ABSTRACT
Corticosteroids inhibit HIV-related immune activation and seem to have a mild favorable effect on immunological recovery in patients with CD4+ counts ≥200 cells/mm3. Data in patients with advanced immunodeficiency are lacking. We analyzed whether corticosteroids negatively influence the short-term CD4+ lymphocyte recovery in patients with CD4+ cell counts <200 cells/mm3 started on combination antiretroviral therapy (cART). We performed a retrospective cohort analysis including all HIV-infected patients under follow-up in our hospital with a documented episode of Pneumocystis Jirovecii Pneumonia (PJP) in the cART era. CD4+ lymphocyte recovery was assessed at three months after the episode of PJP and subsequent start of cART, comparing patients that received adjunctive corticosteroids (AC) versus patients that did not receive corticosteroids (standard care (SC)). In total, 66 patients with an episode of PJP were identified with 38 patients in the AC-group versus 28 patients in the SC-group. Almost all baseline characteristics were similar, including mean CD4+ lymphocyte counts. After three months, the mean CD4+ cell count did not differ; 222 cells/mm3 for the SC-group versus 259 cells/mm3 for the AC-group (p = .29). The use of corticosteroids does not alter CD4+ lymphocyte recovery in HIV-infected patients with advanced immunodeficiency in the first months of antiretroviral therapy.
Introduction
Corticosteroids are frequently used in modern medicine, also in the setting of HIV-related care (Wallis, Citation2007). Immune reconstitution inflammatory syndrome (IRIS) and Pneumocystis jirovecii pneumonia (PJP) with profound hypoxemia are the most common indications for corticosteroids in HIV-infected patients (Ewald et al., Citation2015; Perfect et al., Citation2010; Thwaites et al., Citation2004). Besides side effects like diabetes mellitus, corticosteroids are potent immunosuppressants – leading to concerns of new (opportunistic) infections (Gurwitz et al., Citation1994; Stuck, Minder, & Frey, Citation1989; van Staa, Leufkens, & Cooper, Citation2002).
Several studies described a mildly favorable effect of corticosteroids on CD4+ cell recovery in HIV-infected patients with CD4+ cell counts >200 cells/mm3 (Andrieu, Lu, & Levy, Citation1995; Kasang et al., Citation2016; Kilby et al., Citation1997; Wallis et al., Citation2003). In contrast, data on the impact of corticosteroids in patients with advanced immunodeficiency – e.g., CD4+ cell counts ≤200 cells/mm3 – are limited (McComsey et al., Citation2001). This lack of data is remarkable, considering that especially these patients are likely to receive corticosteroids for the indications mentioned above. As studies in healthy volunteers have shown that corticosteroids induce lymphopenia – in particular in the CD4+ compartment – (Verbruggen, Herman, Ackerman, Mielants, & Veys, Citation1987; Zweiman, Atkins, Bedard, Flaschen, & Lisak, Citation1984), the use of corticosteroids in patients with severe CD4+ lymphopenia might lead to incomplete immune recovery and other opportunistic infections (Kaufmann et al., Citation2005). These observations emphasize the need for a better understanding of the dynamics of CD4+ cell recovery in patients with advanced immunodeficiency using corticosteroids.
In this study, we retrospectively analyzed whether the use of corticosteroids leads to incomplete immunological recovery in HIV-infected patients with CD4+ lymphocyte counts ≤200 cells/mm3 in the first months after the initiation of combination antiretroviral therapy (cART).
Methods
Patients with HIV infection treated for a PJP in the University Medical Center Utrecht (the Netherlands) between 1 January 1996 (start of the cART era) and 1 July 2017 were identified. We chose to include only patients that were diagnosed with PJP in our analysis for two reasons. First of all, the use of corticosteroids as adjunctive treatment in the setting of PJP is standardized with a fixed dosage for three weeks (“Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome,” Citation1990). This is in contrast to the treatment of IRIS that does not have a predefined dosage and duration of therapy, which makes the interpretation of results difficult. PJP patients without profound hypoxemia and therefore no indication for corticosteroids created a control group.
