Abstract
Purpose: Severe chronic hand eczema (CHE) has a debilitating effect on quality of life (QoL). PASSION evaluated the effectiveness of oral alitretinoin on QoL and work productivity in patients with severe CHE following prescribing guidelines.
Methods: A non-interventional, open-label, observational, multicentre study conducted in Germany in fulfilment of German guidelines. Patients (n = 631) were treated with once-daily alitretinoin for ≤24 weeks under standard daily practise conditions. Effectiveness was assessed by Physician Global Assessment (PGA), QoL Assessment (EQ-5D) and work impairment. Tolerability and safety were assessed by adverse event (AE) monitoring.
Results: In total, 279 (44.2%) patients dropped out before Week 24. Of the 631 patients enrolled, 29.8% achieved a PGA rating of clear/almost clear at Week 24. Mean (standard deviation) EQ-5D utility and EQ-5D visual analogue scale scores at baseline were 0.76 (0.25) and 53.6 (23.55), respectively, and increased to 0.94 (0.12) and 80.8 (19.23) at Week 24, indicating improved QoL. At baseline, 49.4%/29.1% of patients reported strong/very strong workplace impairment, respectively, and decreased to 8.5%/1.4%, respectively, at Week 24. AEs were reported in 116 (18.4%) patients. No new safety signals were observed.
Conclusions: Alitretinoin produced marked improvement in the QoL and work productivity of patients with severe CHE.
Introduction
Hand eczema is the most common skin disorder affecting the hands, with an annual prevalence of 7–12% (Citation1–3). Hand eczema is also the most common occupational skin disease and is a pronounced problem in certain irritant- and allergen-exposed professions (Citation4). Approximately, 5–7% of patients with chronic hand eczema (CHE) have disease rated as severe, characterised by extensive, long-lasting or recurrent severe skin changes such as rhagades, lichenification and infiltration, lasting more than three months or relapsing twice or more often per year (Citation5–7). A range of exogenous and endogenous factors contribute to the aetiology of severe CHE including an atopic predisposition, which is a major risk factor (Citation8). Severe CHE is associated with a negative impact on quality of life (QoL) and can lead to psychological problems such as depression, anxiety and low self-esteem (Citation9,Citation10). Severe CHE is reported to reduce work productivity, increase the number of work days missed and markedly increase medical costs (Citation11–13). Total medical costs are reported to increase with disease severity, consequently, severe CHE may cause a significant economic burden (Citation11).
Although some patients with hand eczema may be managed effectively by avoiding irritants, using emollients and by treatment with topical corticosteroids (Citation7,Citation14), patients with severe CHE, that are refractory to potent topical corticosteroids have limited treatment options (Citation6). Alitretinoin (TOCTINO®) is an oral retinoid that is approved for the treatment of severe CHE that is refractory to topical corticosteroids (Citation15) and recommended as second-line treatment (Citation16) Alitretinoin binds to retinoic acid and retinoid X receptors and has anti-inflammatory and immunomodulatory properties (Citation17).
In randomised controlled trials, oral alitretinoin has been demonstrated to be effective at reducing CHE severity in the treatment of severe CHE, refractory to potent topical corticosteroids (Citation18,Citation19). More recently, an open-label study and a non-interventional study reflecting real practise conditions, have supported this finding (Citation20,Citation21). Since severe CHE can have a profound effect on a patient’s QoL and work productivity, it is important to measure how treatment affects these parameters. The objective of the study was to evaluate the effectiveness of oral alitretinoin, in accordance with CHE prescribing guidelines, on the QoL and work productivity of patients with severe CHE refractory to potent topical corticosteroids.
