ABSTRACT
Amantadine is one of the most prescribed antiviral medications. Considering its abuse is life-threatening, monitoring amantadine residues is urgently needed. This study designed a dipstick assay based on lateral flow immunoassay (LFIA) platforms with an indicator range of 50–200 ng/mL. The dipstick consists of four coloured test lines that detect the concentration of amantadine semi-quantitatively in the range of 0–200 ng/mL. The amantadine concentration was categorised as 0–50, 50–100, 100–200, or > 200 ng/mL, depending on the response of the test lines. Furthermore, we measured the amantadine residues in commercial meat samples by this LFIA. The results were highly agreeable with those determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The LFIA shows potential for routine monitoring of amantadine in meat products, and the results of this study provide insight into the development of new LFIA devices for use in agricultural industries.
1. Introduction
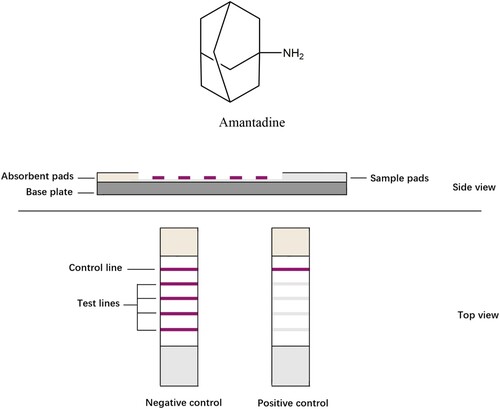
Amantadine () is a widely used pharmaceutical for humans and animals worldwide. It was initially developed in the early 1960s and registered for its anti-influenza A2 activity in 1966 (Maj et al., Citation1974). In addition, patients suffering from Parkinson’s disease showed radical improvement of their symptoms upon taking amantadine. Subsequently, many clinical studies were performed with positive outcomes (Giombini et al., Citation1970), and amantadine was registered as a treatment for Parkinson’s disease. In the next few decades, amantadine became very popular in clinical studies and exhibited many other therapeutic uses, such as anti-tremor (Manyam, Citation1981) and sigma receptor binding activities (Kornhuber et al., Citation1993). In recent years, its efficacy in traumatic brain injury (TBI) and cellular protection has been extensively studied (Brison et al., Citation2014; Khasanova et al., Citation2009; Quarato et al., Citation2014). Moreover, very recently, the putative activity of amantadine against COVID-19 has been widely discussed (Aranda-Abreu, Citation2020; Rejdak & Grieb, Citation2020; Wiwanitkit, Citation2020).
Besides human medicine, amantadine is the most commonly prescribed antiviral drug for the treatment of animal diseases (Liang et al., Citation2016; Speltini et al., Citation2015; Tsuruoka et al., Citation2017; Zhao et al., Citation2017). The therapeutic efficacy is dose-dependent, and the drugs can be transferred through the food chain and consumed by humans. Thus, the abuse of amantadine can lead to potential health risks, such as significant adverse reactions in the gastrointestinal and central nervous systems (Jefferson et al., Citation2000).
Methods for determining amantadine contents include liquid chromatography tandem mass spectrometry (Wang et al., Citation2021), triple quadrupole mass spectrometry (Tang et al., Citation2020), fluorescence (Dong et al., Citation2019; Yu et al., Citation2019), nanochannel sensor (Xie et al., Citation2020), colorimetric immunoassay (Ma et al., Citation2020), and potentiometric analysis (Elzanfaly & Saad, Citation2017). However, these assays often require trained personnel, sophisticated instrumentation, and time-consuming procedures. Therefore, they are not appropriate for rapid analysis of amantadine in the field. In contrast, antibody-based lateral flow immunoassay (LFIA) is user-friendly, rapid, sensitive, portable, and offers high-throughput analysis (Gao et al., Citation2019; Pan et al., Citation2019; Wu et al., Citation2017). Recently, LFIAs have been developed for a broad range of applications, including drug and food safety.
LFIAs are polyvinyl chloride (PVC) plate-based platforms, which contain one basic nitrocellulose (NC) membrane sticking to the plate, the analyte, its antibody/antigen, or their complexes that are often conjugated to a coloured label and immobilised onto the NC membrane as control or test capture reagents. When liquid samples containing the target analyte moves along the strip, the molecules interact with various capture reagents. The coloured lines and their intensities in the test zone can be observed by naked eyes in 5–30 min. Conventional LFIAs have one single test line, determining whether a sample contains target analyte in limit of detections. In this study, we established a LIFA with four test lines to realise semi-quantitative determination.
