ABSTRACT
Okadaic acid (OA), one of marine biotoxins produced by several species of dinoflagellates, can accumulate in marine animals. The consumption of OA-contaminated seafood can cause diarrhoeic shellfish poisoning. Many countries have established regulatory restriction to limit the level of OA in seafood. In the present study, we report a highly sensitive monoclonal antibody (mAb) against OA produced by a new immunogen. Two monoclonal hybridoma cell lines (M8 and M12) that producing mAb with the best sensitivity and specificity were obtained. The obtained mAb-M12 was used to develop an indirect competitive ELISA (icELISA) with 50% inhibition concentrations of 0.15 ng mL−1 and a working range of 0.02–2.74 ng mL−1. The developed icELISA was more sensitive than the previous reports. HPLC-MS confirmed the accuracy and the working range of icELISA, suggesting that the developed icELISA is suitable for the rapid detection of OA in oysters and green mussels.
Introduction
Okadaic acid (OA) is one of the key diarrhoeic shellfish poisoning (DSP) biotoxins (Dickey et al., Citation1990). It is derived from marine dinoflagellates, including Dinophysis acuminata, Prorocentrum lima, and Dinophysis fortii (Dickey et al., Citation1990; Vale et al., Citation2009), and can be enriched in various species of filter-feeding molluscs, including mussels, clams, oysters, and scallops (Ciminiello & Fattorusso, Citation2006). When red tide occurring, the content level of OA in seafood or sea water will present a significant upward trend. Once human beings consume OA-contaminated seafood, it could cause the symptoms of diarrhoea, nausea, vomiting, and abdominal pain (Aune et al., Citation1991). OA is a lipophilic substance and keeps stable at normal cooking temperatures. Studies have shown that OA primarily inhibits the activity of a protein phosphatase which plays an important role in the dephosphorylation of proteins in cells, causing sodium release and subsequent passive loss of fluid, and responsible for diarrhoea symptoms (Takai et al., Citation1992). The DSP caused by OA-contaminated shellfish has become a widespread concern in the global public health and shellfish industry. Various countries have introduced measures to limit the level of OA in seafood. In the European Union (EU), the maximum permissible level of OA is 160 μg kg−1 in bivalve molluscs (Council E U. Regulation (EC) No 853/2004), while the European Food Safety Authority (EFSA) proposed to reduce the maximum permissible level of OA from 160 μg kg−1 to 45 μg kg−1. The National Standard of China proposes that OA content in food exceeding 0.05 MU g −1 is harmful (GB5009.212-2016).
In view of the hazard of OA to human health and aquaculture economy, it is required to develop efficient and sensitive methods for the detection of OA. Among the developed methods, mouse bioassay is still the choice in many countries, because its technology is mature and can intuitively reflect the toxicity. However, there are some problems such as high false positive rate, high cost, and inability to obtain the structure information of toxins. Instrumental analysis methods for determining OA have been developed, such as high-performance liquid chromatography-tandem mass spectrometry determination (HPLC-MS) (Sakaguchi et al., Citation2021) and HPLC (Louppis et al., Citation2010). These methods are sensitive and can qualitatively and quantitatively detect most marine toxins, but they require expensive equipments, complicated pretreatment procedures, and time-consuming. Cytotoxicity assays can be used to evaluate and characterize the toxicity of OA to cells (Oteri et al., Citation1998; Souid-Mensi et al., Citation2008; Tubaro et al., Citation1996), reducing the use of experimental animals. However, live cell lines are easily disturbed by the detection environment. Based on the inhibitory effect of OA for protein phosphatases, the protein phosphatase inhibition assay (PPIA) has been developed to detect OA (Garibo et al., Citation2013; Molinero-Abad et al., Citation2019; Smienk et al., Citation2012). However, the stability of the enzyme is poor, and may present false positive results due to the influence of other compounds in the samples. Immunoassays based on antibody–antigen interaction had been applied for the determination of OA, including enzyme-linked immunosorbent assay (ELISA) (Lin et al., Citation2014; Pang et al., Citation2019; Wang et al., Citation2017), immunosensor (Garibo et al., Citation2014; Li et al., Citation2020; Molinero-Abad et al., Citation2019; Peng et al., Citation2020; Zou et al., Citation2017) and, immunochromatography assay (Fang et al., Citation2016; Ling et al., Citation2019; Zhang et al., Citation2019). Immunoassay is sensitive, fast, and high-throughput. However, the structure and properties of the antibodies used in immunoassays will directly affect the results. The matrix of seafood is usually complicated, it is vital to develop an immunoassay with a highly sensitive and specific antibody.
