ABSTRACT
Orphans and vulnerable children (OVC) programs focusing on improving HIV outcomes for children and adolescents living with HIV (C&ALHIV) may improve viral load (VL) testing coverage, a critical step toward achieving VL suppression. In Mozambique, we conducted a retrospective medical record review comparing VL testing coverage and suppression between C&ALHIV receiving OVC support and two cohorts of non-participants constructed using propensity score matching. We collected data for 25,783 C&ALHIV in Inhambane, Maputo City, Nampula, and Tete between October 2020-September 2021. Unadjusted rates of VL testing were 62.9% among OVC participants compared with 39.2% and 50.4% of non-participants in OVC support and non-OVC support districts, respectively. In multivariate models, OVC participants were 18 and 10 percentage points more likely to have received a VL test than non-participants in OVC districts (p < 0.01) and non-OVC districts (p < 0.01), respectively. OVC participants under 5 years old were significantly more likely to have received a VL test than their same-age counterparts in both comparison groups. Overall, the OVC program did not demonstrate significant effects on VL suppression. This approach could be replicated in other contexts to improve testing coverage. It is crucial that clinical partners and governments continue to share data to enable timely monitoring through OVC programming.
Sustainable Development Goals:
Introduction
Despite adult AIDS-related deaths decreasing over the past 20 years, the global number of orphans and vulnerable children (OVC) increased from 10 million in 2000 to 14.9 million in 2021 (UNICEF, Citation2022). In Eastern and Southern Africa, nearly one-third of orphans were considered OVC (UNICEF, Citation2022), defined as children aged 0–17 having lost at least one parent due to AIDS-related causes or considered vulnerable to shocks due to living with a chronically ill caregiver (Schenk et al., Citation2010). Many OVC are themselves living with HIV, with UNAIDS estimating 1.7 million children (0–14 years) living with HIV in 2020, including 130,000 in Mozambique (UNAIDS, Citation2021). A 2018 modeling study estimated an additional 14.8 million “HIV-exposed uninfected” children globally, or those living in HIV-affected households despite having avoided HIV acquisition in utero and the postnatal period (Slogrove et al., Citation2020). These vulnerable children are eligible to receive OVC services in most contexts.
The magnitude of children living in HIV-affected households, especially in sub-Saharan Africa, contributed to the implementation of OVC programs funded by USAID and PEPFAR, which offer comprehensive services to vulnerable children and their caregivers using case management and the provision of services that contribute to health, safety, schooling, and economic stability. Initially, OVC programs emphasized social protection for all vulnerable children; in 2020, there was a shift toward prioritizing children and adolescents living with HIV (C&ALHIV) and addressing their socio-economic barriers to care. OVC programs have reported effects on HIV-related behaviors for OVC (Thomas et al., Citation2020), including increased uptake of HIV testing through home visits (Thurman et al., Citation2016). However, once C&ALHIV are engaged in care, less is known about whether OVC programs improve HIV treatment outcomes, including viral load (VL) suppression. Scaling up VL testing coverage can lead to improvements in VL suppression (Lecher et al., Citation2015), as more frequent follow-ups encourage adherence to antiretroviral therapy (ART) and facilitate timely detection and regimen-switching for patients in treatment failure (Nicholas et al., Citation2019).
In Mozambique, COVida (Juntos Pelas Crianças [2016-2022]) was a USAID/PEPFAR-funded project that aimed to improve the health, nutritional status, and well-being of OVC aged 0–19. As a PEPFAR project, one of COVida’s priorities was ensuring C&ALHIV adhered to treatment and became virally suppressed. Having achieved 98% of children in the program with known HIV status by 2020, COVida pivoted to improving HIV outcomes for those in treatment. The VL suppression rate among Mozambican C&ALHIV (0-19 years) was 53% at the end of September 2019 (PEPFAR, Citation2020), falling below the UNAIDS target to achieve VL suppression for 95% of those on treatment by 2030 (UNAIDS, Citation2015). COVida used a comprehensive case management approach complementing clinical services to improve linkages to care and ART adherence. For example, COVida provided support related to appointments and medication schedules, assistance accessing VL testing and understanding results, nutritional counseling and access to food packages, transportation support, HIV status disclosure support, and more.
Given the limited evidence on how offering OVC support services to C&ALHIV supports ART adherence and VL suppression status, we conducted a study to assess the effect of COVida programming on VL testing coverage and suppression. We compared participants against non-participants in COVida districts and C&ALHIV in non-COVida districts across four provinces. We aimed to use COVida’s approach to inform services and support for C&ALHIV in Mozambique and sub-Saharan Africa.