To date, our laboratory has developed many highly reliable analytical methods for amantadine and other harmful residues in foods (Jia et al., Citation2018; Mu et al., Citation2016; Weng et al., Citation2020). In this study, we continued the development of our antibody-based dipstick method, based on one antibody against amantadine, into a semi-quantitative, rapid, and multi-range LFIA for amantadine detection.
2. Materials and methods
2.1. Chemicals and reagents
Anti-amantadine antibodies and amantadine conjugated to bovine serum albumin (BSA), as a coating antigen, were obtained from Guangzhou Ucando Biotechnology Co., Ltd. (Guangzhou, China). Goat anti-mouse IgG antibodies and bovine serum albumin (BSA) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Analytical standards of amantadine (CAS number 768-94-5, MW 151.25 g/mol, purity 98%), enrofloxacin (CAS number 93106-60-6, MW 359.39 g/mol, purity 98%), ofloxacin (CAS number 82419-36-1, MW 361.37 g/mol, purity 98%), and florfenicol (CAS number 73231-34-2, MW 358.21 g/mol, purity 98%) were purchased from Alta Technology (Tianjin, China). For dipstick assembly, the precious dispenser and sprayer (BioDot XY-3000), colloidal gold with an average particle diameter of 30 nm, glass fiber-based sample-conjugate pads, nitrocellulose membranes, and cotton linter fiber-based absorbent pads were purchased from Shanghai Jieyi Biotechnology Co., Ltd. (Shanghai, China). HPLC-grade acetonitrile (ACN) and methanol were obtained from Fisher Scientific (Fair Lawn, NJ, USA). HPLC-grade formic acid was obtained from Sigma Aldrich (St. Louis, MO, USA). Ultra-pure water was prepared using a Milli Q-plus system (Billerica, MA, USA). All other chemicals for the preparation of buffer and organic solvents were of analytical grade and were purchased from Beijing Chemical Reagent Co., Ltd (Beijing, China).
2.2. Preparation of anti-amantadine antibodies
The optimum conditions for the antibody were obtained by dialysis. Briefly, 30 kD–2.2 nm dialysis membrane was cut to an appropriate size and sealed at both ends. The antibody dissolved in PBS was transferred to the membrane, and the membrane was sealed. The antibody was dialysed with 2 L of 0.1 M PBS at 4°C for at least 4 h. The process was repeated once. Afterwards, the PBS was replaced by distilled water, and dialysis was continued with 2 L distilled water at 4°C for at least 4 h. This step was repeated once. The purified antibody was lyophilised in a vacuum freezing dryer at 4°C for 24 h, dissolved with ultrapure water, and stored for later use.
2.3. Optimisation of antibody loading on colloidal gold particles
The optimisation of antibody loading on colloidal gold particles was performed by the traditional sodium chloride method. Five 1.5-mL centrifuge tubes were prepared. One millilitre colloidal gold was added to each tube, followed by the slow addition of 50 mL potassium borate with sufficient mixing in order to prevent a rapid reaction. Then, the antibody was dispensed in increasing concentrations (0.5, 1, 1.5, 2, and 2.5 μL) into each tube. Next, 50 mL of 10% sodium chloride solution was added to each tube. The surface of colloidal gold is hydrophobic, and the antibodies can be immobilised with the colloidal gold simply by physical adsorption. If the number of antibodies added was appropriate, the surface of colloidal gold particles would be fully occupied by antibodies; if there were not enough antibodies, the surface of colloidal gold particles will be occupied by NaCl, which made the solution turn from purple to blue. We could judge whether the antibody was enough simply by observing the colour of the colloidal gold solutions.
2.4. Development of a colloidal gold-based assay for amantadine
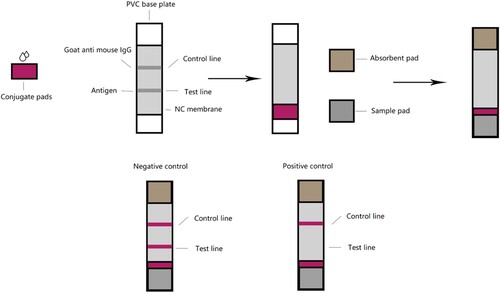
A dipstick was made of four parts: a NC membrane; the PVC plate as backing card for the test strip; and two pads, one for the sample and one for the absorbent. Colloidal gold with an average particle diameter of 30 nm (G30) and 16 nm (G16) was purchased from Shanghai Jieyi Biotechnology. Twenty microliters of aqueous anti-amantadine antibody solution at 1.5 mg/mL were added to 0.5 mL of colloidal gold solution (pH 7.8). After gentle stirring for 10 min, 50 μL of 10% (w/v) BSA was added to the solution. Then, the mixture was centrifuged at 9600 ×g for 20 min. The pellet was resuspended in 500 μL of 0.01 M PBS (pH 7.0) and stored at 4°C. Finally, the conjugate pad was saturated with the gold-antibody conjugate and dried at room temperature. Goat anti-mouse IgG was used as a control capture reagent. Amantadine-BSA antigen in increasing concentrations of 0.1, 0.2, 0.5, and 1 mg/mL, respectively, was used as the capture reagent and was immobilised on the four test lines on the membrane from bottom to top. Goat anti-mouse IgG (1 μL/cm) was immobilised on the nitrocellulose (NC) membrane as a control capture reagent. The distance between the control and each test line was 3 mm. The NC membrane was adhered to the centre of a PVC plate, and the colloidal gold-Ab conjugate pad was applied to the plate by overlapping with the NC membrane. An absorption pad was adhered to the plate at the opposite end, downstream from the control line, and the sample pad was applied by overlapping with the conjugate pad. The card was cut to 3 mm width, sealed in a plastic case with desiccant gel, and stored at 4°C.