Materials and methods
Study design
This was a non-interventional, open-label, observational, multicentre study (GSK Study ATN117218; PASSION) conducted at 233 sites across Germany between November 2011 and 30 June 2013. This study was not monitored nor conducted according to Good Clinical Practise (GCP) but the methodology followed current guidelines for non-interventional studies, which are mandatory for post-marketing surveillance in Germany. The recommendations of the German competent authority, “Bundesinstitut für Arzneimittel und Medizinprodukte” (BfArM) and the “Verband forschender Arzneimittelhersteller e.V”; German Association of Research-based Pharmaceutical Companies (VfA) for improvement of quality and transparency of non-interventional studies were followed. Written, informed consent was obtained from each patient before study procedures were performed. The study was approved by the Ethics Committee of the University of Frankfurt. Eligible patients were treated under normal conditions of practise and in accordance with the product label, with once-daily oral alitretinoin 30 mg capsules for a maximum of 24 weeks. Dose reductions to once-daily alitretinoin 10 mg were permitted at the discretion of the attending physician and in accordance with the product label if the 30 mg dose was not tolerated. The observation plan scheduled a maximum of seven documentation dates, each over a period of approximately four weeks.
Patients
Patients that were already scheduled for treatment with alitretinoin were selected. Study inclusion was at the discretion of the attending physician’s interpretation of having a diagnosis of severe CHE according to summary of product characteristics (SmPC) recommendations on alitretinoin treatment (Citation15), with no limit to the duration of disease. Patients who were not naïve to oral alitretinoin were also included in the study. Prior to treatment, all female patients of childbearing age were required to have a medically supervised pregnancy test, then monthly during treatment and up to five weeks after completion of treatment. Positive pregnancy tests were reported as adverse events (AEs).
Outcomes and assessments
In addition to the initial baseline visit at the start of the observational period, patients returned to study centres approximately every four weeks (standard practise for alitretinoin treatment), for up to 24 weeks (although visits were not mandatory). Primary evaluations at Week 4, 8, 12, 16, 20 and 24 included assessment of disease severity, QoL and work impairment. Disease severity and effectiveness of treatment were assessed using the Physician and Patient Global Assessment (PGA and PaGA, respectively). Physicians’ graded the severity of the CHE (clear/almost clear/mild/moderate/severe) using an assessment tool supported by a photographic guide. In order to ensure a uniform interpretation of clinical severity in line with the PGA assessment, the photographic guide used in this study was modified in line with Coenraads et al. (Citation5). Patients’ graded the change in skin condition compared with the previous visit (worsened/unchanged/slightly improved/almost completely healed/completely healed) using a questionnaire. Patients were treated with alitretinoin until achieving a satisfactory clinical response for up to 24 weeks. A visual analogue scale (VAS) was used to evaluate itch/pain due to CHE, where lower scores indicate no pain or itch (0 – none; 10 – very strong). QoL was evaluated using the standardised, validated, non-skin-specific preference-based QoL (EQ-5D) questionnaire, assessing five areas of QoL (flexibility/mobility, taking care of oneself, general activities, pain/physical complaints and anxiety/depression) (Citation22). From the responses to these questions, an EQ-5D utility score was derived and adjusted using German weighting as calculated and described by Konig et al. (Citation23) and Greiner et al. (Citation24) The EQ-5D utility scores for Germany are between −0.205, for the worst possible health, and 1.0 for the best possible health. The questionnaire additionally contains a VAS for the evaluation of the current health of the patient, where 0 represents the worst imaginable health state and 100, the best imaginable health state. Work incapacity due to CHE was assessed by inability to work, duration of inability to work and degree of impairment of work functionality. Additional assessments included final evaluation of efficacy and tolerability of alitretinoin by physicians and patients at the end of the study, graded as bad, moderate, satisfactory, good or very good. AEs and treatment-related AEs (defined as AEs thought to be caused by the investigational product, including suspicious events) were also monitored and recorded. The length of the observation period for individual patients varied and may have been less than the maximum 24 weeks, if the physician or patient chose to terminate the study early.
