ABSTRACT
Cognitive impairment and chronic pain are amongst the most prevalent neurological sequelae of HIV infection, yet little is understood about the potential bidirectional relationship between the two conditions. Cognitive dysfunction can occur in chronic pain populations whilst those with cognitive impairment can display modified responses to experimentally induced painful stimuli. To date, this has not been explored in HIV cohorts.
This study aimed to identify any contribution of chronic pain to cognitive impairment in HIV and to determine differences in pain characteristics between those with and without cognitive dysfunction.
This was an observational cohort study involving people living with HIV (n = 148) in the United Kingdom. Participants underwent validated questionnaire-based measurement of pain severity, interference and symptom quality as well as conditioned pain modulation and quantitative sensory testing. All participants completed a computer-based cognitive function assessment.
Fifty-seven participants met the criteria for cognitive impairment and 73 for chronic pain. The cognitive impairment group had a higher prevalence of chronic pain (p = 0.004) and reported more neuropathic symptoms (p = 0.001). Those with chronic pain performed less well in emotional recognition and verbal learning domains. The interaction identified between chronic pain and cognitive dysfunction warrants further exploration to identify causal links or shared pathology.
1. Introduction
Early, consistent and successful treatment with highly active antiretroviral therapy (ART) has led to a change in life expectancy of people with HIV to near normal (May et al., Citation2014). However, the clinical consequences of chronic infection in an increasingly ageing population are now being realised. Those with long-term infection appear to have an increased risk of co-morbidity, including cardiovascular disease, cancer and cognitive impairment, despite adequate viral suppression (Burdo et al., Citation2014; Duprez et al., Citation2009; Shiels et al., Citation2009).
Milder forms of HIV-associated neurocognitive disorder (HAND) affect up to 50% of patients (Heaton et al., Citation2010) and those with HIV are at high risk of chronic pain, including musculoskeletal pain, painful neuropathy and headache (Jiao et al., Citation2016; Miaskowski et al., Citation2011; Navis et al., Citation2018; Uebelacker et al., Citation2015). Currently, the role of chronic pain in observed cognitive impairment in HIV is not well understood.
A series of systematic reviews have highlighted the complex relationship between chronic pain and cognitive impairment (Berryman et al., Citation2014, Citation2013; Higgins et al., Citation2018; Moriarty et al., Citation2011). Several cognitive domains, including attention (Oosterman et al., Citation2012), memory (Berryman et al., Citation2013) and executive function (Berryman et al., Citation2014; Moriarty et al., Citation2011) appear to be altered in a variety of chronic pain conditions. Structural brain changes and altered connectivity associated with both self-reported pain measures (Apkarian et al., Citation2004; As-Sanie et al., Citation2012; Malfliet et al., Citation2017; Moriarty et al., Citation2017; Schweinhardt et al., Citation2008) and cognitive performance (Luerding & Bogdahn, Citation2008) are apparent in those reporting chronic pain. However, the relationship between pain and cognitive dysfunction appears to be bi-directional. Experimental pain studies in non-pain populations with cognitive impairment have demonstrated altered pain reporting and psychophysical responses (Binnekade et al., Citation2018; Fletcher et al., Citation2015; Jensen-Dahm et al., Citation2012, Citation2015).
In chronic pain cohorts, psychophysical pain assessments, such as quantitative sensory testing (QST) and conditioned pain modulation (CPM), can be used to demonstrate patterns of sensory change hypothesised to be linked to underlying pain mechanisms, with the potential to guide individualised analgesic treatment (Arendt-Nielsen et al., Citation2017; Baron et al., Citation2017; Campbell et al., Citation2012; Demant et al., Citation2014; Höper et al., Citation2014; Nahman-Averbuch et al., Citation2011; Owens et al., Citation2019; Simpson et al., Citation2010; Westermann et al., Citation2012; Yarnitsky et al., Citation2012). QST is a method of assessing somatosensory function, providing a semi-objective quantification of sensory large and small nerve fibre function (Gracely, Citation1999; Greenspan, Citation2001; Haanpaa et al., Citation1999). CPM is used to quantify endogenous descending pain modulation (Yarnitsky et al., Citation2010), designed to replicate descending noxious inhibitory control (DNIC) identified in animals (Le Bars et al., Citation1979).
These measures require participants to understand instructions, interpret sensory input, report a threshold or intensity, or perform a motor task. Since these tasks require attention, memory, learning, psychomotor and executive function, there is the potential for cognitive dysfunction to influence the outcome of testing. Indeed an international consensus concerning the assessment of neuropathic pain (Backonja et al., Citation2013) acknowledged this issue, stating QST “should be discouraged … in those with clinically relevant cognitive deficits”.
It is, however, uncommon for pain cohorts to undergo cognitive assessment in parallel with psychophysical testing other than as a method for exclusion to the study. A closer examination of the relationship between cognitive function and response to psychophysical testing in chronic pain cohorts is required to elucidate any impact of cognitive dysfunction on test results.
This study aimed:
To explore any contribution of chronic pain to cognitive impairment in people living with HIV
To determine, in a cohort of participants with HIV and chronic pain, any differences in pain characteristics and sensory phenotype between those with and without cognitive impairment
To examine any impact of cognitive impairment on psychophysical testing.
2. Methods
2.1. Study design
This study was performed as part of the HIV-POGO Study (NCT02555930), an observational cross-sectional study approved by the English National Research Ethics Service (14/LO/1574). All participants completed written consent prior to enrolment.
Participants were recruited from HIV outpatient clinics associated with Chelsea & Westminster Hospital NHS Foundation Trust in London, UK and from UK-based patient charities by general advertisement. For inclusion, patients were required to be over the age of 18 and have a serological diagnosis of HIV infection. Exclusion criteria included limited English language skills, pregnancy and co-incident severe neurological conditions (including dementia, but not other types of mild cognitive disorder).
Participants attended a single appointment with a clinical researcher (HIK). Demographic and medical history data were collected, including participant-reported substance misuse. Last recorded serum triglyceride results were recorded from medical records.
Participants underwent structured neurological examination (Philips et al. 2014) quantitative sensory testing, a CPM protocol and sural nerve conduction testing (where available). Computerised cognitive function assessment was delivered via CogState software (Cogstate Ltd, Melbourne Australia). Participants were then provided with a booklet of validated questionnaires to complete.
2.2. Cognitive function assessment
Computerised assessment allows for standardisation between individuals and can be performed by non-psychologists. CogState software has undergone validation compared to traditional neuropsychological testing in HIV cohorts (Overton et al., Citation2011). Eight cognitive domains were assessed: psychomotor function (detection test), attention (identification test), visual learning (one card learning test), working memory (one card back test), executive function (Groton maze learning test), emotional recognition (social–emotional cognition test), verbal learning (International Shopping list), verbal memory (International shopping list delayed recall test).
Raw scores for each test were converted to a standardised T-score using age-adjusted normative data provided by CogState. A composite “global cognitive T-score” was determined as the arithmetic mean of individual test T-scores. Due to variability of a positive outcome being associated with a better or worse performance, dependent on the type of test, scores where a negative difference was associated with a better score were transformed so that all higher scores could be interpreted as “better” cognitive function. The use of T-scores is commonplace in psychometric practice and enables values to be plotted on a scale of 0–100 with a mean of 50(sd 10) (Iverson, Citation2011).
Memory was also assessed using the validated 28-item Everyday Memory Questionnaire (EMQ), a subjective memory measure (Sunderland et al., Citation1984).