In the present study, new immuogen and coating antigen were synthesized through KLH and OVA coupling with OA, respectively. At the same time, a new non-emulsifying quick immune adjuvant was mixed with the immunogen in order to improve the immune response. A highly sensitive monoclonal antibody against OA was obtained and further used to develop an icELISA for the rapid detection of OA in oysters and green mussels.
Materials and methods
Reagents
Okadaic acid and other marine biotoxins were purchased from Beijing Puhuashi Tech. Co. (Beijing, China). Keyhole limpet haemocyanin (KLH), ovalbumin (OVA), anhydrous N,N-dimethylformamide (DMF), N-hydroxysuccinimide (NHS), N,N-dicyclohexylcarbodiimide (DCC), O-phenylenediamine (OPD), polyethylene glycol, hypoxanthine-aminopterin-thymidine (HAT), and hypoxanthine-thymidine (HT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle’s Media (DMEM) and foetal calf serum were purchased from Gibco BRL (Carlsbad, CA, USA). Goat anti-mouse IgG conjugated with horseradish peroxidase (IgG-HRP) is a product from Jackson Immunoresearch Laboratories (West Grove, PA, USA). Quick Antibody-Mouse 5W immune adjuvant was purchased from Suzhou Biodragon Immunotechnologies Co., Ltd (Suzhou, China).
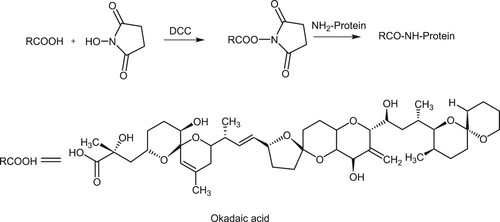
The preparation of OA-protein conjugates.
The chemical synthesis procedure of the antigen was shown in . In brief, to 200 μL DMF was added 3 mg OA, 0.5 mg NHS, and 0.9 mg DCC, and the solution was stirred at room temperature for 3 h. After centrifugation at 6000 rpm for 10 min, a total of 120 μL (80 μL for OA) supernatant was dropwise added to a precooled KLH or OVA solution (10 mg KLH or OVA dissolved in 2 mL of 0.1 M NaHCO3). The reaction solution was magnetically stirred overnight at 4°C and then dialysed against 3 L of PBS (1.5 mM KH2PO4, 8.3 mM Na2HPO4 12H2O, and 154 mM NaCl, pH 7.5) at 4°C for 3 days with 2 times of changes of dialysate each day. The obtained OA-KLH and OA-OVA conjugates were diluted with PBS to 1 mg mL−1 and used as immunogen and coating antigen, respectively.
Preparation of specific monoclonal antibody
The protocol was similar with our previous report (Lan et al., Citation2019) with some modifications. Five BalB/c mice were immunized with OA-KLH conjugate. Each mouse was immunized by injection into the muscle of the hind legs with 25 μL of immunogen mixed with 25 μL of Quick Antibody-Mouse 5W adjuvant. After 21 days, the second injection was given followed the first injection protocol. After two weeks of immunization, icELISA was performed to screen one mouse that gave the best antibody titres and specificity. A booster immunization was performed with 50 μL immunogen without adjuvant 4 days before cell fusion. The spleen cells were fused with myeloma cells Sp2/0 at the ratio of 10:1. After 7 days in culture, the positive hybridoma cells were screened by icELISA, and cloned by limiting dilution method. The monoclonal cell lines showing the greatest titre and sensitivity were used for the production of ascites. According to the results of antibody subtype identification, the protein-G affinity chromatography column was used for monoclonal antibody purification. The purity of purified antibodies was assayed using SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After purification and freeze drying, the monoclonal antibodies were stored at −20°C.