Statistical methods
All patients for whom data were available were included in the analyses. Continuous variables were summarised descriptively (n, mean, median, standard deviation [SD]). Categorical data were summarised using frequency counts and percentages. Efficacy summaries are based on available observations by visit. The last observation carried forward (LOCF) method was applied to deal with missing values. In LOCF analyses, only the values of the follow-up examinations are considered and carried forward from the last non-missing observation, for missing baseline values this approach was not applied. No further imputations for missing values were performed. AEs were coded according to the Medical Dictionary for Regulatory Activities, version 14.1, and summarised by system organ class and preferred term.
Results
Study population
In total, 631 patients received at least one dose of alitretinoin and were included in the analyses. Overall, 279 (44.2%) patients dropped out before Week 24. Of the overall study population, 1.0% withdrew due to clinically-relevant changes in laboratory parameters, 5.9% withdrew due to AEs, 5.9% terminated early due to a lack of effectiveness, 9.7% withdrew consent and 27.1% discontinued for reasons unspecified or not given. The mean age was 49 years (range 18–83 years) and 47.7% were male. The mean (SD) duration of disease was 10.3 (13.8) years, with a majority (61.2%) of patients with eczema diagnosed as hyperkeratotic-rhagadiform. In total, 17.7% patients had previous treatment with alitretinoin. In 40.6% of patients, CHE was possibly or probably job-related and 26.3% of patients were taking part in a dermatologist’s procedure at the start of the study ().
Table 1. Summary of patient demographics and baseline characteristics.
Effectiveness
PGA
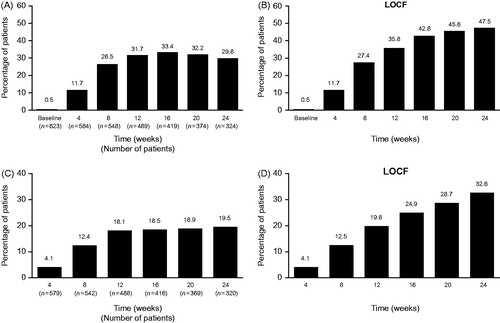
At baseline the majority of patients had CHE characterised on the PGA as moderate (34.1%) or severe (62.0%) and only 3 (0.5%) clear/almost clear. Over the course of the study, the majority of patients demonstrated a marked improvement in symptoms. At Week 12 and Week 24, 31.7% (n = 200) and 29.8% (n = 188), respectively, of all patients receiving at least one dose of alitretinoin (n = 631) had a PGA status of clear/almost clear (). Using the LOCF-approach, 35.8% (n = 226) and 47.5% (n = 300), respectively, of all 631 patients receiving alitretinoin had a PGA status of clear/almost clear at Week 12 and Week 24, respectively (). The mean (SD) and median (range) duration of treatment until a rating of clear/almost clear was 93.4 (51.14) and 84 (1–244) days, respectively. Conversely, the proportion of patients rated as severe on the PGA decreased from 62.0% (n = 391) at baseline, to 3.2% (n = 20) at Week 12 and to 1.1% (n = 7) at Week 24. At Week 24 LOCF, 9.2% (n = 58) of patients were rated as severe, 20.1% (n = 127) as moderate and 23.1% (n = 146) as mild.
Figure 1. Summary of CHE scores of almost completely healed/completely healed by (A) PGA (B) PGA LOCF and almost completely healed by (C) PaGA (D) PaGA LOCF over time. Treatment could end prior to 24 weeks if a rating of clear/almost clear was achieved or if treatment was terminated early. For A and C, analyses were based on patients with observed data, patient numbers differed from visit to visit as illustrated by n values below the x axis. For B and D data presented are LOCF (n = 631). LOCF: last observation carried forward; PGA: Physician Global Assessment; PaGA: Patient Global Assessment.
PaGA
At baseline, the majority of patients rated themselves as having moderate (34.4%) or severe (59.3%) CHE and only 1.0% as [almost] clear. Over the course of the study, patients reported a marked improvement in symptoms. Almost completely healed CHE (compared with the previous visit) was reported by 18.1% and 19.5% of all 631 patients at Week 12 and 24, respectively, and 19.8% and 32.6% of patients at Week 12 and 24 using the LOCF approach (). In total, 17.4% (n = 110) completed treatment with disease evaluated as “worsened”.