2.3. Definition of cognitive impairment
The three most commonly used methods for classification of neurocognitive impairment in HIV are the “Frascati criteria” (Antinori et al., Citation2007), the Global Deficit Score (GDS) (Carey et al., Citation2004) and the Multivariate Normative Comparison (MNC) (Su et al., Citation2015). However, a recent project used a simulated “normative” dataset informed by real-world cognitive data from the “POPPY” (Pharmacokinetic and Clinical Observations in PeoPle Over fiftY) observational study to evaluate the prevalence of cognitive impairment using traditional methods, as well as a novel multivariate method based on the Mahalanobis distance (Underwood et al., Citation2019). The software from this work (https://jonathan-underwood.shinyapps.io/cognitive_calculator/) was used to allocate participants displaying cognitive impairment based on Frascati criteria, GDS and the novel Mahalanobis distance method. Ultimately the Mahalanobis distance technique was used to dichotomise the cohort into those with and without cognitive impairment as this method accounts for covariance between tests, is not biased by the number of cognitive domains tested and does not rely on a study-specific control group, since the calculation is performed based on a hypothesised normative population. It does however place equal weighting on each variable. It has demonstrated the strongest positive predictive value and accuracy and better association with structural brain changes compared to other commonly utilised definitions in very large populations of HIV subjects (Should be Underwood, Cole et al. Citation2017; Underwood Citation2019; Underwood, de Francesco et al. Citation2017b
2.4. Symptom, psychological and quality of life questionnaires
The Neuropathic Pain Symptom Inventory (NPSI) (Bouhassira et al., Citation2004) was used to characterise neuropathic symptoms, and the Brief Pain Inventory (BPI) (Cleeland, Citation1989) to assess pain severity and interference. The Depression Anxiety and Positive Outlook Scale (Pincus et al., Citation2004) was used to assess mood and the Short Form 36 of the Medical Outcomes Study (SF-36) (Ware & Sherbourne, Citation1992) to assess for impact on health-related quality of life.
A simplified body map was used to document body sites with pain. If the diagnostic reason for the pain was known, this was also recorded.
2.5. Psychophysical testing
2.5.1. Quantitative sensory testing
QST was performed to the protocol designed by the German Pain Research Network for Neuropathic Pain (DFNS) (Rolke et al., Citation2006). Testing was performed at the S1 dermatome of the dorsum of the left foot. The DFNS protocol determines quantitative values for cold and warm detection, cold and heat pain thresholds (CDT, WDT, CPT, HPT), the presence of paradoxical heat sensation (PHS), mechanical pain threshold (MPT), mechanical pain sensitivity (MPS), wind up ratio (WUR), mechanical detection threshold (MDT), dynamic mechanical allodynia (DMA), pressure pain threshold (PPT) and vibration detection threshold (VDT). Results were z-transformed to control for age, gender and body site using Equista software version 3.0 (Magerl et al., Citation2010).
2.5.2. Conditioned pain modulation
A handheld algometer was used to measure the test stimulus, pressure pain threshold (PPT), on the dorsum of the right arm prior to and during a conditioning stimulus. The conditioning stimulus involved the participant holding their left hand in a circulating water bath set to 12°C for 90 seconds. At 60 seconds the test stimulus was re-administered. The CPM response was calculated as:
PPT during conditioning stimulus – PPT prior to conditioning stimulus.
2.6. Definition of chronic pain
Participants were classified as having chronic pain if they reported pain that lasted or recurred for at least 3 months, according to the IASP ICD-11 definition (Treede et al., Citation2019), and at least 4 on the BPI “average pain” item at least 4 out of 10, approximating to “at least moderate” chronic pain, thought to be reflective of “clinically meaningful pain” that interferes with function (Paul et al., Citation2005).
2.7. Definition of neuropathy and neuropathic pain
To identify any co-prevalence or interaction of HIV-SN and associated neuropathic pain on cognitive impairment, neuropathic pain was classified following the NeuPSIG grading (Finnerup et al., Citation2016). “Probable” neuropathic pain was based on the presence of pain in a neuroanatomically plausible location (i.e., bilateral foot/lower leg pain) and bilateral signs and symptoms of sensory neuropathy (pain, numbness, reduced vibration detection and reduced ankle reflexes), as described by the validated Clinical HIV-associated Neuropathy Tool (CHANT) (Woldeamanuel et al., Citation2016). “Definite” neuropathic pain was determined if there was neurophysiological evidence of neuropathy from clinical nerve conduction tests or evidence of abnormal sural nerve conduction from hand-held testing using the DPNCheck device (Neurometrix, Waltham, USA).
2.8. Statistical analysis
Data were tested for normality visually and by using the Shapiro Wilk test. Normally distributed data are presented as mean (sd) or, for QST z-scores as mean ±95% confidence interval. Comparison of normal data between groups was conducted using t-tests or ANOVA (LSD post hoc analysis). Non-normally distributed data are presented as median (IQR) and comparison conducted using Mann Whitney or Kruskal Wallis tests (Dunn post hoc analysis). Categorical data were compared using chi-squared or Fisher's exact test (for small groups <5). Correlations between measures were performed using Spearman or Pearson correlations depending on normality. Where multiple comparisons were conducted, and the significance level was adjusted using the Benjamini-Hochberg procedure to decrease the false discovery rate.
Comparison of prevalence of chronic pain and HIV-SN, demographic information, pain characteristics, QST and CPM were performed between those with and without cognitive impairment. Comparisons were repeated in the subgroups with chronic pain and with neuropathy to identify the influence of these factors on any observed differences. Correlations between cognitive domain T-scores and self-reported memory impairment and psychophysical tests were also performed.
3. Results
3.1. Demographic and HIV-related characteristics
148 subjects were enrolled. Due to either time limitation or a request to stop testing, eight were unable to complete enough of the cognitive testing to assess for cognitive impairment. The majority of participants were male (n = 123, 83%), self-reported being of “white” ethnicity (n = 114, 81% (“black” ethnicity n = 18, 13%; “other” n = 8, 6%)) and had an undetectable viral load (n = 132, 89%). Only 5 subjects (3%) were not stable on ART at the time of inclusion. There was a high rate of reported previous illicit drug use (n = 81, 58%) but less than a quarter (n = 31, 22%) reported currently regular use.
According to the Mahalanobis criteria, 57 participants (41%) met the definition for cognitive impairment (proportion based on other criteria presented in Supplemental Table 1). A comparison of demographic and HIV-specific characteristics between those with and without cognitive impairment is presented in . Those without cognitive impairment were older, more likely to be employed and to have completed university-level education. Those with cognitive impairment demonstrated higher serum triglyceride levels.
Table 1. Comparison of demographic information and HIV characteristics between participants with and without cognitive impairment.
3.2. Pain characteristics
Chronic pain was prevalent in this cohort: 73 (52%) participants had chronic pain (as per case definition) and 52 (37%) painful HIV-SN (“probable” (n = 12) and “definite” cases (n = 40)). Nearly three-quarters of subjects (n = 104, 74%) reported another chronic pain diagnosis other than HIV-SN.
Although there was no significant difference in the number of subjects with HIV-SN between those with and without cognitive impairment (37/57 versus 48/83, p = 0.399), significantly more subjects had painful HIV-SN in the cognitively impaired group (29/57 versus 23/83, p = 0.005). A higher proportion of those in the cognitive impairment group met the case definition for chronic pain (38/57 versus 35/83; p = 0.004) but there was no difference in the proportion of subjects using opioids (). Neuropathic symptom intensity (total NPSI score) but not pain severity (BPI severity score) was higher in those with cognitive impairment ().
Table 2. A comparison of pain diagnoses and treatment between participants with and without cognitive impairment.