Protocol of the indirect competitive ELISA (icELISA)
Each well of a microplate was coated with 100 μL OA-OVA diluted in coating buffer at 37°C for 3 h. After washing the plate 3 times with PBST (0.1% (v/v) Tween-20 diluted in PBS), each well was blocked with 10% bovine serum albumin (BSA) at 37°C for 30 min. After washing the plate three times with PBST, 50 μL of multiple concentrations of OA standard or analyte diluted in the PBSTG (0.1% (w/v) gelatine diluted in PBST) was added to the corresponding wells of microplate, followed by adding 50 μL antibody diluted with PBSTG into each well. The mixture was allowed to incubate at 37°C for 30 min. The plate was washed and 100 μL goat anti-mouse IgG-HRP diluted in PBSTG was added to each well. After the microplate was incubated at 37°C for 30 min and washed 3 times with PBST, 100 μL aliquots of o-phenylenediamine/H2O2 solution in substrate buffer solution were added into each well. After 15 min at room temperature, the reaction was stopped by adding 50 μL 2 M H2SO4 into each well. The absorbance values at the wavelength of 490 nm were measured with a microplate reader (Elx808, BioTek, USA). The working range, limit of detection (LOD), and 50% inhibition concentrations (IC50) were calculated by fitting 10–90% B/B0, 90% B/B0, and 50% B/B0 to logistic equation, respectively.
The influence of methanol matrix on ELISA
Each well of a microplate was coated with 100 μL OA-OVA diluted in coating buffer at 37°C for 3 h. After washed 3 times with PBST, 50 μL of multiple concentrations of OA standard was diluted in PBSTG containing 5%, 10%, 20%, and 40% methanol, respectively, followed by adding 50 μL antibody diluted with PBSTG into each well. The followed protocol was the same as that of the icELISA described above.
Sample fortification and recovery
The protocol of sample pretreatment was performed according to the National Standard of China (GB5009.212-2016). In brief, the shellfish meat was ground evenly with a homogenizer, and accurately weighed 5 g sample. Samples were extracted with 25 mL of methanol solution (90%). The spiked concentrations in the shellfish samples were at 80, 40, 20, 10 and 5 μg kg−1, respectively. After centrifuging at 4000 r/min for 15 min, the supernatant was diluted 10 times for icELISA determination. Moreover, the supernatants of Oyster (O-1 to O-9) and Mussel (M-1 to M-7) were diluted 10 times for icELISA test, respectively. The precision and accuracy of icELISA were assessed by the coefficient of variation (CV) and recovery, respectively. CV (%) = SD/mean × 100.
Validation by HPLC-MS
HPLC-MS analysis was used to validate the reliability of the developed icELISA. The sample pretreatment method was referred to the National Standard of China (GB5009.212-2016). Briefly, the chopped sample was homogenized and then accurately weighed 2 g (accurate to 0.01 g) to 9 mL of methanol, followed by vortexing for 1 min. The spiked concentrations in the shellfish samples were at 80, 40, 20, 10, and 5 μg kg−1, respectively. After the sample was extracted by ultrasonic for 10 min, then centrifuged at 8000 r/min for 5 min, and the supernatant solution was transferred into a 20 mL graduated glass tube. The residue was added to 9 mL of methanol and the extraction was repeated once. After the extracts were combined, the volume was adjusted to 20 mL with methanol. To a screw-top sample bottle was added 1 mL of the extract and 125 μL of sodium hydroxide solution (2.5 mol L−1). Then, the bottle was sealed and incubated at 76°C for 40 min, and then cooled to room temperature. Finally, 125 μL of hydrochloric acid solution (2.5 mol L−1) was added to the bottle and mixed well with the extract. The obtained hydrolyzate (1.25 mL equivalent to 0.1 g sample) was passed through a 0.22 μm organic phase microporous membrane for HPLC-MS determination. The chromatographic column was Agilent C18 (2.1 × 100 mm, Φ3.5 μm). Mobile phase A is ammonium formate solution (2 mM), and B is acetonitrile and ammonium formate solution (2 mM) (95:5). Samples were analyzed by electrospray ionization source with negative ion scanning.