2.5. Preparation of standard solution
Stock solutions (1000 mg/L) of target analyte amantadine and the other three analytes, enrofloxacin, ofloxacin, and ciprofloxacin, were dissolved in methanol and stored in the dark at 4°C. Each drug was further diluted, depending on the optimal working conditions of two analysis methods: LFIA and LC-MS/MS. For dipsticks, the LOD concentration of amantadine should be about 500 ng/mL, and for the LC-MS/MS, because of its sensitivity, the concentration of amantadine must be much lower. The working solution for the LFIA was obtained by adding 0.1% BSA and 1% Tween-20 solution to 0.01 M PBS and shaking well. The working solution for the LFIA showed no obvious degradation and was valid for at least 14 days, and it was stored at 4°C.
2.6. Preparation of meat samples for analysis
All meat samples were finely ground and homogenised using a grinding mill and stored at –20°C. Two grams of the sample were weighed in a 50-mL centrifuge tube and extracted with 3.2 mL acetonitrile and 0.8 mL EDTA solution by vortexing for 5 min. The extract was centrifuged at 4°C, 10,000 r/min for 5 min, and the supernatant was transferred in a new 50-mL centrifuge tube. The sediment was extracted again with 3.2 mL 2% acetic acid in acetonitrile and 0.8 mL EDTA solution by vortexing for 5 min, followed by centrifugation at 4°C, 10,000 r/min for 5 min. Finally, the two supernatants were combined, and 1 mL EDTA solution was added. The extract was further diluted with acetonitrile, based on the appropriate concentration for the dipstick and LC-MS/MS instrument.
2.7. Dipstick assay
Amantadine standard methanol solutions at concentrations of 0, 50, 100, 200, and 500 ng/mL were used to determine the sensitivity of the dipstick as described. Sensitivity is expressed as the indicator range of the dipstick, i.e. the lowest concentration of the target analyte at which the test line was not visible. A number of commonly used antimalarial drugs, including enrofloxacin derivatives, were used to evaluate the specificity of the assay. The stock solutions of these drugs were prepared at 1 mg/mL, as described previously, and each sample was analysed in triplicate. The assay results were interpreted according to the colour change of each of the four test lines. For a valid assay, in which the control line was visible, if the test line showed no colour change, the sample contained amantadine residues. According to the different numbers of coloured test lines, the concentration of amantadine is determined. Further testing at a concentration 10 times lower than the concentration at which the test line showed no colour change was generally done to confirm the finding. If the test line showed obvious colour change, the sample was considered containing lower-level amantadine residues, but if the test line showed no colour change, the sample had a high possibility of amantadine contamination.
2.8. LC-MS/MS analysis of amantadine
LC-MS/MS analysis of amantadine standards and other three drug samples, i.e. enrofloxacin, ofloxacin and ciprofloxacin, were performed using a SCIEX Exion LC system equipped with a degasser, a dual pump, a column compartment, and an autosampler (Wilmington, DE, USA). Chromatographic separations were performed on an XDB-C18 column (2.1 mm × 150 mm, 3.5 μm particle size) from Agilent at a flow rate of 0.3 mL/min, at room temperature. The injection volume was 10 μL. The mobile phase consisted of eluent A (0.1% volume ratio of formic acid in water) and eluent B (methanol). The gradient elution programme was as follows: 90% A, linear decrease to 10% A over 2 min, hold for 3 min, and return to 90% A in 0.1 min. The column was equilibrated for 5 min after each run, and the entire cycle time was 10 min.
Mass spectrometric analysis was performed using a QTRAP 6500 + triple quadrupole linear ion trap mass spectrometry (SCIEX, USA). The positive electrospray ionisation voltage was 5.0 kV, the source temperature was 500°C, and nitrogen was the collision gas. The operational software for data collection and analysis was Analyst 1.6.3 (SCIEX). The MRM parameters are described in our previous report (Jia et al., Citation2018).