Materials & methods
We conducted a retrospective clinical record review of VL data comparing cohorts of COVida (hereafter, COVida is generically referred to as the OVC support program) participants and non-participants using propensity score analysis. We collected patient VL data, including test dates and results, from Mozambique’s Electronic Patient Tracking System (EPTS), an electronic medical record system used by public health facilities. VL data for OVC support participants and non-participants were collected between October 1, 2020 and September 30, 2021, representing a 12-month study window. This study was reviewed and approved by the Protections of Human Subjects Committee at FHI 360 and the Comitê Nacional de Bioética em Saúde in Mozambique.
Study setting
The OVC support program operates in seven provinces encompassing 72 PEPFAR-priority districts, including those receiving a tailored monitoring approach to reduce barriers to HIV care (PEPFAR, Citation2020). We conducted this study in four provinces – Inhambane, Maputo City, Tete, and Nampula – which were purposively selected as sites where VL testing first became more consistently available through strengthened relationships with clinical partners. As of 2015, adult HIV prevalence rates in Inhambane, Maputo City, Nampula, and Tete were 14.1%, 16.9%, 5.7%, and 5.2%, respectively (Ministério da Saúde et al., Citation2019). HIV testing coverage among children was 32%, 22%, 4%, and 7%, respectively, despite program support (Ministério da Saúde et al., Citation2019).
Study design
The OVC support program operates in high HIV burden areas and uses eligibility criteria to recruit C&ALHIV of greatest need into the program. Eligibility for OVC support services was assessed at the health facility, with referrals made to the program case managers for all C&ALHIV meeting one the following: 1) newly diagnosed and/or on a new treatment in the past 12 months; 2) being without a documented VL test or not being virally suppressed; or 3) having recently returned to care and treatment. While a randomized study design was infeasible due to the program eligibility criteria and the retrospective nature of the study, we adopted a quasi-experimental design based on propensity score analysis and two distinct control groups to assess program effects. In addition to data from OVC participants, we collected data from 1) C&ALHIV in OVC support districts not participating in the program and 2) C&ALHIV in high priority sites in non-OVC support districts ().
Figure 1. OVC participants and comparison groups, including children ineligible for OVC support services but living in OVC districts and children in non-OVC districts, across Inhambane, Maputo City, Nampula, and Tete.
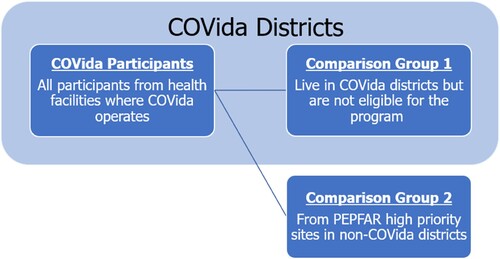
The sample size was not based on statistical considerations; instead, we used all available data collected from participants and non-participants meeting study eligibility criteria across the four provinces (). Generally, C&ALHIV (0-19 years) needed to be on active treatment for at least six months prior to the study window. National VL monitoring guidelines at the time of this study were for C&ALHIV to receive a VL test every six months for new ART initiations or after changing a regimen, then every 12 months for virally suppressed individuals. C&ALHIV on active treatment at least six months in advance of the study window would have been due for a VL test sometime during the study period. For this analysis, all OVC participants from the four provinces were included, with no criteria related to length of OVC support exposure. Children receiving OVC support services typically received monthly home visits, with most receiving ≥9 adherence support visits in the year preceding the study window, which was deemed adequate program exposure for assessing effects.
Table 1. Eligibility criteria for OVC participants and non-participants from two comparison groups.
Data collection & preparation
All health facilities reporting data via EPTS in OVC support and non-OVC support districts across all four provinces were included in the study (n = 174 facilities) (). We conducted a training of regional monitoring and evaluation (M&E) officers who oversaw data collection and quality review in each province. Since EPTS operates on an OpenMRS platform, we provided M&E officers with a list of EPTS queries to run at each facility. Queries included identifiable information (unique identifiers, age, and sex), treatment data (treatment regimen, ART start date), and VL test data (test dates, corresponding results) during the study window. Unique identifiers were compared against the participant roster to create the OVC cohort. Once all eligible C&ALHIV were identified across OVC and non-OVC support districts, datasets were anonymized for analysis.