Pain and itch
At baseline, mean (SD) VAS scores of 5.4 (3.1) and 6.2 (3.0) indicated pain and itch due to CHE. After initiation of treatment, the patient-completed VAS for both pain and itch showed reductions and at Week 4 scores decreased to 3.2 (2.8) and 4.0 (2.8). At Week 24, scores further decreased to 1.6 (2.2) and 2.1 (2.4; ).
Table 2. Summary of pain and itch scores.
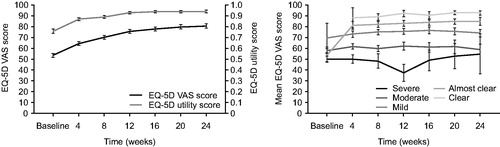
QoL assessment (EQ-5D)
At baseline, EQ-5D VAS indicated severe impairment for patients, with a mean (SD) score of 53.6 (23.55) (Citation23). Over the course of the study, patients demonstrated progressive improvement in EQ-5D VAS scores. By Week 12, EQ-5D VAS scores were 75.6 (20.93), rising to 80.8 (19.23) at Week 24 (). Similarly, baseline EQ-5D utility scores of 0.76 (0.252) indicated impairment. After the initiation of alitretinoin treatment, utility scores increased to 0.87 (0.173) at Week 4 and 0.93 (0.120) at Week 12. These improvements were maintained at Week 24, 0.94 (0.118; ). EQ-5D VAS scores were assessed by PGA rating and demonstrated that increased severity of CHE is associated with lower scores, a result consistent across the study observation period ().
Disability and work incapacity due to severe CHE
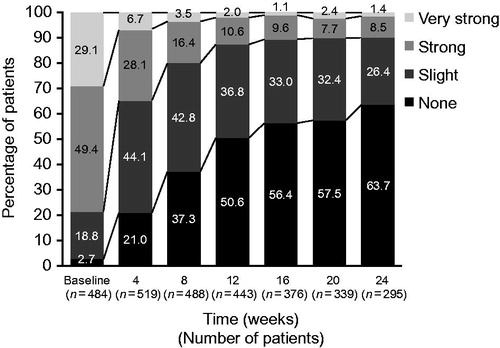
At baseline, 78 (12.4%) patients were disabled due to CHE. Over the course of the observation period there was a progressive decrease in disability due to CHE. At Week 24, 14 (2.2%) patients were disabled due to CHE. At baseline, patients spent a mean (SD) of 22.3 (29.01) consecutive working days unable to work. At Week 24, the mean (SD) was 14.8 (8.09) days, respectively, unable to work since the last visit. There was also a reduction in the degree of workplace impairment from baseline, where 49.4% and 29.1% of patients reported strong or very strong work impairment, respectively, and 2.7% reported none. By Week 12, 10.6% and 2.0% of patients reported strong or very strong work impairment, respectively, and 50.6% of patients reported no impairment. Further reductions were reported at Week 24, with strong or very strong workplace impairment reported by 8.5% and 1.4% of patients, respectively, and no impairment reported by 63.7% of patient ().
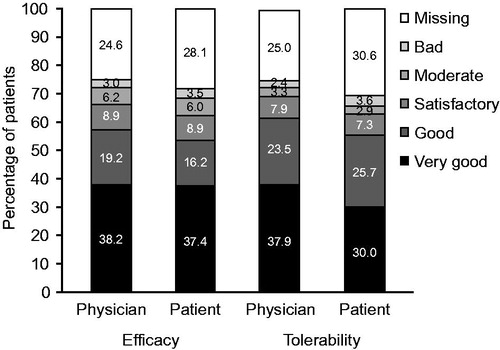
Final evaluation of efficacy and tolerability
Overall, efficacy was evaluated as good or very good by 57.4% of physicians and 53.6% of patients. Tolerability was evaluated as good or very good by 61.3% of physicians and 55.6% of patients ().