3. Results
3.1. Development of the colloidal gold-based LFIA for amantadine
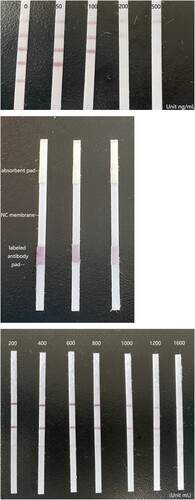
To obtain the labelled specific antibodies against amantadine, colloidal gold particles were employed. With an antigen consisting of amantadine linked to BSA, the four concentration gradients of amantadine-BSA were optimised and were coated on the nitrocellulose membrane as four test lines ((A)). The LFIA was assembled ((B)) and had an indicator range of 50–200 ng/mL for amantadine, and according to the number of test lines, the assay provides semi-quantitative results. When all four coloured test lines appeared, the concentration of amantadine was 0–50 ng/mL. When three coloured test lines appeared, the concentration was 50–100 ng/mL. When two coloured test lines appeared, the concentration was 100–200 ng/mL, and if only one coloured test line appeared, the amantadine concentration was greater than 200 ng/mL ((C)).
Figure 2. The structure, assembly and LOD of the LFIA. (A) Diagram of the lateral flow immunoassay (LFIA) structure. (B) The assembly of the LFIA. (C) Determination of the limit of detection for amantadine. Amantadine was applied to the LFIA at increasing concentrations (0, 50, 100, 200, and 500 ng/mL). Note the disappearance of the test line as amantadine concentration reached 50–200 ng/mL, which is considered the limit of detection for the device.
3.2. Optimisation of the LFIA
A series of experiments were carried out to optimise the experimental conditions of the LFIA. The working conditions, such as the special working solutions, the concentration of potassium borate, the solvent used to dissolve regents, and the drying time and heating temperature of the NC membranes and colloidal gold-labelled antibody conjugate pad are very influential. These variables were optimised by altering one variable at a time and keeping the rest constant.
In order to make the dipsticks visible and make sure that the colloidal gold-antibodies on the conjugate pad were fully dissolved and released, 1% Tween-20, which is a commonly used surfactant, was added to the working solution, and 10% sugar was added to re-dissolve the labelled antibody ((A)). In addition, we optimised the volume of the solvent used to dissolve colloidal gold-labelled antibodies. The optimal composition of the working solution was determined to be 0.1% BSA in 0.01 M PBS solution with 1% Tween-20. The optimal volume to dissolve the labelled antibodies was 400 μL ((B)).
Figure 3. Optimisation of the LFIA. (A) Optimisation of the working solutions for the lateral flow immunoassay (LFIA). LFIA using conventional working solution, from left to right are water, PBS, and 10% alcohol water solution, all of their C lines can’t be coloured. (B) Optimisation of the solvent volume used to dissolve colloidal gold-labelled antibodies. The colour of lines decreased with the concentration of PB increased. Considering the production cost and colour of lines, the optimal volume to dissolve the labelled antibodies was 400 μL. (C) Optimisation of the antigen concentration immobilised on the NC membrane. The concentration of the antigen increased from 0.05 to 3 mg/mL, and the four most recognisable concentrations were finally selected as the scribing concentrations.
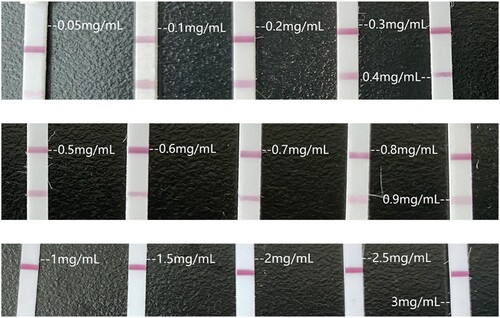
In order to determine the most suitable antigen concentration on the NC membrane surface, we tested a series of antigen concentrations. When the antigen concentration decreased, the colour of the lines faded, making it difficult to interpret the results. On the contrary, at a higher concentration, a higher concentration of amantadine was necessary to make the test lines invisible; in this case, the colour became too strong, which decreased the working range of the dipsticks. Finally, the amantadine-BSA antigen concentrations for test line use were decided to be 0.1, 0.2, 0.5, and 1 mg/mL, respectively ((C)).
3.3. Specificity of lateral flow immunoassay for four commonly used veterinary drugs
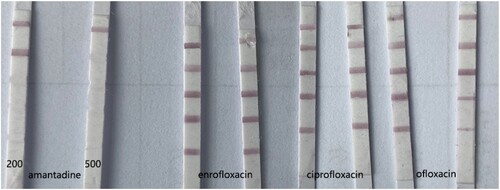
In addition to amantadine, common veterinary drugs such as enrofloxacin, ciprofloxacin and ofloxacin are often detected on livestock and poultry products. On this study, we also verified the specificity of this amantadine LFIA, standard samples of those four veterinary drugs were tested by LIFA, the result was showed on . This result shows that the LIFA dipsticks developed in this study has the ability to specifically detect amantadine.