Results and discussion
Identification of conjugates
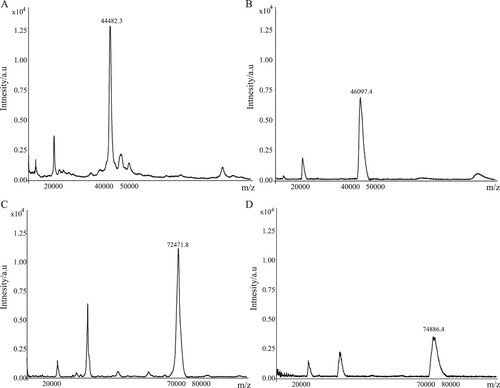
We used the carboxylic acid group of OA molecule to couple with the amino group of protein carriers (KLH or OVA) by the active ester method to synthesize immunogen and coating antigen. The amount of OA coupled with the protein carrier was determined by MALDI TOF/TOF MS analysis. The results showed that the m/z values of the immunogen (OA-KLH) and KLH were 74,886.4 and 72,471.8, respectively, and the m/z values of the coating antigen (OA-OVA) and OVA were 46,097.4 and 44,482.3, respectively (). Therefore, the coupling ratios of OA-KLH and OA-OVA were about 3 and 2, respectively, indicating the success of OA conjugated to the protein carriers.
Characterization of the mAb
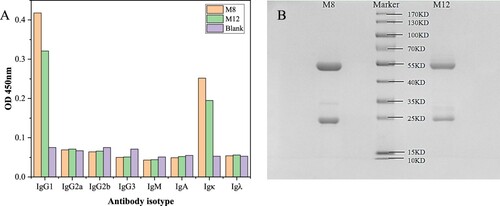
After cell fusion, icELISA was performed to screen hybridomas cells, the hybridoma cell lines M8 and M12 showing high sensitivity and specific inhibition for OA were cloned and used to produce monoclonal antibody. The monoclonal cell lines M8 and M12 were identified by cell subtype detection ((A)), and the results showed that both M8 and M12 were determined as the isotype of IgG1 with a kappa light chain. The antibody was purified by Protein G column, and the purity of the antibody was detected by SDS-PAGE. As shown in (B), M8 and M12 have clear bands with molecular weights around 25KD and 55KD. The band at 25KD is the antibody light chain, and the band at 55KD is the antibody heavy chain. Moreover, there is no obvious impurity protein band, indicating that the purity of the antibody is good.
The analytical performance of icELISA
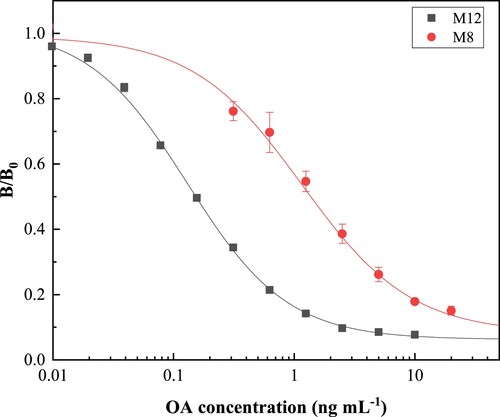
For immunoassays, the half maximum inhibitory concentration (IC50) is often used as a measurement parameter and reflects the sensitivity performance of the analytical method. The standard curves based on the two mAbs were shown in . The icELISA based on M8 antibody provided good detection linearity in the range of 0.12–41.92 ng mL−1 with a IC50 value of 1.37 ng mL−1. The icELISA based on M12 antibody presented the best sensitivity of IC50 value of 0.15 ng mL−1 with a good detection linearity in the range of 0.020–2.74 ng mL−1. Compared with the reported mAbs evaluated by the same immunoassay format (), our M12 mAb shows the best IC50 value (Wang et al., Citation2010; Wang et al., Citation2017). Compared to other reported icELISAs and commercial ELISA kits, the presented icELISA based on the M12 mAb demonstrated the operational and analytical advantages with lower IC50 and wider linear detection range. Moreover, the specificity of M12 mAb was determined with different analogs. As shown in , M12 mAb showed the greatest affinity for OA, and less cross reactivity with other marine toxins.
Table 1. The analytical performance comparison of relevant works.
Table 2. Cross-reactivities of M12 mAb against other marine toxins in the icELISA.