Table 3. Comparison of pain symptoms and interference in participants with chronic pain between those with and without cognitive impairment.
3.3. Type of cognitive dysfunction
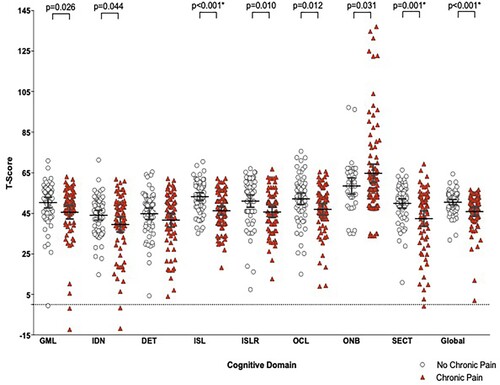
Mean raw scores for each cognitive domain are presented in Supplemental Table 2. Participants with chronic pain performed less well globally during cognitive testing and showed significantly poorer performance in verbal learning and emotional recognition (). Pain severity negatively correlated with psychomotor function (r = −0.423, p = 0.001) and pain interference with verbal memory (r = −0.345, p = 0.004). Evoked pain and pressure symptoms (on NPSI) were associated with a worse psychomotor function (r = −0.403, p = 0.003 and −0.369, p = 0.003). Other correlations were non-significant (for severity r = −0.322–0.164 and interference r = −0.284–0.157). The use of strong opiates in those with chronic pain was not associated with any significant difference in individual or global cognitive scores (p = 0.085–0.990).
Figure 1. Comparison of cognitive domains between participants with and without chronic pain. *indicates a significant difference after correction for multiple comparisons. GML = Groton Maze Learning (function); IDN = Identification Test (attention); DET = Detection Test (psychomotor function); ISL = International Shopping List (verbal learning); ISLR = International shopping list delayed recall test (verbal memory); OCL = One card leaning(visual learning); ONB = One card back test (working memory); SECT = Social and emotional cognition test (emotional recognition); Global = Global T-score.
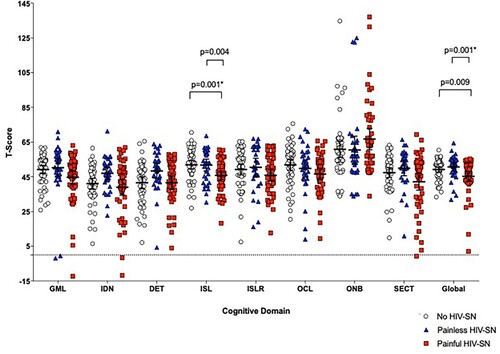
There were no significant differences in cognitive impairment identified between those with painless HIV-SN and those without neuropathy (). However, those with painful HIV-SN performed less well than those without neuropathy in verbal learning and less well globally than those with painless neuropathy suggesting pain, not neuropathy, is associated with worse cognitive performance.
Figure 2. Comparison of cognitive domain scores between those with painful and painless HIV-SN, and those without HIV-SN. *indicates significance after correction for multiple comparisons. GML = Groton Maze Learning (function); IDN = Identification Test (attention); DET = Detection Test (psychomotor function); ISL = International Shopping List (verbal learning); ISLR = International shopping list delayed recall test (verbal memory); OCL = One card leaning(visual learning); ONB = One card back test (working memory); SECT = Social and emotional cognition test (emotional recognition); Global = Global T-score.
3.4. Self-reported memory
Those with chronic pain self-reported poorer memory than those without chronic pain (EMQ score 91.9 (59.3) vs 47.2 (38.9), p = 0.004). EMQ total scores correlated negatively with Cogstate Global Impairment scores (r = −0.369, p = 0.002), i.e., higher self-reported memory problems correlated with worse scores on computerised testing of cognitive function.
3.5. Cognitive function and psychological and quality of life measures
Although those with cognitive impairment reported higher anxiety and lower positive outlook and health-related quality of life, only the difference in positive outlook remained significant after the presence of pain was controlled for (), suggesting pain may contribute to poorer quality of life and anxiety in those with cognitive dysfunction.
Table 4. Comparison of psychological and quality life measures between those with and without cognitive impairment (with results reported separately for those with and without chronic pain).
3.6. Psychophysical measures and cognitive impairment
There were no significant differences in QST z-scores between those with and without cognitive impairment (Supplemental Material Figure 1), even after controlling for the presence of chronic pain and neuropathy. A full CPM paradigm was conducted in 58 participants (28 without cognitive impairment and 30 with cognitive impairment). There was no significant difference in CPM response between those with and without cognitive impairment (0.74 (0.85) vs 0.92 (0.79); 0 = 0.403), and this persisted when the presence of chronic pain was controlled for (p = 0.843). There was however a significant positive correlation between emotional recognition (SECT test) and CPM response in the whole cohort (r = 0.486, p < 0.001) which remained significant when the analysis was repeated in those with chronic pain (r = 0.571, p < 0.001) indicating a greater inhibitory CPM response in those with higher emotional recognition scores.
4. Discussion
This study identified that chronic pain and painful HIV-SN were more prevalent in those with cognitive impairment and that both pain severity and interference correlated with psychomotor function and memory. Those with chronic pain performed less well globally, and on tasks involving verbal learning and emotional recognition. Neuropathic symptom intensity was associated with cognitive impairment, potentially indicating a specific interaction with neuropathic pain. Chronic pain may also account for a self-reported increase in anxiety and poorer quality of life in those with cognitive impairment. Mild cognitive impairment had minimal effect on psychophysical testing thus, supporting use in these cohorts.
4.1. Measurement of cognitive impairment in HIV
Although cognitive impairment is reported in up to 50% of people with HIV (Robertson et al., Citation2007), prevalence is known to be sensitive to the diagnostic criteria (Su et al., Citation2015). There is increasing evidence that earlier reported prevalence of cognitive dysfunction was overestimated. The high rate appears to be at odds with clinical experience (De Francesco et al., Citation2016; Underwood et al., Citation2017) and prevalence has since been found to be lower and more in line with prevalence identified in more “demographically-matched” HIV negative controls (Mcdonnell et al., Citation2014; Su et al., Citation2015).
In our cohort, 41% met the study definition for cognitive impairment, a slightly higher prevalence than that identified in demographically comparable HIV cohorts in the UK using similar methods ([Underwood et al., Citation2017] prevalence: 22–35%; [Mcdonnell et al., Citation2014]: 21–32%). This may be due to the high rate of chronic pain, or differences in psychosocial factors such as depression, anxiety or substance abuse that have been associated with cognitive dysfunction in HIV cohorts (De Francesco et al., Citation2019).
Our findings demonstrate that emotional recognition was impaired in those with chronic pain – this type of dysfunction is thought to be a very early, subclinical marker of cognitive dysfunction so our test battery may have identified those with subtle symptoms (Virtanen et al., Citation2017). The median global T-score in a large study of an aging Western European HIV population, (De Francesco et al., Citation2016) was 48.7, compared to 49.6 in this cohort suggesting a similar severity of impairment.
4.2. Cognitive impairment and pain
Although attempts have been made to assess the impact of cognitive function on physical and mental function (Underwood et al., Citation2017) and quality of life (Gorman et al., Citation2009) little is known about the association between pain and cognitive impairment in HIV. In the POPPY study, self-reported pain recall over a one-month period, rather than the more typical definition of chronic pain at 3 months was collected (Sabin et al., Citation2018). Although cognitive function and pain data were both collected, we are not aware of any published analysis including both outcomes.