3.4. Semi-quantitative analysis of the commercial meat samples
Amantadine residues in eight commercial meat samples were analysed using the optimised LFIA. Amantadine contents in these samples were first determined by LC-MS/MS (), and five samples had amantadine contents consistent with the residue limits of national standards, namely there are no amantadine residues in those five samples. The contents in the other three samples exceeded the threshold value, and the meats were considered harmful if consumed. For validation of the LFIA, all four coloured test lines appeared for five of the samples, three coloured test lines appeared for two of the samples, and two coloured test lines appeared for one sample, indicating that the concentrations were not detectable, 50–100 ng/mL, and 100–200 ng/mL, respectively (). The results of the two methods were in agreement, indicating that our LFIA method is reliable and could have commercial applications ().
Figure 5. Three of all the mass spectra for amantadine in commercial livestock and poultry products.
Figure 6. Images of the LFIA results of the analysis of commercial livestock and poultry products. Each sample was analysed in triplicate. Sample 1–5 had no residue, whereas sample 6–8 showed no colour of the four test lines, indicating the amantadine contamination. Upper and lower arrows indicate the control line (C) and the four test lines (T), respectively.
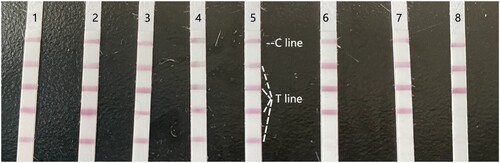
Table 1. Validation of the lateral flow immunoassay (LFIA) for amantadine determination via LC-MS/MS.
4. Discussion
Amantadine is widely used in veterinary medicine as an antiviral drug; however, amantadine residues in meat products pose serious health risks. The chemical structure is too simple to carrier proteins for rendering its immunogenic (Guo et al., Citation2021), which made the antigen design difficult, and this poses a significant tricky in obtaining the antibody of amantadine for our laboratory. Therefore, we employed the antibody and corresponding antigen, which coated the surface of gold NPs. The antibody was dialysed for 4 h to remove salts and glycerin before conjugation with colloidal gold particles.
The development of LFIAs for several drugs has been reported (Chen et al., Citation2021; Walter et al., Citation2021; Zou et al., Citation2021). Although many LFIAs have been reported, the design of dipsticks and the technology are still the same. Here, we report a novel LFIA design, consisting of one control line and four test lines, to provide a semi-quantitative analysis of amantadine. The four test lines were achieved by coating a gradient concentration of antigen, i.e. amantadine-BSA conjugate. The four test lines, from bottom to top, contained 0.1, 0.2, 0.5, and 1 mg/mL of antigen, respectively. When the sample flowed through the nitrocellulose membrane via capillary action, amantadine in the sample interacted with the four test lines sequentially. The first test line, from the bottom, had minimum antigen, meaning that even a low concentration of amantadine will generate a colour change. The fourth test line, from the bottom, contained the highest concentration of the antigen. Therefore, it could determine small concentrations of amantadine remaining in the sample after interacting with the other three test lines. For higher concentrations of amantadine, the sample has to flow through the fourth test line, which means that a part of the amantadine and its colloid golden-antibodies should be attached to the antigen on the fourth test line; then, the sample continues along the NC membrane and reaches the third test line, if there is enough amantadine, there should be remainder colloid golden-antibody here attached with antigen, so besides the fourth test line, the third test line will also be coloured. This is why we set four gradient concentrations of antigen-coated test lines on the nitrocellulose membrane from great to small, from top to bottom.
Potassium borate was used as a buffer to facilitate the conjugation of antibodies with colloidal gold particles. When the potassium borate concentration increased, the antibody did not attach to the gold particles, and the number of antibodies used increased. Since the reaction stoichiometry was still not clear, further investigation is needed.
Due to the hydrophobicity of amantadine, both the colloidal gold antibody and antigen are hard to dissolve in water, making it difficult to flow with the samples along the PVC plate when analysing. Tween-20 was added to the working solution to solve this problem, and sugar was added to make the colloidal gold-labelled antibodies easier to re-dissolve. Tween-20 is a commonly used surfactant that improves the solubility of the antigen in the working solution and enhances the antibody interaction at the test lines. Thus, the colour change at the test lines was easier to observe. Sugar acted as a buffer for the labelled antibody before application on the conjugate pad, preventing the antibody from attaching too vigorously so that it could flow easily.