Safety
Overall, 116 (18.4%) patients reported AEs, 104 (16.5%) patients reported AEs considered treatment-related and eight patients (1.3%) reported serious AEs (SAEs). SAEs included alopecia, headache, nausea and abdominal discomfort, renal pain, myocardial infarction, hepatic enzymes and lipids increased (n = 1 for each), and increased blood triglycerides (n = 2). A total of 45 (7.1%) AEs led to withdrawal, 42 (6.7%) of which were treatment-related. The five most common AEs leading to treatment discontinuation were myalgia, depression, hypercholesterolaemia and nausea (all n = 4) and headache (n = 9). There were no deaths during the study. The most commonly reported AEs included headache, increased triglycerides, increased cholesterol, erythema, nausea and myalgia ().
Table 3. Summary of most common (≥1% patients) treatment-emergent AEs.
Discussion
In this non-interventional, open-label, multicentre, observational, real-world study, alitretinoin was shown as an effective treatment for patients with severe CHE, refractory to potent topical corticosteroids. Improvements in QoL and reduction in work incapacity were demonstrated throughout the study. Furthermore, the majority of patients and physicians evaluated alitretinoin effectiveness and tolerability as good or very good.
The patient demographics and characteristics including disease duration and the type of CHE were similar to those of previous studies of alitretinoin but there were important differences (Citation18–21). In PASSION, 17.7% of patients had received alitretinoin in the previous 12 months before the study, compared with the naïve population used in a Phase III clinical trial (Citation18). The SmPC for alitretinoin indicates its use in the treatment of severe CHE, refractory to potent topical corticosteroids (Citation15). In the current study, 62% of patients had PGA ratings of severe; with other PGA ratings of moderate, mild and almost clear comprising 34.1%, 2.2% and 0.5% of patients, respectively.
The effectiveness of alitretinoin was consistent across all parameters, with marked improvements in PGA and PaGA, in addition to reduced pain and itch. The median (range) time to a rating of clear/almost clear on the PGA was 84 days (1, 244), similar to the 86.5 days reported in a previous open-label Phase III trial (Citation20). By Week 24 the proportion of patients achieving a rating of clear/almost clear on the PGA was 29.8%. This is lower than the 50% previously reported in a Phase III study, using a comparable alitretinoin dose (Citation18). Similarly, 47–57% of patients treated with alitretinoin 30 mg or 10 mg, were rated as clear/almost clear at Week 24 in two open-labelled studies (Citation20,Citation21). The lower proportion of patients rated as clear/almost clear at Week 24 in this study may be due to 44.2% of patients leaving the study before the end of the observation period (Week 24). Of the total patient population, 11.8% dropped-out due to AEs or lack of efficacy and 27.1% dropped-out without specifying a reason. Therefore, it was not possible to determine whether drop-out was due to effectiveness and tolerability issues or due to treatment success. The proportion of patients (19.5%) with PaGA ratings of [almost] clear at Week 24 is lower than the reported rating of 40% in a clinical trial with alitretinoin 30 mg treatment (Citation18). As discussed above, the high drop-out rate may have been a factor in this difference. Finally, a previous Phase III open-label study with alitretinoin 30 mg demonstrated immediate decreases in pain and itch VAS scores after four weeks, with a continued reduction in these symptoms to the end of the study at Week 24 (Citation20). The results of the current study support these findings.
An observational, open-label study in 15 patients with severe CHE demonstrated that QoL, measured by EQ-5D and the Dermatology Life Quality Index, improves with alitretinoin 30 mg administered for three months (Citation10) The results of the current study support these findings. Patients reported mean EQ-5D VAS scores at baseline of 53.6, lower than the 77.4 reported in the general German population, indicating impairment in their QoL.[Citation23]. Progressive improvements in QoL, in the form of EQ-5D utility scores were seen until Week 12 and maintained until Week 24. EQ-5D VAS scores continued to improve until the end of the observation period. When EQ-5D VAS scores were assessed by PGA severity rating, better QoL scores were associated with decreased CHE severity. This suggests that effective treatment of severe CHE in accordance with the relevant stages and guidelines can markedly improve QoL. Similar findings have been reported in studies of other dermatological conditions such as psoriasis (Citation25,Citation26).