In our study, although chronic pain and painful neuropathy were more prevalent in the cognitive impairment group, it is not possible to elucidate any direction of causality or direct association. There are a number of possible hypotheses for the association. Firstly, chronic pain could directly cause cognitive impairment. In other chronic pain cohorts, markers of dysregulated pain processing, including deep tissue allodynia and facilitatory descending pain control were associated with poorer cognitive performance (Coppieters et al., Citation2015; Galvez-Sanchez et al., Citation2018). Chronic pain is most associated with deficits in attention, memory and executive function and these are also the most prevalent cognitive deficits identified in contemporary HIV populations (De Francesco et al., Citation2019; Heaton et al., Citation2010). This highlights an overlap of features between two potentially independent conditions. The high prevalence of chronic pain in the HIV population (Parker et al., Citation2014) means previous studies examining cognitive impairment in HIV likely included subjects with chronic pain, potentially influencing results.
Alternatively, those with cognitive impairment could be particularly prone to perceiving or reporting pain. However, it appears those with frank cognitive impairment, for example in dementia (Achterberg et al., Citation2010), report less pain (Hadjistavropoulos et al., Citation2014). Experimental pain studies in participants with cognitive impairment, but without chronic pain, demonstrate a variety of results. Some indicated altered temperature responsiveness (Fletcher et al., Citation2015) and thermal detection thresholds (Monroe et al., Citation2017) whilst others suggested lower cold pain (Jensen-Dahm et al., Citation2015) or pressure pain tolerance (Jensen-Dahm et al., Citation2014) with normal detection.
Chronic pain and cognitive dysfunction may share a common underlying neuropathology in HIV. For example, a neuroimmune process, such as microglial activation, has been implicated in the pathogenesis of both HAND and painful HIV-SN (Wallace et al., Citation2007; Williams et al., Citation2014). Disordered lipid metabolism has also been associated with both HIV-SN (Phillips et al., Citation2014) and HAND (Bandaru et al., Citation2013) and, although our study was not designed to specifically identify an association, serum triglyceride levels were higher in those with cognitive dysfunction.
Our results suggest an increase in neuropathic symptoms specifically in those with a cognitive impairment which either supports a common pathology in the nervous system or suggests that neuropathic symptoms, known to more heavily influence pain interference (Attal et al., Citation2011), are more impactful on cognitive dysfunction.
Finally, risk factors associated with chronic pain in HIV overlap with those identified in HAND, including mental health co-morbidity, lower educational attainment, increasing age and substance misuse, thereby increasing the risk of both conditions within the same individual (Fazeli et al., Citation2013; Lawson et al., Citation2015; Miaskowski et al., Citation2011; Weber et al., Citation2013).
The presence of chronic pain appeared to account for the effect of cognitive impairment on both anxiety and quality of life and the complex interplay between pain, cognitive impairment and psychosocial outcomes warrants further investigation. The collection of robust pain-related data in cognitive impairment studies, as well as cognitive function data in chronic pain cohorts, would enhance understanding.
4.3. Cognitive function and psychophysical testing
Studies, including participants with Alzheimer's disease (but without chronic pain diagnoses), demonstrated a minimal difference in QST results compared to healthy controls, and good reliability (Jensen-Dahm et al., Citation2014). Our results similarly did not demonstrate a difference in QST parameters in those with cognitive impairment, further supporting the use of this type of testing in those with milder cognitive impairment, also important in other conditions such as painful diabetic polyneuropathy and chemotherapy-induced neuropathy (Biessels et al., Citation2006; Hutchinson et al., Citation2012).
Our findings are in contrast to a study involving patients with fibromyalgia which demonstrated a correlation between positive CPM efficiency and measures of psychomotor function, attention and choice reaction. This may be due to the inherent differences in pain processing between underlying pain conditions, limited sample size or use of different experimental methods. Many environmental and subject-related factors can influence CPM. Our finding that emotional recognition correlated positively with CPM response, indicating those with higher ability to recognise emotion in interpersonal communication showed a more inhibitory response, supports the hypothesis that subtle changes in participant-researcher dynamic may affect results obtained.
4.4. Strengths and limitations
The computerised Cogstate testing is comprehensive, easy to understand, requires no specialised neuropsychological training, demonstrates good test-rest reliability and has been validated against traditional neuropsychological tests (Fredrickson et al., Citation2010; Maruff et al., Citation2009). Age-adjusted normative data are also available. However, Cogstate is limited by potentially low face validity in comparison to traditional neuropsychological testing in certain tasks (Crook et al., Citation2009) and, due to limitations on assessment time, we did not control for generalised intelligence (IQ) which may influence this type of testing (Kataja et al., Citation2017).
The cohort recruited was predominantly male, of “white” ethnicity, and in general had long-standing, but well-controlled HIV. The educational level attained was also very high and these factors should be taken into account when comparing to other cohorts. Due to limited previous work on this topic, this study was exploratory and therefore may have been underpowered to show significance in some areas.
5. Conclusion
Chronic pain appears to be an important, yet under-researched correlate of cognitive performance in people with HIV. Verbal learning and emotional recognition were particularly influenced by chronic pain, and neuropathic symptoms were more prominent in those with cognitive impairment. A further investigation of the bi-directional relationships with psychosocial variables and an identification of causal links or shared pathology between the two conditions have the potential to yield targets for therapeutic intervention in both realms.
Contributions
HIK, NWSD, WS and ASCR were involved in the design of the study. DLK contributed to the design of the conditioned pain modulation paradigm. HIK recruited and tested all participants. JV assisted with statistical advice and analysis. All authors contributed to writing reviewing and refining the manuscript.
Supplemental Material
Download MS Word (355 KB)Acknowledgments
This work was funded by the European Commission, under the NeuroPain FP7 Grant EC (#2013-602891). DLK's contribution was funded by a National Institute for Health Research and Health Education England Clinical Doctoral Research Fellowship. WS's contribution was supported by the National Institute for Health Research (NIHR Postdoctoral Fellowship, Dr Whitney Scott, PDF-2015-08-059). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care. The authors would like to thank Dr Jonathan Underwood for the use of his online software and the clinical staff at the Chelsea & Westminster NHS Foundation Trust outpatient services for their advice on recruitment.
Disclosure statement
HIK: Nothing to declare.
DLK: Nothing to declare.
JV: Jan Vollert has received personal fees from CASQUAR, outside of the submitted work.
NWSD: Nothing to declare.
WS: Nothing to declare.
ASCR: Orion Pharma funding; Consultancy and advisory board work for Imperial College Consultants including remunerated work for Pharmanovo, Galapagos, Toray, Quartet, Lateral, Novartis, Pharmaleads, Orion, Asahi Kasei & Theranexis; Owner of share options in Spinifex Pharmaceuticals from which personal benefit accrued upon the acquisition of Spinifex by Novartis in July 2015 and from which future milestone payments may occur. Inventor on patents; Rice ASC, Vandevoorde S. and Lambert D.M. Methods using N-(2-propenyl) hexadecanamide and related amides to relieve pain. WO 2005/079771; Okuse K. et al Methods of treating pain by inhibition of vgf activity EP13702262.0/ WO2013 110945.