Table 2. Number of health facilities reporting data in OVC and non-OVC districts across Inhambane, Maputo City, Nampula, and Tete.
Data queries from each facility were first exported into separate files, then compiled into a single analytic dataset using Python. Collaborating with M&E teams, we generated a standard query in EPTS, which identified time frames and pre-defined variables available across all facilities using EPTS. Despite the consistency that EPTS offered in terms of variable definitions and availability, formatting and datatypes were not always standardized across districts and health facilities, especially between facilities participating in the OVC support program and those that did not. The data team reviewed facility files as they were submitted on a rolling basis, then conferred with the regional M&E officer to clarify any issues that were presented during the data cleaning and standardization process (e.g., confirming and re-labeling non-standardized indicator names). Once all queries were addressed, we consolidated health facility level data into a unified dataset.
Analysis
We addressed selection bias by adopting an analysis based on the propensity to receive the treatment; specifically, we used an inverse-probability of treatment weighted (IPTW) regression model (Cattaneo et al., Citation2013). We used participant gender, age, province, ART start date and participation in an optimized ART regimen to model and predict treatment status (i.e., propensity to receive the treatment). We then computed inverse-probability (treatment) weights using propensity scores and estimated the average treatment effect (ATE) using a weighted linear regression model for our outcomes of interest: VL coverage and VL suppression (). These outcomes are defined using PEPFAR definitions, with VL coverage measured as the number of C&ALHIV with a VL test result divided by the number of C&ALHIV eligible for a viral load test (on ART for ≥6 months). VL suppression is measured as the number of C&ALHIV with a viral load under 1000 copies/milliliter divided by the number of C&ALHIV with a documented viral load test.
Table 3. Outcome measures and control variables.
For each outcome, we separately compared OVC participants first to non-participants in OVC districts, then to C&ALHIV in non-OVC districts. Further, we separated the analysis by province, gender, and age group to understand program effects for distinct groups. Since all participants had at least one VL test during the study window, all were included in the VL coverage analysis. However, participants with a missing value for their VL test result were dropped from the suppression analysis. Test results could be missing for various facility-level reasons, including delays in laboratories returning test results or delays in entering the results into the EPTS. Given the large sample sizes and the multiple models and comparisons included in the analysis, we interpret results as significant using a probability threshold of p = 0.01.
Results
Demographic characteristics
We collected medical record information for 25,783 C&ALHIV (0-19 years) on ART across four provinces (). Of all C&ALHIV, 28.7% were OVC participants and 28.6% and 42.7% were non-participants from OVC districts and non-OVC districts, respectively. These percentages varied between districts and provinces. Overall, roughly two-thirds were C&ALHIV from Maputo City (29.5%) or Nampula (37.6%), with Tete reporting data for the fewest C&ALHIV (13.7%). As anticipated due to selection into the OVC program, there were significant differences between the unweighted samples of OVC participants and non-OVC counterparts. Mean age was 10.5 years across all C&ALHIV, with OVC participants averaging 9.6 years compared to 12.0 and 10.0 years for non-participants in OVC districts and non-OVC districts, respectively (p < 0.01). Consistently across groups, more C&ALHIV were female (56.7%). Overall, 85.3% of C&ALHIV were on an optimized treatment regimen, with the proportion on an optimized regimen greater than 92% for C&ALHIV in OVC districts (both OVC participants and non-participants), compared with 80.3% of C&ALHIV in non-OVC districts.
Table 4. Descriptive statistics for the unweighted sample of C&ALHIV participating in OVC support services compared with non-participants from OVC districts and non-OVC districts, October 1, 2020 – September 31, 2021.
Viral load coverage
We descriptively examined VL coverage, which was highest among OVC participants at 62.9% (), followed by non-participants in non-OVC districts at 50.4% and non-participants in OVC districts at 39.2%. From the IPTW regression models, we found that, overall, OVC participants were 18 percentage points (95% confidence interval [CI]: 0.17-0.20; p < 0.01) more likely to have received a VL test than non-participants in OVC districts (). Examined by province, these differences ranged from 33 percentage points in Nampula (95% CI: 0.29-0.36; p < 0.01) to 6 percentage points in Maputo City (95% CI: 0.03-0.08; p < 0.01). Compared to non-participants in the same districts, female OVC participants were 18 percentage points (95% CI: 0.16-0.20; p < 0.01) and male OVC participants were 19 percentage points (95% CI: 0.16-0.21; p < 0.01) more likely to have received a VL test than female and male non-participants, respectively. OVC participants in all age bands were more likely to have received a VL test than non-participants in OVC districts. OVC participants under 5 were 29 percentage points (95% CI: 0.25-0.33; p < 0.01) more likely to have received a VL test than their same-age counterparts in OVC districts not receiving support services.