LFIAs have rapidly been widely used to detect antibiotics and veterinary drug residues in animal-based foods. The LFIA platform presented here highlights the potential for future optimisation and deployment of these assays as qualitative and semi-quantitative assays for rapid screening of amantadine residues in endemic settings. To evaluate the applicability of this LFIA, actual meat samples were analysed for amantadine residues. The LFIA has an indicator range of 0–200 ng/mL. When all four test lines changed colour, the concentration of amantadine was 0–50 ng/mL. When three test lines changed colour, the concentration was 50–100 ng/mL. When two test lines changed colour, the concentration was 100–200 ng/mL. Finally, when one test line changed colour, the concentration was > 200 ng/mL.
Unlike the traditional LFIA design, our four–train platform provides semi-quantitative results. In addition, our LFIA also provides concrete results, benefiting from the interaction between the amantadine and its hapten-protein conjugates on the NC membrane. Thus, amantadine can be distinguished from other commonly used veterinary drugs. Although amantadine has minimal molecular weight, the indicator range of this assay can reach as low as 50–200 ng/mL. Traditional LFIAs, which typically have only one test line, is observed by the naked eye. Therefore, the interpretation may differ from individual to individual, leading to inaccurate determination of drug concentrations. In this study, we have minimised operator error by comparing the test lines with amantadine concentration gradients and four test lines on one device. The results were obtained within 10 min, much faster than other detection methods. The rapid and user-friendly process makes this LFIA suitable for high-throughput amantadine determination for market regulators and lays people.
Despite the achievements of this novel design, further optimisation of the LFIA is needed. Sufficient stability under ambient temperature is required since many testing sites do not have refrigeration. Regarding the poor water solubility of amantadine, we have used organic solvents, such as acetonitrile, for extraction in the lab and recommend using pure ethanol to prepare stock drug extracts for field use. The product performance under these conditions will be evaluated in the future.
5. Conclusions
Amantadine monitoring is a part of food and medicine product regulations, and its results should be provided rapidly and indicatively. A specific antibody for amantadine was immobilised on the surface of the NC membrane. The antibody presented low cross-reactivity to enrofloxacin, ciprofloxacin, and ofloxacin. An immunoconjugate based on colloidal gold was produced for measuring amantadine concentration. A specific LFIA dipstick was developed using this colloidal gold-labelled antibody, which has an indicator range of 50–200 ng/mL for amantadine. The assay provides semi-quantitative analysis by assessing the colour change of four test lines. When the four test lines retained their colour, the amantadine concentration in the sample was 0–50 ng/mL. When three test lines were coloured, the concentration was 50–100 ng/mL. When two test lines retained colour, the concentration was 100–200 ng/mL. If only one test line was coloured, the amantadine concentration in the sample was more significant than 200 ng/mL. Semi-quantitative analysis of amantadine in commercial edible livestock products was performed, and the results were validated via an established LC-MS/MS method. The results of the LFIA for amantadine in commercial pork agreed with those determined by HPLC. Thus, the assay shows excellent potential as a regulation device for monitoring veterinary drug residues in livestock and poultry products.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aranda-Abreu, G. (2020). The sars-Cov-2 Viroporin E and its interaction with amantadine an analysis. Journal of Pharmaceutics & Pharmacology, 8(1). https://doi.org/10.13188/2327-204X.S200006
- Brison, E., Jacomy, H., Desforges, M., & Talbot, P. J. (2014). Novel treatment with neuroprotective and antiviral properties against a neuroinvasive human respiratory virus. Journal of Virology, 88(3), 1548–1563. https://doi.org/10.1128/JVI.02972-13
- Chen, K., Ma, B. A., Li, J. L., et al. (2021). A rapid and sensitive Europium nanoparticle-based lateral flow immunoassay combined with recombinase polymerase amplification for simultaneous detection of three food-borne pathogens. International Journal of Environmental Research and Public Health, 18(9). https://doi.org/10.3390/ijerph18094574
- Dong, B., Li, H., Sun, J., Mari, G. M., Yu, X., Ke, Y., Li, J., Wang, Z., Yu, W., Wen, K., & Shen, J. (2019). Development of a fluorescence immunoassay for highly sensitive detection of amantadine using the nanoassembly of carbon dots and MnO2 nanosheets as the signal probe. Sensors and Actuators B: Chemical, 286, 214–221. https://doi.org/10.1016/j.snb.2019.01.100
- Elzanfaly, E. S., & Saad, A. S. (2017). Green in-line Ion selective electrode potentiometric method for determination of amantadine in dissolution media and in pharmaceutical formulations. Acs Sustainable Chemistry & Engineering, 5(5), 4381–4387. https://doi.org/10.1021/acssuschemeng.7b00421
- Gao, Y. F., Wu, X. L., Wang, Z. X., Luo, P., Xu, L., Zheng, Q., & Kuang, H. (2019). A sensitive lateral flow immunoassay for the multiple residues of five adamantanes. Food and Agricultural Immunology, 30(1), 647–661. https://doi.org/10.1080/09540105.2019.1612331
- Giombini, S., Solero, C., Bazzan, A., Ferrari, P., & Robotti, E. (1970). [Amantadine in the treatment of Parkinson's disease]. Il Fracastoro, 63, 869–881.PMID: 5519639.