Additional information
Funding
References
- Achterberg, W. P., Gambassi, G., Finne-soveri, H., Liperoti, R., Noro, A., Frijters, D. H. M., Cherubini, A., Dell’Aquila, G., & Ribbe, M. W. (2010). Pain in European long-term care facilities: Cross-national study in Finland, Italy and the Netherlands. Pain, 148(1), 70–74. https://doi.org/10.1016/j.pain.2009.10.008
- Antinori, A., Arendt, G., Becker, J. T., Brew, B. J., Byrd, D. A., Cherner, M., Clifford, D. B., Cinque, P., Epstein, L. G., Goodkin, K., Gisslen, M., Grant, I., Heaton, R. K., Joseph, J., Marder, K., Marra, C. M., McArthur, J. C., Nunn, M., Price, R. W., … Wojna, V. E. (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69(18), 1789–1799. https://doi.org/10.1212/01.WNL.0000287431.88658.8b
- Apkarian, A. V., Sosa, Y., Sonty, S., Levy, R. M., Harden, R. N., Parrish, T. B., & Gitelman, D. R. (2004). Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. Journal of Neuroscience, 24(46), 10410–10415. https://doi.org/10.1523/JNEUROSCI.2541-04.2004
- Arendt-Nielsen, L., Jiang, G. L., DeGryse, R., & Turkel, C. C. (2017). Intra-articular onabotulinumtoxinA in osteoarthritis knee pain: Effect on human mechanistic pain biomarkers and clinical pain. Scandinavian Journal of Rheumatology, 46(4), 303–316. https://doi.org/10.1080/03009742.2016.1203988
- As-Sanie, S., Harris, R. E., Napadow, V., Kim, J., Neshewat, G., Kairys, A., Williams, D., Clauw, D., & Schmidt-Wilcke, T. (2012). Changes in regional gray matter volume in women with chronic pelvic pain: A voxel-based morphometry study. Pain, 153(5), 1006–1014. https://doi.org/10.1016/j.pain.2012.01.032
- Attal, N., Lanteri-Minet, M., Laurent, B., Fermanian, J., & Bouhassira, D. (2011). The specific disease burden of neuropathic pain: Results of a French nationwide survey. Pain, 152(12), 2836–2843. https://doi.org/10.1016/j.pain.2011.09.014
- Backonja, M. M., Attal, N., Baron, R., Bouhassira, D., Drangholt, M., Dyck, P. J., Edwards, R. R., Freeman, R., Gracely, R., Haanpaa, M. H., Hansson, P., Hatem, S. M., Krumova, E. K., Jensen, T. S., Maier, C., Mick, G., Rice, A. S., Rolke, R., Treede, R.-D., … Ziegler, D. (2013). Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain, 154(9), 1807–1819. https://doi.org/10.1016/j.pain.2013.05.047
- Bandaru, V. V. R., Mielke, M. M., Sacktor, N., McArthur, J. C., Grant, I., Letendre, S., Chang, L., Wojna, V., Pardo, C., Calabresi, P., Munsaka, S., & Haughey, N. J. (2013). A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology, 81(17), 1492–1499. https://doi.org/10.1212/WNL.0b013e3182a9565e
- Baron, R., Maier, C., Attal, N., Binder, A., Bouhassira, D., Cruccu, G., Finnerup, N. B., Haanpää, M., Hansson, P., Hüllemann, P., Jensen, T. S., Freynhagen, R., Kennedy, J. D., Magerl, W., Mainka, T., Reimer, M., Rice, A. S. C., Segerdahl, M., Serra, J., … Treede, R.-D. (2017). Peripheral neuropathic pain: A mechanism-related organizing principle based on sensory profiles. Pain, 158(2), 261–272. https://doi.org/10.1097/j.pain.0000000000000753
- Berryman, C., Stanton, T. R., Bowering, K. J, Tabor, A., McFarlane, A., & Moseley, G. L. (2013). Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain, 154(8), 1181–1196. https://doi.org/10.1016/j.pain.2013.03.002
- Berryman, C., Stanton, T. R., Bowering, K. J., Tabor, A., Mcfarlane, A., & Moseley, G. L. (2014). Do people with chronic pain have impaired executive function? A meta-analytical review. Clinical Psychology Review, 34(7), 563–579. https://doi.org/10.1016/j.cpr.2014.08.003
- Biessels, G. J., Staekenborg, S., Brunner, E., Brayne, C., & Scheltens, P. (2006). Risk of dementia in diabetes mellitus: A systematic review. The Lancet Neurology, 5(1), 64–74. https://doi.org/10.1016/S1474-4422(05)70284-2
- Binnekade, T. T., Scherder, E. J. A., Maier, A. B., Lobbezoo, F., Overdorp, E. J., Rhebergen, D., & Perez, R. S. G. M. (2018). Pain in patients with different dementia subtypes, mild cognitive impairment, and subjective cognitive impairment. Pain Medicine, 19(5), 920–927. https://doi.org/10.1093/pm/pnx162
- Bouhassira, D., Attal, N., Fermanian, J., Alchaar, H., Gautron, M., Masquelier, E., Rostaing, S., Lanteri-Minet, M., Collin, E., Grisart, J., & Boureau, F. (2004). Development and validation of the neuropathic pain symptom inventory. Pain, 108(3), 248–257. https://doi.org/10.1016/j.pain.2003.12.024
- Burdo, T., Weiffenbach, A., Woods, S., Letendre, S. L., Ellis, R. J., & Williams, K. (2014). Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS, 27(9), 1–16.
- Campbell, C. M., Kipnes, M. S., Stouch, B. C., Brady, K. L., Kelly, M., Schmidt, W. K., Petersen, K. L., Rowbotham, M. C., & Campbell, J. N. (2012). Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain, 153(9), 1815–1823. https://doi.org/10.1016/j.pain.2012.04.014
- Carey, C., Woods, S., Gonzalez, R., Conover, E., Marcotte, T., Grant, I., Heaton, R.K., & HNRC Group. (2004). Predicitive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology, 26(3), 307–319. https://doi.org/10.1080/13803390490510031
- Cleeland, C. (1989). Measurement of pain by subjective report. In C. Chapman & J. Loeser (Eds.), Advances in pain research and therapy (Vol. 12, pp. 391–403). Raven Press.
- Coppieters, I., Ickmans, K., Cagnie, B., Nijs, J., De Pauw, R., Noten, S., & Meeus, M. (2015). Cognitive performance is related to central sensitization and health-related quality of life in patients with chronic whiplash-associated disorders and fibromyalgia. Pain Phys, 18(3), E389–E402.
- Crook, T., Kay, G., & Larrabee, G. (2009). Computer-based cognitive testing. In I. Grant & K. Adams (Eds.), Neuropsychological assessment of neuropsychiatric and neuromedical disorders (pp. 84–100). Oxford University Press.