The debilitating effects of CHE have a significant impact on both healthcare costs and work productivity (Citation6,Citation11,Citation12). Two studies in Germany have assessed the cost of illness in patients with CHE. One study demonstrated that the direct costs to patients and the healthcare system increase with disease severity and are greater than for other dermatological conditions such as moderate-to-severe chronic psoriasis vulgaris. In terms of occupational costs, the study demonstrated that one-third of patients with CHE have reported taking sick leave in the last year, with an average of 7.2 days, despite only 31% of patients having severe disease (Citation11,Citation27). In a study of occupational hand eczema, nearly 68% of patients rated severe reported at least one sickness absence, with an average of 76 days. It also demonstrated that nearly 70% of societal costs of occupational eczema are due to loss of productivity (Citation28). The results of the current study support these findings and together, they highlight the need for treatment options to minimise work impairment. In the current study, treatment with alitretinoin reduced workplace impairment and the number of strong and very strong impairment ratings. Alitretinoin also showed beneficial changes in reducing the number of people rated as disabled. These results provide evidence that alitretinoin can reduce work incapacity, when used at clinically approved doses.
In the current study, the overall incidence of AEs was lower than reported in the previous Phase III trial (BAP00089) (Citation18) but similar to a previous open-label study (Citation21). The lower incidence of AEs in studies conducted under real-world clinical practise conditions is likely due to differences in AE reporting. In this study, only AEs believed by physicians to be treatment-related were documented, whereas in clinical trials all AEs are recorded regardless of causality. Additionally, the current study used an AE reporting design where AE information was not explicitly requested and instead only prompted by a reminder for the physician at each time point to document any treatment-related AEs that occurred during therapy. The most common AEs reported, including headache and hypertriglyceridaemia, were typical of retinoid class drugs and broadly consistent with previous studies (Citation18,Citation20,Citation21). Though AEs were demonstrated to be manageable in the majority of patients, 7.1% of AEs led to withdrawal, 6.7% of which were deemed to be treatment-related. This rate is lower than the 14.1% previously reported in an open-label interventional Phase III study (Citation21), but similar to the 8.5% in a non-interventional study (Citation20). Patients and physicians had similar perceptions of the effectiveness and tolerability of alitretinoin, with the majority rating it as good or very good.
The main limitation of this study, particularly compared with interventional studies, is that it was not monitored or conducted according to GCP, but the methodology followed the current guidelines for non-interventional studies, which are mandatory for post-marketing surveillance in Germany. However, measures to improve data quality were implemented including a Data Handling Manual and a Data Validation Plan. Additionally, patients were treated in accordance with CHE prescribing guidelines and on that basis it may be considered indicative of a real-world setting. The alitretinoin label suggests that physicians should consider the discontinuation of treatment in patients with severe disease if no improvements have been made after 12 weeks. However, a number of patients continued therapy in this study and further improvements were seen. At Week 24, the number of patients rated as severe decreased to 7 from the 20 at Week 12. Another limitation was the use of the LOCF method to address missing data in the study analysis. The LOCF method has several limitations which have been described elsewhere (Citation29). However, since this was a non-interventional and observational study and over half of patients withdrew before Week 24, this method is appropriate for addressing the bias introduced by missing data. Moreover, there is no best method for dealing with missing values.
In summary, the QoL of patients with CHE is significantly influenced by clinical disease severity, measured by PGA. Alitretinoin treatment resulted in marked and rapid improvements in the severity of CHE, coupled with increased QoL and reduced work impairment for patients. The safety profile of alitretinoin is consistent with the marketed product label. The majority of physicians and patients were in agreement in their final evaluation of the effectiveness and tolerability of alitretinoin, rating it good or very good, making alitretinoin a useful therapy in patients’ refractory to potent topical corticosteroid treatment.
