, B., & Han, Q. (2019b). Development of a monoclonal antibody-based immunoaffinity chromatography and a sensitive immunoassay for detection of spinosyn A in milk, fruits, and vegetables. Food Control, 95, 196–205. https://doi.org/10.1016/j.foodcont.2018.08.002
- Li, X., Chen, X., Wu, J., Liu, Z., Wang, J., Song, C., Zhao, S., Lei, H., & Sun, Y. (2021). Portable, rapid, and sensitive time-resolved fluorescence immunochromatography for on-site detection of dexamethasone in milk and pork. Foods (basel, Switzerland), 10(6), 1339. https://doi.org/10.3390/foods10061339
- Liu, Y., Peng, W., Hu, M., Xu, M., & Wu, C. (2016). The pharmacology, toxicology and potential applications of arecoline: A review. Pharmaceutical Biology, 54(11), 2753–2760. https://doi.org/10.3109/13880209.2016.1160251
- Lu, W., Lin, P., & Hai, P. (2018). Determination of the arecoline content in Tibetan medicine Anshen pills by high performance liquid chromatography-cation exchange chromatography. Journal of Food Safety and Quality, 9(9), 2113–2117. http://www.chinafoodj.com/ch/reader/view_abstract.aspx?file_no=20180130007&flag=1
- Moutasim, K. A., Jenei, V., Sapienza, K., Marsh, D., Weinreb, P. H., Violette, S. M., Lewis, M. P., Marshall, J. F., Fortune, F., Tilakaratne, W. M., Hart, L. R., & Thomas, G. J. (2011). Betel-derived alkaloid up-regulates keratinocyte alphavbeta6 integrin expression and promotes oral submucous fibrosis. The Journal of Pathology, 223(3), 366–377. https://doi.org/10.1002/path.2786
- Mukunzi, D., Suryoprabowo, S., Song, S., Liu, L., & Kuang, H. (2018). Development of an indirect enzyme-linked immunosorbent assay and lateral-flow test strips for pefloxacin and its analogues in chicken muscle samples. Food and Agricultural Immunology, 29(1), 484–497. https://doi.org/10.1080/09540105.2017.1406460
- Nuntawong, P., Horikawa, T., Ochi, A., Wada, S., Tsuneura, Y., Tanaka, H., Sakamoto, S., & Morimoto, S. (2021). A monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay to quantify swertiamarin and related compounds in Swertia japonica Makino. Phytochemical Analysis: PCA, 32(4), 512–520. https://doi.org/10.1002/pca.2999
- Pan, H., Huang, L., Li, Y., Zhou, X., Lu, Y., & Shi, F. (2017). Liquid chromatography-tandem mass spectrometric assay for determination of unstable arecoline in rat plasma and its application. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1070, 112–116. https://doi.org/10.1016/j.jchromb.2017.10.026
- Papke, R. L., Horenstein, N. A., & Stokes, C. (2015). Nicotinic activity of arecoline, the psychoactive element of “betel nuts”, suggests a basis for habitual use and anti-inflammatory activity. PLoS One, 10(10), e0140907. https://doi.org/10.1371/journal.pone.0140907
- Prabhu, R. V., Prabhu, V., Chatra, L., Shenai, P., Suvarna, N., & Dandekeri, S. (2014). Areca nut and its role in oral submucous fibrosis. Journal of Clinical and Experimental Dentistry, 6(5), e569–e575. https://doi.org/10.4317/jced.51318
- Singh, K. V., Kaur, J., Varshney, G. C., Raje, M., & Suri, C. R. (2004). Synthesis and characterization of hapten-protein conjugates for antibody production against small molecules. Bioconjugate Chemistry, 15(1), 168–173. https://doi.org/10.1021/bc034158v
- Tsai, Y. S., Lee, K. W., Huang, J. L., Liu, Y. S., Juo, S. H., Kuo, W. R., Chang, J. G., Lin, C. S., & Jong, Y. J. (2008). Arecoline, a major alkaloid of areca nut, inhibits p53, represses DNA repair, and triggers DNA damage response in human epithelial cells. Toxicology, 249(2-3), 230–237. https://doi.org/10.1016/j.tox.2008.05.007
- Volgin, A. D., Bashirzade, A., Amstislavskaya, T. G., Yakovlev, O. A., Demin, K. A., Ho, Y. J., Wang, D., Shevyrin, V. A., Yan, D., Tang, Z., Wang, J., Wang, M., Alpyshov, E. T., Serikuly, N., Wappler-Guzzetta, E. A., Lakstygal, A. M., & Kalueff, A. V. (2019). Dark classics in chemical neuroscience: Arecoline. ACS Chemical Neuroscience, 10(5), 2176–2185. https://doi.org/10.1021/acschemneuro.8b00711
- Wang, F., Li, N., Zhang, Y., Sun, X., Hu, M., Zhao, Y., & Fan, J. (2021). Preparation and directed evolution of anti-ciprofloxacin scFv for immunoassay in animal-derived food. Foods (basel, Switzerland), 10(8), 1933. https://doi.org/10.3390/foods10081933
- Wei, X., Zhang, J., Niu, J., Zhou, X., Li, J., & Li, B. (2015). Evaluation of arecoline hydrobromide toxicity after a 14-day repeated oral administration in wistar rats. PLoS One, 10(4), e0120165. https://doi.org/10.1371/journal.pone.0120165
- WHO IARC. (1985). Tobacco habits other than smoking; betal quid and areca nut chewing; and some related nitrosamines. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, 37, 1–295.
- WHO IARC. (2004). Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 85, 1–334.
- Yang, X., Chen, Z., & Liu, Y. (2015). Determination of arecoline in areca catechu by microfluidic chip with contactless conductivity detection. Lishizhen Medicine and Materia Medica Research, 26(6), 1327–1329. https://doi.org/10.3969/j.issn.1008-0805.2015.06.018
- Yu, C. C., Yu, C. H., & Chang, Y. C. (2016). Aberrant SSEA-4 upregulation mediates myofibroblast activity to promote pre-cancerous oral submucous fibrosis. Scientific Reports, 6(1), 37004. https://doi.org/10.1038/srep37004
- Zhao, J., Li, G., Wang, B. M., Liu, W., Nan, T. G., Zhai, Z. X., Li, Z. H., & Li, Q. X. (2006). Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for the analysis of glycyrrhizic acid. Analytical and Bioanalytical Chemistry, 386(6), 1735–1740. https://doi.org/10.1007/s00216-006-0780-z