- De Francesco, D., Underwood, J., Bagkeris, E., Boffito, M., Post, F. A., Mallon, P. W. G., Vera, H. H., Williams, I., Anderson, J., Johnson, M., Sabin, C. A., Winston, A., & Pharmacokinetic and Clinical Observations in People over Fifty (POPPY) Study. (2019). Depression, lifestyle factors and cognitive function in people living with HIV and comparable HIV-negative controls. HIV Medicine, 20(4), 274–285. https://doi.org/10.1111/hiv.12714
- De Francesco, D., Underwood, J., Post, F. A., Vera, J. H., Williams, I., Boffito, M., Sachikonye, M., Anderson, J., Mallon, P. W. G., Winston, A., Sabin, C. A., & POPPY Study Group. (2016). Defining cognitive impairment in people- living-with-HIV: The POPPY study. BMC Infectious Diseases, 16(1), 617. https://doi.org/10.1186/s12879-016-1970-8
- Demant, D. T., Lund, K., Vollert, J., Maier, C., Segerdahl, M., Finnerup, N. B., Jensen, T. S., & Sindrup, S. H. (2014). The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double-blind, placebo-controlled phenotype-stratified study. Pain, 155(11), 2263–2273. https://doi.org/10.1016/j.pain.2014.08.014
- Duprez, D. A., Kuller, L. H., Tracy, R., Otvos, J., Cooper, D. A., Hoy, J., Neuhaus, J., Paton, N. I., Friis-Moller, N., Lampe, F., Liappis, A. P., Neaton, J. D., & INSIGHT SMART Study Group. (2009). Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis, 207(2), 524–529. https://doi.org/10.1016/j.atherosclerosis.2009.05.001
- Fazeli, P. L., Crowe, M., Ross, L. A., Wadley, V., Ball, K., & Vance, D. E. (2013). Cognitive functioning in adults aging with HIV: A cross-sectional analysis of cognitive subtypes and influential factors. Journal of Clinical Research In HIV AIDS And Prevention, 1(4), 54–68. https://doi.org/10.14302/issn.2324-7339.jcrhap-13-191
- Finnerup, N. B., Haroutounian, S., Kamerman, P., Baron, R., Bennett, D. L., Bouhassira, D., Cruccu, G., Freeman, R., Hansson, P., Nurmillo, T., Raja, S. N., Rice, A. S. C., Serra, J., Smith, B. H., Treede, R.-D., & Jensen, T. S. (2016). Neuropathic papin: Updated grading system for research and clinical practice. Pain, 157(8), 1599–1606. https://doi.org/10.1097/j.pain.0000000000000492
- Fletcher, P. D., Downey, L. E., Golden, H. L., Clark, C. N., Slattery, F., Paterson, R. W., Rohrer, J. D., Schott, J. M., Rossor, M. N., & Warren, J. D. (2015). Pain and temperature processing in dementia: A clinical and neuroanatomical analysis. Brain, 138(11), 3360–3372. https://doi.org/10.1093/brain/awv276
- Fredrickson, J., Maruff, P., Woodward, M., Moore, L., Fredrickson, A., Sach, J., & Darby, D. (2010). Evaluation of the usability of a brief computerized cognitive screening test in older people for epidemiological studies. Neuroepidemiology, 34(2), 65–75. https://doi.org/10.1159/000264823
- Galvez-Sanchez, C. M., Ladron de Guevara, C., Montoro, C., Fernandez-Serrano, M., Duschek, S., & Reyes, G. A. (2018). Cognitive deficits in fibromyalgia syndrome are associated with pain responses to low intensity pressure stimulation. PLoS ONE, 13(8), e0201488.
- Gorman, A. A., Foley, J. M., Ettenhofer, M. L., Hinkin, C. H., & Van Gorp, W. G. (2009). Functional consequences of HIV-associated neuropsychological impairment. Neuropsychology Review, 19(2), 186–203. https://doi.org/10.1007/s11065-009-9095-0
- Gracely, R. (1999). Pain measurement. Acta Anaesthesiologica Scandinavica, 43(9), 897–908. https://doi.org/10.1034/j.1399-6576.1999.430907.x
- Greenspan, J. (2001). Quantitative assessment of neuropathic pain. Current Pain and Headache Reports, 5(2), 107–113. https://doi.org/10.1007/s11916-001-0078-y
- Haanpaa, M., Laippala, P., & Nurmikko, T. (1999). Thermal and tactile perception thresholds in acute herpes zoster. European Journal of Pain, 3(4), 375–386. https://doi.org/10.1016/S1090-3801(99)90019-8
- Hadjistavropoulos, T., Herr, K., Prkachin, K., Craig, K., Gibson, S., Lukas, A., & Smith, J. (2014). Pain assessment in elderly adults with dementia. Lancet Neurology, 13(12), 1216–1227. https://doi.org/10.1016/S1474-4422(14)70103-6
- Heaton, R., Clifford, D., Franklin, D., Woods, S., Ake, C., Vaida, F., Ellis, R., Letendre, S. L., Marcotte, T. D., Atkinson, J. H., Rivera-Mindt, M., Vigil, O. R., Taylor, M. J., Collier, A. C., Marra, C. M., Gelman, B. B., McArthur, J. C., Morgello, S., Simpson, D. M., … Grant, I. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087–2096. https://doi.org/10.1212/WNL.0b013e318200d727
- Higgins, D. M., Martin, A. M., Baker, D. G., Vasterling, J. J., & Risbrough, V. (2018). The relationship between chronic pain and neurocognitive function: A systematic review. The Clinical Journal of Pain, 34(3), 262–275. https://doi.org/10.1097/AJP.0000000000000536
- Höper, J., Helfert, S., Heskamp, M.-L. S., Maihöfner, C. G., & Baron, R. (2014). High concentration capsaicin for treatment of peripheral neuropathic pain: Effect on somatosensory symptoms and identification of treatment responders. Current Medical Research and Opinion, 30(4), 565–574. https://doi.org/10.1185/03007995.2013.869491
- Hutchinson, A. D., Hosking, J. R., Kichenadasse, G., Mattiske, J. K., & Wilson, C. (2012). Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treatment Reviews, 38(7), 926–934. https://doi.org/10.1016/j.ctrv.2012.05.002
- Iverson, G. (2011). T scores. In J. Kreutzer, J. DeLuca, & B. Caplan (Eds.), Encyclopedia of Clinical Neuropsychology, 181–182. US: Springer.
- Jensen-Dahm, C., Vogel, A., Waldorff, F. B., & Waldemar, G. (2012). Discrepancy between self- and proxy-rated pain in Alzheimer’s disease: Results from the Danish Alzheimer intervention study. Journal of the American Geriatrics Society, 60(7), 1274–1278. https://doi.org/10.1111/j.1532-5415.2012.04036.x
- Jensen-Dahm, C., Werner, M. U., Ballegaard, M., Andersen, B. B., Høgh, P., & Waldemar, G. (2015). Discrepancy between stimulus response and tolerance of pain in Alzheimer disease. Neurology, 84(15), 1575–1581. https://doi.org/10.1212/WNL.0000000000001465
- Jensen-Dahm, C., Werner, M. U., Dahl, J. B., Jensen, T. S., Ballegaard, M., Hejl, A. M., & Waldemar, G. (2014). Quantitative sensory testing and pain tolerance in patients with mild to moderate Alzheimer disease compared to healthy control subjects. Pain, 155(8), 1439–1445. https://doi.org/10.1016/j.pain.2013.12.031
- Jiao, J., So, E., Jebakumar, J., George, M. C., Simpson, D. M., & Robinson-Papp, J. (2016). Chronic pain disorders in HIV primary care: Clinical characteristics and association with healthcare ultilisation. Pain, 157(4), 931–937. https://doi.org/10.1097/j.pain.0000000000000462
- Kataja, E. L., Karlsson, L., Tolvanen, M., Parsons, C., Schembri, A., Kiiski-Mäki, H., & Karlsson, H. (2017). Correlation between the cogstate computerized measure and WAIS-IV among birth cohort mothers. Archives of Clinical Neuropsychology, 32(2), 252–258. https://doi.org/10.1093/arclin/acw099
- Lawson, E., Sabin, C., Perry, N., Richardson, D., Gilleece, Y., Churchill, D., Dean, G., Williams, D., Fisher, M., & Walker-Bone, K. (2015). Is HIV painful? An epidemiologic study of the prevalence and risk factors for pain in HIV-infected patients. The Clinical Journal of Pain, 31(9), 813–819. https://doi.org/10.1097/AJP.0000000000000162
- Le Bars, D., Dickenson, A. H., & Besson, J. (1979). Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain, 6(3), 283–304. https://doi.org/10.1016/0304-3959(79)90049-6
- Luerding, R., & Bogdahn, U. (2008). Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: Structural correlates of pain-cognition interaction. Brain, 131(12), 3222–3231. https://doi.org/10.1093/brain/awn229
- Magerl, W., Krumova, E. K., Baron, R., Tölle, T., Treede, R. D., & Maier, C. (2010). Reference data for quantitative sensory testing (QST): Refined stratification for age and a novel method for statistical comparison of group data. Pain, 151(3), 598–605. https://doi.org/10.1016/j.pain.2010.07.026
- Malfliet, A., Coppieters, I., Van Wilgen, P., Kregel, J., De Pauw, R., Dolphens, M., & Ickmans, K. (2017). Brain changes associated with cognitive and emotional factors in chronic pain: A systematic review. European Journal of Pain, 21(5), 769–786. https://doi.org/10.1002/ejp.1003
- Maruff, P., Thomas, E., Cysique, L., Brew, B., Collie, A., Snyder, P., & Pietrzak, R. H. (2009). Validity of the CogState brief battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Archives of Clinical Neuropsychology, 24(2), 165–178. https://doi.org/10.1093/arclin/acp010
- May, M. T., Gompels, M., Delpech, V., Porter, K., Orkin, C., & Kegg, S. (2014). Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS, 28(8), 1193–1202. https://doi.org/10.1097/QAD.0000000000000243
- Mcdonnell, J., Haddow, L., Daskalopoulou, M., Lampe, F., Speakman, A., Gilson, R., Phillips, A., Sherr, L., Wayal, S., Harrison, J., Antinori, A., Maruff, P., Schembri, A., Johnson, M., Collins, S., Roger, A., & CIPHER Study Group. (2014). Minimal cognitive impairment in UK HIV-positive men who have sex with men: Effect of case definitions and comparison with the general population and HIV-negative men. JAIDS, 67(2), 120–127.