Authorship contributions
Diamant Thaçi contributed to the study concept and design and was involved in data acquisition, analysis and interpretation. Matthias Augustin participated in data acquisition, analysis and interpretation. Anja Kamps contributed to the study concept and design and was involved in data analysis and interpretation. Bernd Westermayer and Michael Hennig participated in planning and conducting data analyses and interpretation. All authors were involved in the preparation and review of the manuscript and approved the final version to be submitted.
Role of the funding source
The study (GSK 117218) was funded by Stiefel, a GSK company, and Basilea Pharmaceutica Deutschland GmbH. Basilea Pharmaceutica planned and conducted this alitretinoin study. GSK acquired the alitretinoin asset from Basilea Pharmaceutica.
Acknowledgements
The authors wish to thank RTI Health Solutions for additional analysis of data (funded by GSK) and Alex Lowe, PhD, and Sarah Brown, BSc (Hons), from Fishawack Indicia Ltd, who provided editorial assistance with developing this manuscript (in the form of writing assistance, including development of the initial draft, assembling tables and figures, collating authors comments, grammatical editing and referencing), funded by GSK.
Disclosure statement
Diamant Thaçi has received consulting/advisory board agreements or honoraria for lecturing with AbbVie, Amgen, Biogen-Idec, Boehringer-Ingelheim, Janssen-Cilag, Celgene Corp., Dignity, Dermira, GSK, Leo, Pfizer, Regeneron, Sanofi, Sandoz, MSD, Mitsubishi, Medimmune, Novartis, UCB and Lilly.
Matthias Augustin has received grants and/or honoraria as a consultant, speaker, and/or advisory board member from AbbVie, Almirall, Amgen, Astellas, Basilea, Biogen, Celgene, Eli Lilly, GSK, Janssen Biotech, Inc., Leo Pharma, Merck Sharp Dohme, Merck-Serono, MedImmune, Novartis, Pfizer, Pohl-Boskamp, Sandoz, Stiefel, UCB, and Xenoport.
Bernd Westermayer and Michael Hennig are employees of GSK and hold stocks/shares in GSK.
Anja Kamps was an employee of Stiefel, a GSK company at the time of the study and holds stocks/shares in GSK.
References
- Coenraads PJ, Nater JP, van der Lende R. Prevalence of eczema and other dermatoses of the hands and arms in the Netherlands. Association with age and occupation. Clin Exp Dermatol. 1983;8:495–503.
- Fowler JF, Duh MS, Chang J, et al. A survey-based assessment of the prevalence and severity of chronic hand dermatitis in a managed care organisation. Cutis. 2006;77:385–92.
- Meding B, Jarvholm B. Hand eczema in Swedish adults - changes in prevalence between 1983 and 1996. J Invest Dermatol. 2002;118:719–23.
- Diepgen TL. Occupational skin-disease data in Europe. Int Arch Occup Environ Health. 2003;76:331–8.
- Coenraads PJ, Van Der Walle H, Thestrup-Pedersen K, et al. Construction and validation of a photographic guide for assessing severity of chronic hand dermatitis. Br J Dermatol. 2005;152:296–301.
- Diepgen TL, Agner T, Aberer W, et al. Management of chronic hand eczema. Contact Dermatitis. 2007;57:203–10.
- Diepgen TL, Andersen KE, Chosidow O, et al. Guidelines for diagnosis, prevention and treatment of hand eczema – short version. J Dtsch Dermatol Ges. 2015;13:77–84.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7–13.
- Cvetkovski RS, Zachariae R, Jensen H, et al. Quality of life and depression in a population of occupational hand eczema patients. Contact Dermatitis. 2006;54:106–11.
- Gola M, D'Erme AM, Milanesi N, et al. Effects of alitretinoin on quality of life of patients having chronic hand eczema: an observational study. Dermatitis. 2013;24:166–9.
- Augustin M, Kuessner D, Purwins S, et al. Cost-of-illness of patients with chronic hand eczema in routine care: results from a multicentre study in Germany. Br J Dermatol. 2011;165:845–51.