Table 5. Impact estimates for viral load coverage comparing weighted samples of OVC participants to non-participants in Groups 1 and 2 in four provinces in Mozambique (October 1, 2020 – September 30, 2021).
OVC participants were also 10 percentage points more likely to have received a VL test than C&ALHIV in non-OVC districts (95% CI: 0.08-0.11; p < 0.01). Respectively, OVC participants in Nampula and Tete were 15 (95% CI: 0.12-0.18; p < 0.01) and 24 percentage points (95% CI: 0.21-0.27; p < 0.01) more likely to have received a VL test than C&ALHIV in non-OVC districts. However, there were no program effects compared to C&ALHIV in non-OVC districts in Inhambane or Maputo City. OVC participants of both sexes and all age bands were significantly more likely to have received a VL test than C&ALHIV in non-OVC districts, with children under 5 being 20 percentage points (95% CI: 0.16-0.23; p < 0.01) more likely to have gotten a VL test if they received OVC services.
Viral load suppression
We did not identify significant effects when examining rates of VL suppression. Overall, our descriptive analysis () found 73.3% of OVC participants were virally suppressed compared with 77.1% of non-participants in OVC districts and 70.0% of C&ALHIV in non-OVC districts. In IPTW regression models, we did not identify significant program effects for VL suppression (), with the exception of OVC participants in Tete being 12 percentage points (95% CI: 0.07-0.17; p < 0.01) more likely to be virally suppressed than Tete-based C&ALHIV in non-OVC districts.
Table 6. Impact estimates for viral load suppression (under 1,000 copies/mL) comparing weighted samples of OVC participants to non-participants in Groups 1 and 2 in four provinces in Mozambique (October 1, 2020 – September 30, 2021).
Discussion
Since the World Health Organization recommended conducting VL monitoring at 6 and 12 months after ART initiation, then yearly thereafter (World Health Organization, Citation2021), HIV programs have worked to increase access to VL testing. This study supports the contribution of OVC programs in improving HIV treatment outcomes among C&ALHIV, including through reducing barriers to treatment and VL testing. The OVC support program assessed here demonstrated higher rates of VL testing coverage among participants compared to non-participants in both OVC and non-OVC districts. These results are consistent with findings from South Sudan (Coard et al., Citation2022) and Eswatini (EGPAF, Citation2021), indicating OVC support services increased VL testing coverage.
The OVC support program achieved statistically significant increases in VL coverage through data triangulation and close collaboration with clinical partners and activistas (OVC case workers) (Sousa et al., Citation2020). Beginning in 2020, the OVC support program worked with clinical partners to generate lists of children eligible for VL testing using the EPTS. Lists were shared with activistas, who educated caregivers on the importance of follow-up VL testing and provided transport support to health facilities. Results indicate this approach served especially effective for improving coverage for children under 5, which is crucial given that national VL coverage for children under 5 was the lowest across age bands in Mozambique (<14% as of the end of September 2021) (Wate, Citation2023). That the OVC support program was able to deliver significant gains in coverage by implementing their procedures for less than one year prior to the study window suggests the strength and feasibility of this approach, which could be replicated in other contexts seeking to increase VL coverage and monitoring for C&ALHIV.
The OVC support program’s ability to increase VL coverage varied by province. In Mozambique, most VL testing occurs at hospitals in provincial capitals. The smaller program effect reported in the capital may be due to Maputo City already operating with adequate resources and strategies for VL monitoring, making additional OVC services less influential. Although VL coverage among OVC participants was roughly 20 percentage points lower than the 95-95-95 target, it is notable that the testing window included the start of the COVID-19 pandemic when resources shifted away from HIV and other routine services to support COVID-19 testing and treatment. VL testing coverage for all PEPFAR-supported countries dropped 8 percentage points in January-March 2020 compared to rates at the end of 2019, then slowly recovered over the course of the year (Lecher et al., Citation2021). C&ALHIV coverage rates in Mozambique were likely affected by COVID-19 and may be higher in non-pandemic years. In fact, the OVC support program continued to adapt its programming to increase VL suppression, and data from the end of September 2022 estimated a VL suppression rate closer to 92% among participants (COVida, Citation2022).