- Guo, L. C., Liu, M. X., Zhang, S. X., Wang, Z. H., & Yu, X. Z. (2021). Multi-wavelength fluorescence polarization immunoassays for simultaneous detection of amantadine and ribavirin in chicken and human serum. Food and Agricultural Immunology, 32(1), 321–335. https://doi.org/10.1080/09540105.2021.1940877
- Jefferson, T. O., Demicheli, V., Deeks, J. J., & Rivetti, D. (2000). Amantadine and rimantadine for preventing and treating influenza A in adults. Cochrane Database of Systematic Reviews, (3), CD001169.
- Jia, Q., Li, D., Wang, X. L., Yang, S. M., Qian, Y. Z., & Qiu, J. (2018). Simultaneous determination of amantadine and rimantadine in feed by liquid chromatography-Qtrap mass spectrometry with information-dependent acquisition. Analytical and Bioanalytical Chemistry, 410(22), 5555–5565. https://doi.org/10.1007/s00216-018-1022-x
- Khasanova, D. R., Saikhunov, M. V., Kitaeva, E. A., Khafizianova, R. K., Islamov, R. R., & Demin, T. V. (2009). Amantadine sulfate (PK-Merz) in the treatment of ischemic stroke: A clinical-experimental study. Zhurnal Nevrologii I Psikhiatrii Imeni S S Korsakova, 109(5), 37–43.
- Kornhuber, J., Schoppmeyer, K., & Riederer, P. (1993). Affinity of 1-aminoadamantanes for the σ binding site in post-mortem human frontal cortex. Neuroscience Letters, 163(2), 129–131. https://doi.org/10.1016/0304-3940(93)90362-O
- Liang, Z., Zhaob, Z., Sun, T., Shi, W., & Cui, F. (2016). Adsorption of quinolone antibiotics in spherical mesoporous silica: Effects of the retained template and its alkyl chain length. Journal of Hazardous Materials, 305, 8–14. https://doi.org/10.1016/j.jhazmat.2015.11.033
- Ma, X.M., He, S., Zhang, Y., Xu, J.G., Zhang, H.F., Wang, Z., Xue, J., Li, X., Yu, W.B., Fan, X.L., (2020). Signal-off tuned signal-on (SF-T-SN) colorimetric immunoassay for amantadine using activity-metalmodulated peroxidase-mimicking nanozyme. Sensors and Actuators B-Chemical, 311. https://doi.org/10.1016/j.snb.2020.127933
- Maj, J., Sowińska, H., Baran, L., & Sarnek, J. (1974). Pharmacological effects of 1,3-dimethyl-5-aminoadamantane, a new adamantane derivative. European Journal of Pharmacology, 26(1), 9–14. https://doi.org/10.1016/0014-2999(74)90067-3
- Manyam, B. V. (1981). Amantadine in essential tremor. Annals of Neurology, 9(2), 198–199. https://doi.org/10.1002/ana.410090219
- Mu, P. Q., Xu, N. N., Chai, T. T., Jia, Q., Yin, Z.Q., Yang, S.M., Qian, Y.Z., Qiu, J., (2016). Simultaneous determination of 14 antiviral drugs and relevant metabolites in chicken muscle by UPLC-MS/MS after QuEChERS preparation. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 1023, 17–23. https://doi.org/10.1016/j.jchromb.2016.04.036
- Pan, M. F., Yang, J. Y., Li, S. J., Wang, G. Z., Wang, J. P., & Wang, S. (2019). Indirect competitive ELISA and colloidal gold-based immunochromatographic strip for amantadine detection in animal-derived foods. Analytical Methods, 11(15), 2027–2032. https://doi.org/10.1039/C8AY02806K
- Quarato, G., Scrima, R., Ripoli, M., Agriesti, F., Moradpour, D., Capitanio, N., & Piccoli, C. (2014). Protective role of amantadine in mitochondrial dysfunction and oxidative stress mediated by hepatitis C virus protein expression. Biochemical Pharmacology, 89(4), 545–556. https://doi.org/10.1016/j.bcp.2014.03.018
- Rejdak, K., & Grieb, P. (2020). Adamantanes might be protective from COVID-19 in patients with neurological diseases: Multiple sclerosis, parkinsonism and cognitive impairment. Multiple Sclerosis and Related Disorders, 42, 102163. https://doi.org/10.1016/j.msard.2020.102163