- Diepgen TL, Purwins S, Posthumus J, et al. Cost-of-illness analysis of patients with chronic hand eczema in routine care in Germany: focus on the impact of occupational disease. Acta Derm Venereol. 2013;93:538–43.
- Apfelbacher CJ, Akst W, Molin S, et al. CARPE: a registry project of the German Dermatological Society (DDG) for the characterization and care of chronic hand eczema. J Dtsch Dermatol Ges. 2011;9:682–8.
- Soost S, Abdollahnia M, Kostev K, et al. Topical therapy of hand eczema - analysis of the prescription profile from dermatologists in private practice. J Dtsch Dermatol Ges. 2012;10:180–4.
- Stiefel. Alitretinoin (Toctino®) Summary of Product Characteristics. Available from https://www.medicines.org.uk/emc/medicine/21177. Accessed March 1, 2015.
- Diepgen TL, Andersen KE, Chosidow O, et al. Guidelines for diagnosis, prevention and treatment of hand eczema. J Dtsch Dermatol Ges. 2015;13:e1–22.
- Bissonnette R, Diepgen TL, Elsner P, et al. Redefining treatment options in chronic hand eczema (CHE). J Eur Acad Dermatol Venereol. 2010;24:1–20.
- Ruzicka T, Lynde CW, Jemec GB, et al. Efficacy and safety of oral alitretinoin (9-cis retinoic acid) in patients with severe chronic hand eczema refractory to topical corticosteroids: results of a randomized, double-blind, placebo-controlled, multicentre trial. Br J Dermatol. 2008;158:808–17.
- Fowler JF, Graff O, Hamedani AG. A phase 3, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of alitretinoin (BAL4079) in the treatment of severe chronic hand eczema refractory to potent topical corticosteroid therapy. J Drugs Dermatol. 2014;13:1198–204.
- Dirschka T, Reich K, Bissonnette R, et al. An open-label study assessing the safety and efficacy of alitretinoin in patients with severe chronic hand eczema unresponsive to topical corticosteroids. Clin Exp Dermatol. 2011;36:149–54.
- Diepgen TL, Pfarr E, Zimmermann T. Efficacy and tolerability of alitretinoin for chronic hand eczema under daily practice conditions: results of the TOCCATA open study comprising 680 patients. Acta Derm Venereol. 2012;92:251–5.
- Yang Y, Brazier J, Longworth L. EQ-5D in skin conditions: an assessment of validity and responsiveness. Eur J Health Econ 2015;16:927–39.
- Konig HH, Bernert S, Angermeyer MC. Health status of the German population: results of a representative survey using the EuroQol questionnaire. Gesundheitswesen. 2005;67:173–82.
- Greiner WCC. Der EQ-5D der EuroQol-Gruppe. In: Schöffski OS, J. Matthias Graf, eds. Gesundheitsökonomische Evaluationen. Berlin, Heidelberg: Springer, 2007. p 403–14.
- Kalb RE, Blauvelt A, Sofen HL, et al. Effect of infliximab on health-related quality of life and disease activity by body region in patients with moderate-to-severe psoriasis and inadequate response to etanercept: results from the PSUNRISE trial. J Drugs Dermatol. 2013;12:874–80.
- Norlin JM, Steen Carlsson K, Persson U, et al. Switch to biological agent in psoriasis significantly improved clinical and patient-reported outcomes in real-world practice. Dermatology. 2012;225:326–32.
- Berger K, Ehlken B, Kugland B, et al. Cost-of-illness in patients with moderate and severe chronic psoriasis vulgaris in Germany. J Dtsch Dermatol Ges. 2005;3:511–18.
- Diepgen TL, Scheidt R, Weisshaar E, et al. Cost of illness from occupational hand eczema in Germany. Contact Dermatitis. 2013;69:99–106.
- Streiner D, Geddes J. Intention to treat analysis in clinical trials when there are missing data. Evid Based Ment Health. 2001;4:70–1.