All patients with a minimal follow-up time of three months after the episode of PJP were considered for the analysis. The diagnosis of PJP was based on clinical history with either microbiological or cytological evidence. HIV-positive patients with CD4+ counts ≤200 cells/mm3 presenting with a clinical picture highly suspicious for PJP – severe hypoxemia and ground glass opacities on the imaging studies – and with clinical recovery, while given anti-PJP therapy were also considered eligible for this analysis. Patients were excluded in the following situations: (1) not naïve for antiretroviral therapy at the time of PJP; (2) no initiation of cART within 8 weeks after PJP diagnosis; (3) corticosteroids for a period longer than 21 days. Baseline demographical, clinical and biochemical characteristics were retrieved from patient’s charts, as were the CD4+ cell counts in the year after the PJP diagnosis. The primary outcome of this analysis was the difference in the mean CD4+ cell count at three months after the diagnosis of PJP between patients receiving adjunctive corticosteroids (AC) – prednisone dosage according to the NEJM consensus statement – next to antimicrobial treatment compared to those who only received antimicrobial therapy (standard care (SC)) for the treatment of PJP. Furthermore, we evaluated the occurrence of other AIDS-defining conditions – e.g., Toxoplasmosis, Mycobacterium avium complex infection, candidiasis and Kaposi sarcoma – in the first year. We used chi-square test to compare dichotomous variables and Student’s t-test to compare means – also for mean CD4+ counts during follow-up. A two-sided p < .05 was regarded as significant. For calculations SPSS (version 22.0, Chicago, IL) was used, the figure was created with GraphPad Prism 7.
Results
We identified 89 patients with PJP of whom 23 were excluded – flow-chart is shown in the supplement. Of the remaining 66 patients, 38 patients received AC and 28 patients received SC. All patients were alive and in follow-up after 12 months. At baseline, patients did not differ between groups (AC versus SC) regarding age, gender and HIV transmission route (). The mean CD4+ cell count at baseline was 47 cells/mm3 in the AC group versus 59 cells/mm3 in the SC-group (p = .35). As expected, more patients from the AC group were admitted at the Intensive Care Unit (ICU) during the episode of PJP compared to patients receiving SC (26.3% vs. 3.6% p = .02), but there was no difference in mean baseline CD4 cell count between these patients (ICU versus non-ICU: 42.9 vs. 54.2 cells/mm3 (p = .46)). All patients received initially trimethoprim/sulfamethoxazole (TMP/SMX), but a significant part (24.4%) was switched to pentamidine because of TMP/SMX-associated toxicity.
Table 1. Baseline characteristics.
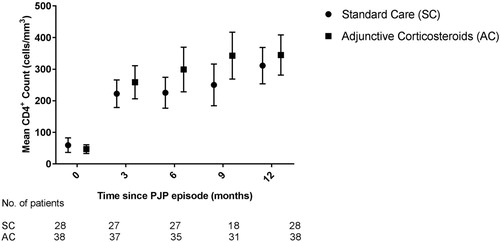
At three months after the PJP diagnosis, the mean CD4+ count between both groups did not differ; 222 cells/mm3 for the SC group versus 259 cells/mm3 for the AC group (p = .29). Analysis at 12 months after PJP diagnosis did not reveal a significant difference either: 311 cells/mm3 (SC) versus 345 cells/mm3 (AC) (p = .43) (). Separate sub-analyses for time eras (1996–2002; 2003–2007 and 2008–2017) or cART backbone did not reveal significant differences between both groups (data not displayed).
The percentage of patients reaching a CD4+ count of >200 cells/mm3 within the first year was 84.2% in the AC-group versus 71.4% for the patients from the SC-group (p = .24). All but one patient (1.5%) had a viral load <400 copies/ml after one year.
There was no difference in the percentage of patients that suffered from another AIDS-defining condition; 21.4% for patients receiving SC versus 13.2% of those from the AC-group (p = .50) in the first year.
Discussion
In this retrospective cohort study in HIV-infected patients with advanced immunodeficiency, the use of corticosteroids had no impact on CD4+ cell recovery in the first months after the initiation of cART.