Despite the OVC support program’s success in improving testing coverage, it did not significantly improve VL suppression during the analysis period. Overall rates of VL suppression were slightly lower among OVC participants compared with counterparts within OVC districts, though slightly higher than C&ALHIV in non-OVC districts. Significant effects were not identified in regression models, except for some provincial level differences in Tete. One reason for a lack of overall program effects on VL suppression may be the late nation-wide transition to dolutegravir-based treatment regimens that occurred toward the end of the study window. Ideally, C&ALHIV would have been on an optimized dolutegravir-based regimen for at least six months before the study window. A longer period after the regimen transition could have better captured treatment effects on VL suppression. Moreover, since increased VL testing coverage is a precursor to identifying improvements in suppression, it may be more appropriate to measure these effects within different time periods. Future studies might measure these outcomes using a 6-month lag for suppression rates to allow time for ART adherence and clinical decisions (e.g., regimen switching, etc.) to affect suppression rates.
Selection bias due to the lack of randomization into OVC support services is a consideration when interpreting results, especially comparing suppression rates between participants and C&ALHIV in their districts. The OVC support program’s eligibility requirements entail that participants have generally poorer HIV outcomes than non-participants, thus making them eligible for services. It may require longer time frames to quantify the effect of support services on VL suppression when comparing against C&ALHIV that have a higher likelihood of being virally suppressed at baseline. Children from non-OVC districts could also be more virally suppressed given their district was not flagged as a high priority district in need of an OVC program like COVida. However, our inclusion of two comparison groups strengthens our results interpretation given that findings were similar across comparator groups per outcome. Specifically, participants experienced increased rates of testing coverage over both comparison groups, while suppression rates were not significantly different from either group. Had there been significant differences in one comparator over the other for either outcome, results may have been explained more by selection bias than the influence of OVC support programming.
Limitations
This study represents a collaborative effort across multiple partners to leverage clinical data to assess HIV outcomes among C&ALHIV. These promising results should be interpreted considering some limitations. First, a randomized design was infeasible given that eligibility into OVC support services was determined a priori based on specific criteria. For this retrospective analysis, we attempted to address selection bias by including two comparison groups and using an IPTW regression adjustment. However, group differences in indicate that groups are indeed dissimilar in important ways. We also sought to reduce bias by controlling for observed variables in multivariable models (i.e., province, age, and treatment regimen). However, as we were limited to data available in the EPTS – which does not contain household, demographic, or other social support variables – there are likely omitted variables uncontrolled for in this analysis. Another challenge with EPTS data was data missingness and non-standardization. We undertook an intensive data cleaning and merging process, though data quality was not consistent across health facilities. The number of C&ALHIV who were missing VL test results and subsequently dropped from the suppression analysis presents limitations due to the potential loss in statistical power and challenges generalizing findings to the entire population. However, even after those cases were dropped, the sample size was still adequate for detecting small effects and none were identified. Also, we compared the demographic characteristics of those individuals who were missing VL test results against those for whom we had results and did not find substantial differences between groups.
Conclusions
With the recent prioritization of C&ALHIV, OVC programming has provided essential support services yielding improvements in HIV outcomes. This study highlights the important effect of OVC services on improving VL testing coverage, especially for children under 5. The OVC support program’s data triangulation and case management approaches can be replicated in other contexts to improve VL monitoring for C&ALHIV of all ages. Although we did not identify improvements in VL suppression, future analyses that use staggered or longer time periods may identify effects on VL suppression, especially as children transition to optimized regimens. It is crucial that clinical partners and governments share data and resources to enable VL monitoring for C&ALHIV.
Acknowledgements
This work would not have been possible without the commitment of clinical partners across Inhambane, Maputo City, Nampula, and Tete, especially provincial monitoring and evaluation officers at participating health facilities who facilitated data collection and quality review. The authors are grateful for their willingness to collaborate in sharing data and resources to support this effort. The authors are also thankful to members of the Ministry of Health for providing access to C&ALHIV data for this study and for facilitating all documentation needed for ethical approvals.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cattaneo, M. D., Drukker, D. M., & Holland, A. D. (2013). Estimation of multivalued treatment effects under conditional independence. The Stata Journal: Promoting communications on statistics and Stata, 13(3), 407–450. https://doi.org/10.1177/1536867X1301300301
- Coard, E., Oliver, D., & Monday, F. (2022). HIV outcomes within the context of orphans and vulnerable children programing: The 4Children project in South Sudan. BMC Infectious Diseases, 22(1), 186. https://doi.org/10.1186/s12879-022-07172-1
- COVida. (2022). COVida - Together for Children Program Data (Q4, FY22). FHI 360.