Influence of methanol matrix
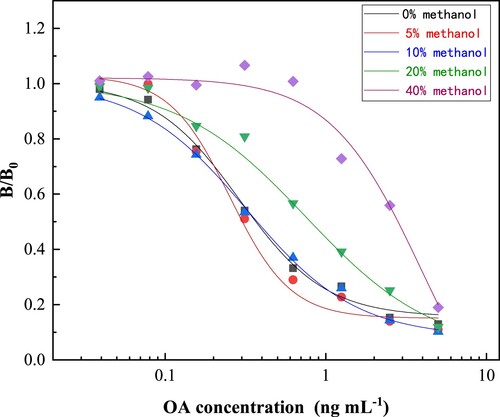
Because methanol is an effective solvent for the extraction of lipophilic shellfish toxins, the effect of methanol content on the icELISA was tested by comparing several standard inhibition curves established under different conditions. As shown in , the results showed that the methanol concentrations of 5% and 10% had little effect on the standard curves. However, when the methanol concentrations were from 20% to 40%, the IC50 values were also increased. Therefore, methanol residues of less than 10% in the PBSTG are recommended to minimize its interference with the icELISA. In the present study, the samples were extracted with 90% methanol, and then diluted in PBSTG by at least 10 times, which makes the methanol concentration in the test solution acceptable.
Detection and verification of spiked sample recoveries
To verify the accuracy and precision of icELISA, recovery tests were performed on real samples using icELISA and HPLC-MS methods, respectively (Wang et al., Citation2017). Oyster and mussel samples were fortified with different concentrations of OA standard, and each sample was processed and analyzed three times. As shown in , the recoveries of spiked shellfish samples measured by icELISA varied from 88.43% to 114.89% for OA, and the CV varied from 2.18% to 6.04%. These results demonstrated that the recoveries obtained from icELISA using M12 mAb were within acceptable ranges of accuracy and precision (Poungmalai et al., Citation2021). The recoveries of spiked shellfish samples measured by HPLC-MS ranged from 91.69% to 98.35% for OA, and the CV varied from 2.20% to 4.97%. The spiked recovery results of icELISA and HPLC-MS have a good correlation, indicating that the developed icELISA has good accuracy and reproducibility. In the previous study, Pang et al. reported immunoassay recoveries for OA ranging from 96.56% to 102.5% (Pang et al., Citation2019). Therefore, the sample recoveries of the icELISA and CVs in this study meet the requirements of immunoassay detection.
Table 3. Average recoveries of okadaic acid spiked in oyster and mussel samples.
Detection and verification of fresh shellfish samples
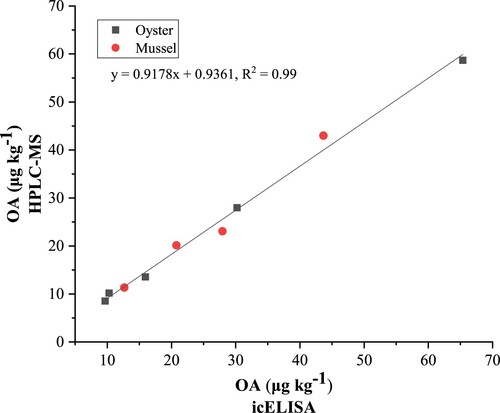
Several oyster and mussel samples were purchased from seafood markets in Hainan Island. Samples O-1 to O-4 and M-1 to M3 were purchased in August 2020, and the other samples were obtained in April 2021. These samples were tested by the developed icELISA and HPLC-MS, respectively. The comparison of the analysis results based on the two methods is shown in . The detection result of real samples showed that the developed icELISA had similar results obtained by HPLC-MS, indicating that the icELISA has reliable performance and accuracy. We further analyzed the correlation between icELISA and HPLC-MS based on the analysis results of oysters and mussels (). The linear regression equation is y = 0.9178x + 0.9361. The correlation coefficient was 0.99 (R2 = 0.99), indicating that the icELISA detection results are accurate and can be used for the detection of real samples (Wang et al., Citation2022). Since OA is commonly accumulated in shellfish, its accurate and rapid detection will help reduce the occurrence of related poisoning events and economic losses in aquaculture industry.
Table 4. Detection of real samples by icELISA and HPLC-MS.