- Miaskowski, C., Penko, J. M., Guzman, D., Mattson, J. E., Bangsberg, D. R., & Kushel, M. B. (2011). Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. The Journal of Pain, 12(9), 1004–1016. https://doi.org/10.1016/j.jpain.2011.04.002
- Monroe, T. B., Beach, P. A., Bruehl, S. P., Dietrich, M. S., Rogers, B. P., Gore, J. C., Atalla, S. W., & Cowan, R. L. (2017). The impact of Alzheimer’s disease on the resting state functional connectivity of brain regions modulating pain: A cross sectional study. Journal of Alzheimer’s Disease, 57(1), 71–83. https://doi.org/10.3233/JAD-161187
- Moriarty, O., McGuire, B. E., & Finn, D. P. (2011). The effect of pain on cognitive function: A review of clinical and preclinical research. Progress in Neurobiology, 93(3), 385–404. https://doi.org/10.1016/j.pneurobio.2011.01.002
- Moriarty, O., Ruane, N., O’Gorman, D., Maharaj, C. H., Mitchell, C., Sarma, K. M., Finn, D. P., & McGuire, B. E. (2017). Cognitive impairment in patients with chronic neuropathic or radicular pain: An interaction of pain and age. Frontiers in Behavioral Neuroscience, 11, 100. https://doi.org/10.3389/fnbeh.2017.00100
- Nahman-Averbuch, H., Yarnitsky, D., Granovsky, Y., Sprecher, E., Steiner, M., Tzuk-Shina, T., & Pud, D. (2011). Pronociceptive pain modulation in patients with painful chemotherapy-induced polyneuropathy. Journal of Pain and Symptom Management, 42(2), 229–238. https://doi.org/10.1016/j.jpainsymman.2010.10.268
- Navis, A., Jiao, J., George, M. C., Simpson, D., & Robinson-Papp, J. (2018). Comorbid pain syndromes in HIV-associated peripheral neuropathy. Pain Medicine, 19(7), 1445–1450. https://doi.org/10.1093/pm/pnx129
- Oosterman, J., Derksen, L. C., van Wijck, A. J. M., Kessels, R. P. C., & Veldhuijzen, D. S. (2012). Executive and attentional functions in chronic pain: Does performance decrease with increasing task load? Pain Research and Management, 17(3), 159–165. https://doi.org/10.1155/2012/962786
- Overton, E. T., Kauwe, J. S. K., Paul, R., Tashima, K., Tate, D. F., Patel, P., Carpenter, C., Patty, D., Brooks, J. T., & Clifford, D. B. (2011). Performances on the CogState and standard neuropsychological barriers among HIV patients without dementia. AIDS and Behavior, 15(8), 1902–1909. https://doi.org/10.1007/s10461-011-0033-9
- Owens, M. A., Parker, R., Rainey, R. L., Gonzalez, C. E., White, D. M., Ata, A. E., Okunbor, J. I., Heath, S. L., Merlin, J. S., & Goodin, B. R. (2019). Enhanced facilitation and diminished inhibition characterizes the pronociceptive endogenous pain modulatory balance of persons living with HIV and chronic pain. Journal of NeuroVirology, 25(1), 57–71. https://doi.org/10.1007/s13365-018-0686-5
- Parker, R., Stein, D. J., & Jelsma, J. (2014). Pain in people living with HIV/AIDS: A systematic review. Journal of the International AIDS Society, 17(10), 18719. https://doi.org/10.7448/IAS.17.1.18719
- Paul, S. M., Zelman, D. C., Smith, M., & Miaskowski, C. (2005). Categorizing the severity of cancer pain: Further exploration of the establishment of cutpoints. Pain, 113(1–2), 37–44. https://doi.org/10.1016/j.pain.2004.09.014
- Phillips, T., Brown, M., Ramirez, J., Perkins, J., Woldeamanuel, Y., Williams, A., Orengo, C., Bennett, D. L. H., Bodi, I., Cox, S., Maier, C., Krumova, E. K., & Rice, A. S. C. (2014). Sensory, psycholgical and metabolic dysfunction in HIV-associated sensory peripheral neuropathy: A cross-sectional deep profiling study. Pain, 155(9), 1846–1860. https://doi.org/10.1016/j.pain.2014.06.014
- Pincus, T., Williams, A. C. D. C., Vogel, S., & Field, A. (2004). The development and testing of the depression, anxiety, and positive outlook scale (DAPOS). Pain, 109(1), 181–188. https://doi.org/10.1016/j.pain.2004.02.004
- Robertson, K. R., Smurzynski, M., Parsons, T. D., Wu, K., Bosch, R. J., Wu, J., McArthur, J. C., Collier, A. C., Evans, S. R., & Ellis, R. J. (2007). The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS, 21(14), 1915–1921. https://doi.org/10.1097/QAD.0b013e32828e4e27
- Rolke, R., Magerl, W., Campbell, K. A., Schalber, C., Caspari, S., Birklein, F., & Treede, R. D. (2006). Quantitative sensory testing: A comprehensive protocol for clinical trials. European Journal of Pain, 10(1), 77–88. https://doi.org/10.1016/j.ejpain.2005.02.003
- Sabin, C. A., Harding, R., Bagkeris, E., Nkhoma, K., Post, F. A., Sachikonye, M., Boffito, M., Anderson, J., Mallon, P. W. G., Williams, I., Vera, J., Johnson, M., Babalis, D., & Winston, A. (2018). Pain in people living with HIV and its association with healthcare resource use, well being and functional status. AIDS, 32(18), 2697–2706. https://doi.org/10.1097/QAD.0000000000002021
- Schweinhardt, P., Kuchinad, A., Pukall, C. F., & Bushnell, M. C. (2008). Increased gray matter density in young women with chronic vulvar pain. Pain, 140(3), 411–419. https://doi.org/10.1016/j.pain.2008.09.014
- Shiels, M. S., Cole, S. R., Kirk, G. D., & Poole, C. (2009). A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. JAIDS Journal of Acquired Immune Deficiency Syndromes, 52(5), 611–622. https://doi.org/10.1097/QAI.0b013e3181b327ca
- Simpson, D. M., Schifitto, G., Clifford, D. B., Murphy, T. K., Durso De-Cruz, E., Glue, P., Whalen, E., Emir, B., Scott, G. N., & Freeman, R. (2010). Pregabalin for painful HIV neuropathy: A randomized, double-blind, placebo-controlled trial. Neurology, 74(5), 413–420. https://doi.org/10.1212/WNL.0b013e3181ccc6ef
- Su, T., Schouten, J., Geurtsen, G. J., Wit, F. W., Stolte, I. G., Prins, M., Portegies, P., Caan, M. W. A., Reiss, P., Majoie, C. B., Schmand, B. A. & AGEhIV Cohort Study Group. (2015). Multivariate normative comparison, a novel method for more reliably detecting cognitive impairment in HIV infection. AIDS, 29(5), 547–557. https://doi.org/10.1097/QAD.0000000000000573