- EGPAF. (2021). Achieving success of high viral load coverage, suppression, and retention among children and adolescents living with HIV in Eswatini.
- Lecher, S., Ellenberger, D., Kim, A. A., Fonjungo, P. N., Agolory, S., Borget, M. Y., Broyles, L., Carmona, S., Chipungu, G., De Cock, K. M., Deyde, V., Downer, M., Gupta, S., Kaplan, J. E., Kiyaga, C., Knight, N., MacLeod, W., Makumbi, B., Muttai, H., … Nkengasong, J. (2015). Scale-up of HIV Viral Load Monitoring — Seven Sub-Saharan African Countries. MMWR. Morbidity and Mortality Weekly Report, 64(46), 1287–1290. https://doi.org/10.15585/mmwr.mm6446a3
- Lecher, S. L., Naluguza, M., Mwangi, C., N'tale, J., Edgil, D., Alemnji, G., & Alexander, H. (2021). Impact of the COVID-19 response on scale-up of HIV viral load testing - PEPFAR-supported countries, January-June 2020. MMWR. Morbidity and Mortality Weekly Report, 70(21), 794–795. https://doi.org/10.15585/mmwr.mm7021a3
- Ministério da Saúde, Instituto Nacional de Estatística, & ICF. (2019). Survey of Indicators on Immunization, Malaria and HIV/AIDS in Mozambique (IMASIDA) 2015: Supplemental Report Incorporating Antiretroviral Biomarker Results.
- Nicholas, S., Poulet, E., Wolters, L., Wapling, J., Rakesh, A., Amoros, I., Szumilin, E., Gueguen, M., & Schramm, B. (2019). Point-of-care viral load monitoring: Outcomes from a decentralized HIV programme in Malawi. Journal of the International AIDS Society, 22(8), e25387. https://doi.org/10.1002/jia2.25387
- PEPFAR. (2020). Mozambique PEPFAR COP20.
- Schenk, K. D., Michaelis, A., Sapiano, T. N., Brown, L., & Weiss, E. (2010). Improving the lives of vulnerable children: Implications of Horizons research among orphans and other children affected by AIDS. Public health reports (Washington, D.C. : 1974), 125(2), 325–336. https://doi.org/10.1177/003335491012500223
- Slogrove, A. L., Powis, K. M., Johnson, L. F., Stover, J., & Mahy, M. (2020). Estimates of the global population of children who are HIV-exposed and uninfected, 2000–18: A modelling study. The Lancet Global Health, 8(1), e67–e75. https://doi.org/10.1016/S2214-109X(19)30448-6
- Sousa, B., Chiale, S., Bryant, H., & Medrano, T. (2020). Triangulating OVC and health facility data to identify gaps in HIV care and treatment for children and adolescents living with HIV.
- Thomas, T., Tan, M., Ahmed, Y., & Grigorenko, E. L. (2020). A Systematic Review and Meta-Analysis of Interventions for Orphans and Vulnerable Children Affected by HIV/AIDS Worldwide. Annals of Behavioral Medicine, 54(11), 853–866. https://doi.org/10.1093/abm/kaaa022
- Thurman, T. R., Luckett, B., Taylor, T., & Carnay, M. (2016). Promoting uptake of child HIV testing: An evaluation of the role of a home visiting program for orphans and vulnerable children in. South Africa [Journal Article]. AIDS Care, 28(Suppl. 2), 7–13. http://www.tandfonline.com/loi/caic20.
- UNAIDS. (2015). Understanding Fast-Track: Accelerating Action to End the AIDS Epidemic by 2030.
- UNAIDS. (2021). UNAIDS Data 2021 (https://www.unaids.org/en/resources/documents/2021/2021_unaids_data.
- UNICEF. (2022). UNICEF Data: Orphanhood. https://data.unicef.org/topic/hivaids/orphanhood/.
- Wate, A. (2023). Apresentação: Ponto de Situação do TARV Pediatrico e Adolescente.
- World Health Organization. (2021). Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring: March 2021.