Conclusion
In the present study, OA-KLH conjugate was synthesized as a new immunogen and was mixed with a new non-emulsifying immune adjuvant to improve the immune response of mice. A highly sensitive and specific mAb against OA was obtained and used for icELISA development. To our knowledge, the developed icELISA showed an IC50 of 0.15 ng mL−1 against OA which is more sensitive than other icELISA results previously reported. The results determined by icELISA and HPLC-MS/MS of spiked samples or real samples had a good correlation. The developed mAb-based icELISA was highly sensitive, which has been demonstrated that suitable for high-throughput detection of OA in oysters and green mussels to reduce the risk of residues.
Acknowledgements
This work was supported by Hainan Provincial Science and Technology Special Fund (ZDYF2022SHFZ082), Natural Science Foundation of Hainan Province (821RC556), and the special fund of the State Key Joint Laboratory of Environmental Simulation and Pollution Control (20K10ESPCT) from Tsinghua University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Conceptualization, H.Z. and Y.J.; methodology, C.L. and Y.J; validation, C.L., J.M and C.Z.; resources, C.Z.; data curation, C.Z.; writing-original draft preparation, C.L and Y.J.; writing-review and editing, H.Z.; visualization, C.L.; supervision, C.Z.; funding acquisition, Y.J and C.Z. All authors have read and agreed to the published version of the manuscript.
Additional information
Funding
References
- Aune, T., Yasumoto, T., & Engeland, E. (1991). Light and scanning electron microscopic studies on effects of marine algal toxins toward freshly prepared hepatocytes. Journal of Toxicology and Environmental Health, Part A Current Issues, 34(1), 1–9. https://doi.org/10.1016/j.jhazmat.2017.06.030
- Ciminiello, P., & Fattorusso, E. (2006). Bivalve molluscs as vectors of marine biotoxins involved in seafood poisoning. Molluscs, 53–82. https://doi.org/10.1007/978-3-540-30880-5_3
- Dickey, R. W., Bobzin, S. C., Faulkner, D. J., Bencsath, F. A., & Andrzejewski, D. (1990). Identification of okadaic acid from a Caribbean dinoflagellate, Prorocentrum concavum. Toxicon, 28(4), 371–377. https://doi.org/10.1016/0041-0101(90)90074-H
- Fang, J., Qiu, X., Wan, Z., Zou, Q., Su, K., Hu, N., & Wang, P. (2016). A sensing smartphone and its portable accessory for on-site rapid biochemical detection of marine toxins. Analytical Methods, 8(38), 6895–6902. https://doi.org/10.1039/C6AY01384H
- Garibo, D., Campbell, K., Casanova, A., De La Iglesia, P., Fernández-Tejedor, M., Diogène, J., Elliott, C. T., & Campàs, M. (2014). SPR immunosensor for the detection of okadaic acid in mussels using magnetic particles as antibody carriers. Sensors and Actuators B: Chemical, 190, 822–828. https://doi.org/10.1016/j.snb.2013.09.037
- Garibo, D., de la Iglesia, P., Diogene, J., & Campas, M. (2013). Inhibition equivalency factors for dinophysistoxin-1 and dinophysistoxin-2 in protein phosphatase assays: Applicability to the analysis of shellfish samples and comparison with LC-MS/MS. Journal of Agricultural and Food Chemistry, 61(10), 2572–2579. https://doi.org/10.1021/jf305334n
- Lan, J., Wang, M., Ding, S., Fan, Y., Diao, X., Li, Q., & Zhao, H. (2019). Simultaneous detection of carbofuran and 3-hydroxy-carbofuran in vegetables and fruits by broad-specific monoclonal antibody-based ELISA. Food and Agricultural Immunology, 30(1), 1085–1096. https://doi.org/10.1080/09540105.2019.1664997
- Li, R., Cao, L., Liang, C., Sun, S., Liu, H., & Yan, P. (2020). Development and modeling of an ultrasensitive label-free electrochemical immunosensor for okadaic acid based on polythionine-modified three-dimensional gold nanoelectrode ensembles. Ionics, 26(9), 4661–4670. https://doi.org/10.1007/s11581-020-03599-1