In the past, several randomized controlled trials studied the effects of corticosteroids in HIV-infected patients in respect to clinical and immunological outcomes. Most of these reports describe dynamics in CD4+ counts and immune activation in the setting of HIV-infection with CD4+ counts >200 cells/mm3(Andrieu et al., Citation1995; Kasang et al., Citation2016; Kilby et al., Citation1997; Wallis et al., Citation2003). For example, the largest cohort by Kasang et al. (Citation2012) with a total number of 326 untreated HIV-infected patients with a CD4+ count ≥300 cells/mm3, were randomized to 5 mg prednisolone per day or placebo for 2 years. After two years, the rise in CD4+ count compared to baseline in patients receiving corticosteroids was significantly higher than the control group – an increase 77.4 cells/mm3 versus a decline of 37.4 cells/mm3 (p < .01). Furthermore, several markers for immune activation in the prednisone-group were significantly lower compared to placebo. It was hypothesized that corticosteroids inhibit HIV-related immune activation – slowing down CD4+ destruction.
However, information on the effects of corticosteroid use in HIV-infected patients with advanced immunodeficiency are lacking despite that these data in this vulnerable population are needed. After all, two major indications for corticosteroids in HIV-infected patients – treatment of IRIS and adjunctive therapy in PJP with profound hypoxemia – mainly occur in patients with advanced immunodeficiency and this population is at risk for new opportunistic infections. To our best knowledge, only McComsey et al. (Citation2001) describes the short-term dynamics of CD4+ cell recovery exclusively in patients with advanced immunodeficiency. In this placebo-controlled trial, 41 HIV-infected patients with a mean baseline CD4+ count of 129 cells/mm3 were randomized to 8 weeks 0.5 mg/kg body weight prednisone daily or placebo. Almost all patients were on cART for at least two months before randomization, with a median of 207 days for the prednisone group. After 8 weeks, there was no significant difference between both groups; the mean CD4+ cell count in the placebo group remained stable and there was a slight decrease of 3 cells/mm3 in patients receiving corticosteroids. There were no data available on CD4+ cell dynamics after eight weeks. We built on the data by McComsey et al. by describing the impact of corticosteroids on CD4+ cell recovery in the setting of subsequent start of cART. As the largest increase in CD4+ cells tend to happen in the first three months, it is likely that possible negative effects of the corticosteroids will be visualized during this period (Notermans et al., Citation1999). Furthermore, the observation period in our study was extended up to 12 months after the course of corticosteroids. Long follow-up time is needed, considering that the immunosuppressive effects of corticosteroids can persist up till three months after discontinuing prednisone – especially when the cumulative dose of 700 mg is exceeded (Stuck et al., Citation1989).
A limitation of our study is the small sample size, which results in reduced statistical power. Considering the trend towards better CD4+ cell recovery in patients treated with corticosteroids it seems unlikely that a negative effect is missed as a result of a lack of power. In addition, the described study period is long – this poses a risk for bias due to changes in (antiretroviral) therapy over the years. However, sub-analyses per time era or cART backbone did not reveal any differences.
In conclusion, although corticosteroids have several potential side effects, its use does not alter CD4+ cell recovery in HIV-infected patients with advanced immunodeficiency in the first months of antiretroviral therapy.
Supplemental Material
Download JPEG Image (88.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Berend J. van Welzen http://orcid.org/0000-0001-5601-4207
References
- Andrieu, J. M., Lu, W., & Levy, R. (1995). Sustained increases in CD4 cell counts in asymptomatic human immunodeficiency virus type 1-seropositive patients treated with prednisolone for 1 year. The Journal of Infectious Diseases, 171(3), 523–530. doi: 10.1093/infdis/171.3.523
- Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome. (1990). The New England Journal of Medicine, 323(21), 1500–1504. doi.org/10.1056/NEJM199011223232131
- Ewald, H., Raatz, H., Boscacci, R., Furrer, H., Bucher, H. C., & Briel, M. (2015). Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. The Cochrane Database of Systematic Reviews, (4), CD006150. doi.org/10.1002/14651858.CD006150.pub2
- Gurwitz, J. H., Bohn, R. L., Glynn, R. J., Monane, M., Mogun, H., & Avorn, J. (1994). Glucocorticoids and the risk for initiation of hypoglycemic therapy. Archives of Internal Medicine, 154(1), 97–101. doi: 10.1001/archinte.1994.00420010131015
- Kasang, C., Kalluvya, S., Majinge, C., Kongola, G., Mlewa, M., Massawe, I., … Scheller, C. (2016). Effects of prednisolone on disease progression in antiretroviral-untreated HIV infection: A 2-year randomized, double-blind placebo-controlled clinical trial. PLoS One, 11(1), e0146678. doi.org/10.1371/journal.pone.0146678
- Kasang, C., Ulmer, A., Donhauser, N., Schmidt, B., Stich, A., Klinker, H., … Scheller, C. (2012). HIV patients treated with low-dose prednisolone exhibit lower immune activation than untreated patients. BMC Infectious Diseases, 12, 14. doi.org/10.1186/1471-2334-12-14
- Kaufmann, G. R., Furrer, H., Ledergerber, B., Perrin, L., Opravil, M., Vernazza, P., … Battegay, M. (2005). Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America, 41(3), 361–372. doi.org/10.1086/431484
- Kilby, J. M., Tabereaux, P. B., Mulanovich, V., Shaw, G. M., Bucy, R. P., & Saag, M. S. (1997). Effects of tapering doses of oral prednisone on viral load among HIV-infected patients with unexplained weight loss. AIDS Research and Human Retroviruses, 13(17), 1533–1537. doi.org/10.1089/aid.1997.13.1533
- McComsey, G. A., Whalen, C. C., Mawhorter, S. D., Asaad, R., Valdez, H., Patki, A. H., … Lederman, M. M. (2001). Placebo-controlled trial of prednisone in advanced HIV-1 infection. AIDS (London, England), 15(3), 321–327. doi: 10.1097/00002030-200102160-00004
- Notermans, D. W., Pakker, N. G., Hamann, D., Foudraine, N. A., Kauffmann, R. H., Meenhorst, P. L., … Danner, S. A. (1999). Immune reconstitution after 2 years of successful potent antiretroviral therapy in previously untreated human immunodeficiency virus type 1-infected adults. The Journal of Infectious Diseases, 180(4), 1050–1056. doi.org/10.1086/315013
- Perfect, J. R., Dismukes, W. E., Dromer, F., Goldman, D. L., Graybill, J. R., Hamill, R. J., … Sorrell, T. C. (2010). Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America, 50(3), 291–322. doi.org/10.1086/649858
- Stuck, A. E., Minder, C. E., & Frey, F. J. (1989). Risk of infectious complications in patients taking glucocorticosteroids. Reviews of Infectious Diseases, 11(6), 954–963. doi: 10.1093/clinids/11.6.954
- Thwaites, G. E., Nguyen, D. B., Nguyen, H. D., Hoang, T. Q., Do, T. T. O., Nguyen, T. C. T., … Farrar, J. J. (2004). Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. The New England Journal of Medicine, 351(17), 1741–1751. doi.org/10.1056/NEJMoa040573
- van Staa, T. P., Leufkens, H. G. M., & Cooper, C. (2002). The epidemiology of corticosteroid-induced osteoporosis: A meta-analysis. Osteoporosis International : A Journal Established as Result of Cooperation Between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 13(10), 777–787. doi.org/10.1007/s001980200108
- Verbruggen, G., Herman, L., Ackerman, C., Mielants, H., & Veys, E. M. (1987). The effect of low doses of prednisolone on T-cell subsets in rheumatoid arthritis. International Journal of Immunopharmacology, 9(1), 61–67. doi: 10.1016/0192-0561(87)90111-1
- Wallis, R. S. (2007). Corticosteroids and HIV infection: A review of experience. Current Opinion in HIV and AIDS, 2(3), 213–218. doi.org/10.1097/COH.0b013e3280eec766
- Wallis, R. S., Kalayjian, R., Jacobson, J. M., Fox, L., Purdue, L., Shikuma, C. M., … Lederman, M. M. (2003). A study of the immunology, virology, and safety of prednisone in HIV-1-infected subjects with CD4 cell counts of 200 to 700 mm(-3). Journal of Acquired Immune Deficiency Syndromes, 32(3), 281–286. doi: 10.1097/00126334-200303010-00006
- Zweiman, B., Atkins, P. C., Bedard, P. M., Flaschen, S. L., & Lisak, R. P. (1984). Corticosteroid effects on circulating lymphocyte subset levels in normal humans. Journal of Clinical Immunology, 4(2), 151–155. doi: 10.1007/BF00915049