- Speltini, A., Sturini, M., Maraschi, F., Consoli, L., Zeffiro, A., & Profumo, A. (2015). Graphene-derivatized silica as an efficient solid-phase extraction sorbent for pre-concentration of fluoroquinolones from water followed by liquid-chromatography fluorescence detection. Journal of Chromatography A, 1379, 9–15. https://doi.org/10.1016/j.chroma.2014.12.047
- Tang, M., Zhao, Y., Chen, J., & Xu, D. (2020). On-line multi-residue analysis of fluoroquinolones and amantadine based on an integrated microfluidic chip coupled to triple quadrupole mass spectrometry. Analytical Methods, 12(44), 5322–5331. https://doi.org/10.1039/D0AY01641A
- Tsuruoka, Y., Nakajima, T., Kanda, M., Hayashi, H., Matsushima, Y., Yoshikawa, S., Nagata, M., Koike, H., Nagano, C., Sekimura, K., Hashimoto, T., Takano, I., & Shindo, T. (2017). Simultaneous determination of amantadine, rimantadine, and memantine in processed products, chicken tissues, and eggs by liquid chromatography with tandem mass spectrometry. Journal of Chromatography B, 1044-1045, 142–148. https://doi.org/10.1016/j.jchromb.2017.01.014
- Walter, A., Bodendoerfer, E., Kolesnik-Goldmann, N., & Mancini, S. (2021). Rapid identification of CTX-M-type extended-spectrum beta-lactamase-producing enterobacterales from blood cultures using a multiplex lateral flow immunoassay. Journal of Global Antimicrobial Resistance, 26, 130–132. https://doi.org/10.1016/j.jgar.2021.05.013
- Wang, Z.D., Wang, X.R., Wang, Y.H., Wu, C.L., & Zhou, J.H. (2021). Simultaneous determination of five antiviral drug residues and stability studies in honey using a two-step fraction capture coupled to liquid chromatography tandem mass spectrometry. Journal of Chromatography A, 1638. https://doi.org/10.1016/j.chroma.2021.461890
- Weng, R., Lou, S. T., Pang, X., Song, Y., Su, X., Xiao, Z., & Qiu, J. (2020). Multi-residue analysis of 126 pesticides in chicken muscle by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Chemistry, 309, 125503. https://doi.org/10.1016/j.foodchem.2019.125503
- Wiwanitkit, V. (2020). Amantadine, COVID-19 and parkinsonism. Archives of Medical Research, 51(7), 714–714. https://doi.org/10.1016/j.arcmed.2020.07.001
- Wu, S. S., Zhu, F. F., Hu, L. M., Xia, J., Xu, G., Liu, D., Guo, Q., Luo, K., & Lai, W. (2017). Development of a competitive immunochromatographic assay for the sensitive detection of amantadine in chicken muscle. Food Chemistry, 232, 770–776. https://doi.org/10.1016/j.foodchem.2017.04.058
- Xie, Z. P., Yang, M. F., Luo, L., Lv, Y.P., Song, K.J., Liu, S.M., Chen, D.Q., Wang, J.H., (2020). Nanochannel sensor for sensitive and selective adamantanamine detection based on host-guest competition. Talanta, 219, 8. https://doi.org/10.1016/j.talanta.2020.121213
- Yu, W.B., Li, Y., Xie, B., Ma, M.F., Chen, C.C., Li, C.L., Yu, X.Z., Wang, Z.H., Wen, K., Tang, B.Z., (2019). An aggregation-induced emission-based indirect competitive immunoassay for fluorescence “turn-On” detection of drug residues in foodstuffs. Frontiers in Chemistry, 7. https://doi.org/10.3389/fchem.2019.00228
- Zhao, Y., Li, W., Liu, J., Huang, K., Wu, C., Shao, H., Chen, H., & Liu, X. (2017). Modification of garlic peel by nitric acid and its application as a novel adsorbent for solid-phase extraction of quinolone antibiotics. Chemical Engineering Journal, 326, 745–755. https://doi.org/10.1016/j.cej.2017.05.139
- Zou, M. Y., Su, F. Y., Zhang, R., Jiang, X.L., Xiao, H., Yan, X.J., Yang, C.K., Fan, X.B., Wu, G.Q., (2021). Rapid point-of-care testing for SARS-CoV-2 virus nucleic acid detection by an isothermal and nonenzymatic signal amplification system coupled with a lateral flow immunoassay strip. Sensors and Actuators B-Chemical, 342. https://doi.org/10.1016/j.snb.2021.129899