- Lin, C., Liu, Z. S., Wang, D. X., Ren, H. L., Li, Y. S., Hu, P., Zhou, Y., Guo, Y. P., Meng, X. M., & Lu, S. Y. (2014). Sensitive and reliable micro-plate chemiluminescence enzyme immunoassay for okadaic acid in shellfish. Analytical Methods, 6(18), 7142–7148. https://doi.org/10.1039/C4AY01063A
- Ling, S., Li, X., Zhang, D., Wang, K., Zhao, W., Zhao, Q., Wang, R., Yuan, J., Xin, S., & Wang, S. (2019). Detection of okadaic acid (OA) and tetrodotoxin (TTX) simultaneously in seafood samples using colloidal gold immunoassay. Toxicon, 165, 103–109. https://doi.org/10.1016/j.toxicon.2019.04.011
- Louppis, A. P., Badeka, A. V., Katikou, P., Paleologos, E. K., & Kontominas, M. G. (2010). Determination of okadaic acid, dinophysistoxin-1 and related esters in Greek mussels using HPLC with fluorometric detection, LC-MS/MS and mouse bioassay. Toxicon, 55(4), 724–733. https://doi.org/10.1016/j.toxicon.2009.10.026
- Lu, S. Y., Zhou, Y., Li, Y. S., Lin, C., Meng, X. M., Yan, D. M., Li, Z. H., Yu, S. Y., Liu, Z. S., & Ren, H. L. (2012). Production of monoclonal antibody and application in indirect competitive ELISA for detecting okadaic acid and dinophytoxin-1 in seafood. Environmental Science and Pollution Research, 19(7), 2619–2626. https://doi.org/10.1007/s11356-012-0819-y
- Lu, S. Y., Zhou, Y., Li, Y. S., Zhang, D. H., Yu, G., Yu, S. Y., & Reng, H. L. (2009). ELISA and HPLC-MS/MS for detecting okadaic acid in shellfish. Food Science, 30(08|8), 158–162. https://doi.org/10.3321/j.issn:1002-6630.2009.08.032
- Molinero-Abad, B., Perez, L., Izquierdo, D., Escudero, I., & Arcos-Martinez, M. J. (2019). Sensor system based on flexible screen-printed electrodes for electrochemical detection of okadaic acid in seawater. Talanta, 192, 347–352. https://doi.org/10.1016/j.talanta.2018.09.072
- Mouratidou, T., Kaniou-Grigoriadou, I., Samara, C., & Kouimtzis, T. (2006). Detection of the marine toxin okadaic acid in mussels during a diarrhetic shellfish poisoning (DSP) episode in Thermaikos Gulf, Greece, using biological, chemical and immunological methods. Science of the Total Environment, 366(2–3), 894–904. https://doi.org/10.1016/j.scitotenv.2005.03.002
- Oteri, G., Stammati, A., Zampaglioni, F., & Zucco, F. (1998). Evaluation of the use of two human cell lines for okadaic acid and DTX-1 determination by cytotoxicity assays and damage characterization. Natural Toxins, 6(5), 197–209. https://doi.org/10.1002/(SICI)1522-7189(199809/10)6:5<197::AID-NT21>3.0.CO;2-N
- Pang, L., Quan, H., Sun, Y., Wang, P., Ma, D., Mu, P., Chai, T., Zhang, Y., & Hammock, B. D. (2019). A rapid competitive ELISA assay of okadaic acid level based on epoxy-functionalized magnetic beads. Food and Agricultural Immunology, 30(1), 1286–1302. https://doi.org/10.1080/09540105.2019.1689231
- Pasquazzi, F. M., Sanfilippo, L., Moscetta, P., & Chiavarini, S. (2019). Re-modeling ELISA kits embedded in an automated system suitable for on-line detection of algal toxins in seawater. Sensors and Actuators B: Chemical, 283, 865–872. https://doi.org/10.1016/j.snb.2018.12.083
- Peng, J., Zhao, Z., Zheng, M., Su, B., Chen, X., & Chen, X. (2020). Electrochemical synthesis of phosphorus and sulfur co-doped graphene quantum dots as efficient electrochemiluminescent immunomarkers for monitoring okadaic acid. Sensors and Actuators B: Chemical, 304, 127383. https://doi.org/10.1016/j.snb.2019.127383
- Poungmalai, P., Buakeaw, A., Puthong, S., & Khongchareonporn, N. (2021). A specific monoclonal antibody for chlortetracycline detection in milk and honey samples based on ELISA. Food and Agricultural Immunology, 32(1), 163–173. https://doi.org/10.1080/09540105.2021.1897531