- Sunderland, A., Harris, J., & Gleave, J. (1984). Memory failures in everyday life following severe head injury. Journal of Clinical Neuropsychology, 6(2), 127–142. https://doi.org/10.1080/01688638408401204
- Treede, R., Rief, W., Barke, A., Aziz, Q., Bennett, M. I., Benoliel, R., Cohen, M., Evers, S., Finnerup, N. B., First, M. B., Giamberardino, M. A., Kaasa, S., Korwisi, B., Kosek, E., Lavand’homme, P., Nicholas, M., Perrot, S., Scholz, J., Schug, S., … Wang, S. (2019). Chronic pain as a symptom or a disease: The IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain, 160(1), 19–27. https://doi.org/10.1097/j.pain.0000000000001384
- Uebelacker, L. A., Weisberg, R. B., Herman, D. S., Bailey, G. L., Pickston-Camp, M. M., & Stein, M. D. (2015). Chronic pain in HIV-infected patients: Relationship to depression, substance use, and mental health and pain treatment. Pain Medicine, 16(10), 1870–1881. https://doi.org/10.1111/pme.12799
- Underwood, J., Cole, J. H., Caan, M., De Francesco, D., Leech, R., van Zoest, R. A., Su, T., Geurtsen, G. J., Schmand, B. A., Portegies, P., Prins, M., Wit, F. W. N., Sabin, C. A., Majoie, C., Reiss, P., Winston, A., Sharp, D. J., & The COBRA Collaboration. (2017). Gray and white matter abnormalities in treated human immunodeficiency virus disease and their relationship to cognitive function, Clinical Infectious Diseases, 65(3), 422–432. https://doi.org/10.1093/cid/cix301
- Underwood, J., De, D., Post, F. A., Vera, J. H., Williams, I., Boffito, M., Mallon, P. W., Anderson, J., Sachikonye, M., Sabin, C., & Winston, A. & the POPPY Study Group. (2017). Associations between cognitive impairment and patient- reported measures of physical / mental functioning in older people living with HIV. HIV Med 18(5), 363–369.
- Underwood, J., De Francesco, D., Cole, J. H., Caan, M. W. A., Van Zoest, R. A., Schmand, B. A., Sharp, D. J., Sabin, C. A., Reiss, P., Winston, A., Reiss, P., Wit, F. W. N. M., Schouten, J., Kooij, K. W., van Zoest, R. A., Elsenga, B. C., Janssen, F. R., Heidenrijk, M., Zikkenheiner, W., … Matthews, C. (2019). Validation of a novel multivariate method of defining HIV-associated cognitive impairment. Open Forum Infectious Diseases, 6(6), 1–9. https://doi.org/10.1093/ofid/ofz198
- Underwood, J., De Francesco, D., Leech, R., Sabin, C. A., Winston, A., & Cysique, L. A. (2018). Medicalising normality? Using a simulated dataset to assess the performance of different diagnostic criteria of HIV-associated cognitive impairment. PLoS ONE, 13(4), e0194760. https://doi.org/10.1371/journal.pone.0194760
- Underwood, J, De Francesco, D., Post, F. A., Vera, J. H., Williams, I., Boffito, M., Mallon, P. W., Anderson, J., Sachikonye, M., Sabin, C., Winston, A., & The POPPY Study Group. (2017b). Associations between cognitive impairment and patient- reported measures of physical / mental functioning in older people living with HIV. HIV Medicine, 18(5):363–369. https://doi.org/10.1111/hiv.12434
- Virtanen, M., Singh-Manoux, A., Batty, G. D., Ebmeier, K. P., Jokela, M., Harmer, C. J., Kivimäki, M., & Aleman, A. (2017). The level of cognitive function and recognition of emotions in older adults. PLoS ONE, 12(10), e0185513. https://doi.org/10.1371/journal.pone.0185513
- Wallace, V. C. J., Blackbeard, J., Segerdahl, A. R., Hasnie, F., Mcmahon, S. B., & Rice, A. S. C. (2007). Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain, 130(10), 2688–2702. https://doi.org/10.1093/brain/awm195
- Ware, J. J., & Sherbourne, C. (1992). The MOS 36-item short-form health survey (SF-36).I. Conceptual framework and item selection. Medical Care, 30(6), 473–483. https://doi.org/10.1097/00005650-199206000-00002
- Weber, E., Morgan, E. E., Iudicello, J. E., Blackstone, K., Grant, I., Ellis, R. J., Letendre, S. L., Little, S., Morris, S., Smith, D. M., Moore, D. J., Woods, S. P., & The TMARC Group.(2013). Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. Journal of NeuroVirology, 19(1), 65–74. https://doi.org/10.1007/s13365-012-0141-y
- Westermann, A., Krumova, E. K., Pennekamp, W., Horch, C., Baron, R., & Maier, C. (2012). Different underlying pain mechanisms despite identical pain characteristics: A case report of a patient with spinal cord injury. Pain, 153(7), 1537–1540. https://doi.org/10.1016/j.pain.2012.02.031
- Williams, D., Veenstra, M., Gaskill, P., Morgello, S., Calderon, T., & Berman, J. (2014). Monocytes mediate HIV neuropathogenesis: Mechanisms that contribute to HIV associated neurocognitive disorders. Current HIV Research, 12(2), 85–96. https://doi.org/10.2174/1570162X12666140526114526
- Woldeamanuel, Y. W., Kamerman, P. R., Veliotes, D. G. A., Phillips, T. J., Asboe, D., Boffito, M., Rice, A. S. C., & Sommer, C. (2016). Development, validation, and field-testing of an instrument for clinical assessment of HIV-associated neuropathy and neuropathic pain in resource-restricted and large population study settings. PLoS ONE, 11(10), e0164994. https://doi.org/10.1371/journal.pone.0164994
- Yarnitsky, D., Arendt-Nielsen, L., Bouhassira, D., Edwards, R. R., Fillingim, R. B., Granot, M., Hansson, P., Lautenbacher, S., Marchand, S., & Wilder-Smith, O. (2010). Recommendations on terminology and practice of psychophysical DNIC testing. European Journal of Pain, 14(4), 339–339. https://doi.org/10.1016/j.ejpain.2010.02.004
- Yarnitsky, D., Granot, M., Nahman-Averbuch, H., Khamaisi, M., & Granovsky, Y. (2012). Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain, 153(6), 1193–1198. https://doi.org/10.1016/j.pain.2012.02.021