- Sakaguchi, Y., Kawamura, R., Nakayama, E., Ako, K., Kawasue, S., Koga, R., Yoshida, H., & Nohta, H. (2021). Selective analysis of the okadaic acid group in shellfish samples using fluorous derivatization coupled with liquid chromatography-tandem mass spectrometry. Journal of Chromatography B, 1173, 122681. https://doi.org/10.1016/j.jchromb.2021.122681
- Smienk, H. G., Calvo, D., Razquin, P., DomÃnguez, E., & Mata, L. (2012). Single laboratory validation of a ready-to-use phosphatase inhibition assay for detection of okadaic acid toxins. Toxins, 4(5), 339–352. https://doi.org/10.3390/toxins4050339
- Souid-Mensi, G., Moukha, S., Mobio, T. A., Maaroufi, K., & Creppy, E. E. (2008). The cytotoxicity and genotoxicity of okadaic acid are cell-line dependent. Toxicon, 51(8), 1338–1344. https://doi.org/10.1016/j.toxicon.2008.03.002
- Takai, A., Murata, M., Torigoe, K., Isobe, M., Mieskes, G., & Yasumoto, T. (1992). Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochemical Journal, 284(2), 539–544. https://doi.org/10.1042/bj2840539
- Tubaro, A., Florio, C., Luxich, E., Vertua, R., & Yasumoto, T. (1996). Suitability of the MTT-based cytotoxicity assay to detect okadaic acid contamination of mussels. Toxicon, 34(9), 965–974. https://doi.org/10.1016/0041-0101(96)00073-6
- Vale, P., & Sampayo, M. A. D. M. (1999). Comparison between HPLC and a commercial immunoassay kit for detection of okadaic acid and esters in Portuguese bivalves. Toxicon, 37(11), 1565–1577. https://doi.org/10.1016/S0041-0101(99)00105-1
- Vale, P., Veloso, V., & Amorim, A. (2009). Toxin composition of a Prorocentrum lima strain isolated from the Portuguese coast. Toxicon, 54(2), 145–152. https://doi.org/10.1016/j.toxicon.2009.03.026
- Wang, L., Sang, Y. X., & Wang, X. H. (2011). Enzyme-linked immunosorbent assay for okadaic acid: Investigation of analytical conditions and sample matrix on assay performance. Journal of AOAC International, 94(5), 1531–1539. https://doi.org/10.5740/jaoacint.10-412
- Wang, Q., Yan, Y. N., & Huang, K. H. (2010). Preparation of monoclonal antibody against okadaic acid and establishment of icELISA for detecting its residues. Animal Husbandry & Veterinary Medicine (in Chinese), 42(08|8), 1–5. https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjIwNDE1Eg54bXlzeTIwMTAwODAwMRoIOHFteDZycmY%3D
- Wang, R., Zeng, L., Yang, H., Zhong, Y., Wang, J., Ling, S., farhan Saeed, A., Yuan, J., & Wang, S. (2017). Detection of okadaic acid (OA) using ELISA and colloidal gold immunoassay based on monoclonal antibody. Journal of Hazardous Materials, 339, 154–160. https://doi.org/10.1016/j.jhazmat.2017.06.030
- Wang, Y., Ding, M., Ma, H., Wu, J., Zhao, H., & Wan, Y. (2022). Development of a specific monoclonal antibody-based icELISA for detection of arecoline in traditional Chinese medicines and fresh areca nuts. Food and Agricultural Immunology, 33(1), 113–126. https://doi.org/10.1080/09540105.2021.2025347
- Zhang, H., Luo, J., Beloglazova, N., Yang, S., De Saeger, S., Mari, G. M., Zhang, S., Shen, J., Wang, Z., & Yu, X. (2019). Portable multiplex immunochromatographic assay for quantitation of two typical algae toxins based on dual-color fluorescence microspheres. Journal of Agricultural and Food Chemistry, 67(21), 6041–6047. https://doi.org/10.1021/acs.jafc.9b00011
- Zou, L., Tian, Y., Zhang, X., Fang, J., Hu, N., & Wang, P. (2017). A competitive love wave immunosensor for detection of okadaic acid based on immunogold staining method. Sensors and Actuators B: Chemical, 238, 1173–1180. https://doi.org/10.1016/j.snb.2016.05.030