Abstract
Background: Actinic keratoses (AKs) are a consequence of chronic exposure to ultraviolet radiation. Treatment of chronically photo-damaged skin and AKs is driven by risk of progression to squamous cell carcinoma, as well as for symptomatic relief. Conventional photodynamic therapy (c-PDT) is indicated when AKs are multiple or confluent and if patients respond poorly or are unable to tolerate other therapies. c-PDT is limited by the field size that can be treated in single sessions and can cause significant discomfort.
Objective: Recent studies investigated daylight illumination to activate protoporphyrin IX and daylight-PDT (d-PDT) is now licensed in the UK for face and scalp AKs. A group of experts met to discuss application of d-PDT with methyl aminolevulinate (MAL) and develop a UK consensus statement, specific to UK weather conditions.
Methods: The UK consensus recommendations were reached among eight experts, who reviewed recent studies on d-PDT, assessed UK meteorological data and discussed personal experiences of d-PDT for AKs.
Results: Recommendations from these discussions provide guidance on d-PDT use, specifically regarding patient selection, therapeutic indications, when to treat, skin preparation, MAL application and daylight exposure for patients with AKs.
Conclusions: This UK expert consensus provides practical guidance for UK application of d-PDT.
Introduction
Actinic keratoses (AKs) arising as a consequence of chronic exposure to ultraviolet (UV) radiation appear most frequently on photoexposed sites of the head, neck, forearms and legs (Citation1,Citation2). Patients may present for treatment because lesions are symptomatic or for cosmetic reasons. However, the potential of AKs to progress to invasive squamous cell carcinoma (SCC) is also usually a consideration. Within areas of chronically photodamaged skin, there are invariably visible AK lesions and subclinical areas of dysplasia, which are not seen by the naked eye (field change cancerisation [field AKs]). Management can therefore be divided into lesion-directed or field-directed therapy. Treatment of field AKs is essential to treat subclinical disease but requires larger areas and numbers of treatments (Citation3). A field change approach offers treatment of not only evident AKs but also subclinical disease (Citation4,Citation5).
The prevalence of AKs increases with age and cumulative sunlight exposure and is also higher in fair-skinned individuals and men (Citation1,Citation2,Citation6). Field AKs are markedly increased in patients who are immunosuppressed, with 40% of patients who have undergone organ transplantation developing AKs within five years of the procedure (Citation7) and a much greater risk (52- to 250-fold higher) of lesions progressing to invasive SCC in the immunocompromised population (Citation5,Citation8,Citation9). AKs are a marker of significant UV exposure and thus total skin examination is recommended and patients should be educated regarding sun avoidance and protection.
A range of treatments are available for AKs. These include topical field-directed treatments such as 5-fluorouracil, imiquimod, ingenol mebutate and diclofenac in sodium hyaluronate; destructive therapies, including chemical peels, cryosurgery, dermabrasion and laser; and photodynamic therapy (PDT) (Citation4). Topical treatments are widely used in primary care in the UK but have limitations, particularly with the need for the patient to comply with their treatment application. This can be of lengthy duration and cause significant inflammatory adverse effects, which can also impact on treatment compliance and completion (Citation4). If patients are not able to comply with topical therapies, or have failed other therapies, then PDT is an appropriate field-directed treatment option.
Conventional PDT (c-PDT)
c-PDT involves the photoactivation of protoporphyrin IX (PpIX); after pro-drug application and uptake, PpIX selectively accumulates in the dysplastic or malignant cells, evident by visualisation of the localised crimson red PpIX fluorescence. On illumination in the presence of oxygen, photoactivation and photobleaching of PpIX occurs, generating oxidative stress and reactive oxygen species (ROS). This in turn initiates inflammation and cytotoxicity, resulting in apoptosis and necrosis of abnormal cells (Citation10). c-PDT involves removal of any superficial crusting and scaling around the AKs and then applying the photosensitiser pro-drug (such as methyl aminolevulinate [MAL]). This treatment is applied not only to visible lesions but also to the surrounding field in order to include subclinical lesions. Pro-drug absorption and conversion into PpIX take place over a 3-h period with the cream under occlusion. Photoactivation of PpIX is then undertaken with narrowband red light (∼630 nm, light dose of 37.5 J/cm2), generally using a light emitting diode (LED; e.g. Aktilite CL128) (Citation11,Citation12). The only licensed pro-drugs in the UK for PDT for AK are MAL; Metvix (Galderma, Watford, UK) and 5-aminolaevulinic acid in nanocolloid emulsion ALA; Ameluz (Biofrontera, Leverkusen, Germany). PDT in the UK employs red light irradiation, generally using LEDs, such as Aktilite or RhodoLED, although many different types of red light source could be used. In the US, ALA is licensed as Levulan Kerastick for use with fluorescent blue light for PD for AK and Metvixia is licensed for PDT for AK using a red light illumination source.
PDT is a highly effective method of treating AKs, achieving up to 90% clearance following one or two treatments, and is also an important treatment option for superficial basal cell carcinoma (BCC) and Bowen’s disease when surgical excision is considered less appropriate (Citation10,Citation13). PDT is now included in several guidelines for the treatment of AKs from the National Institute for Health and Care Excellence (NICE), the Primary Care Dermatology Society and the British Association of Dermatologists (Citation4,Citation14,Citation15). High efficacy, good cosmetic effects and the fact that PDT is generally well tolerated result in high patient satisfaction for PDT (Citation3).
There are two main aspects of the treatment that affect patients’ amenability to the procedure: the duration of the treatment and the degree of discomfort during illumination. With c-PDT, pro-drug must be applied to the treatment area, occluded and incubated prior to irradiation. The area that can be treated in a single session is limited by the area of the static LED used and is therefore limited to a 10 × 20 cm field. However, it is possible to use multiple LEDs at the same time, although this is often not practical as most centres do not have multiple sources and this limits the number of patients that can be treated at any given time. As most patients with field AKs have large areas of multiple lesions, multiple visits to the hospital are required for c-PDT sessions, resulting in inconvenience for patients and a significant commitment for PDT clinics with regards to space and staff.
The discomfort of topical PDT has both neuropathic (Citation16) and inflammatory components and appears to be initiated during the generation of ROS. The discomfort is usually described as a burning, prickling, neuropathic-like pain. Pain is most severe in patients having large areas treated, during treatment of the face and scalp, and treatment for AK lesions and methods to reduce pain are of limited efficacy (Citation17–19).
However, there is emerging evidence that low irradiance PDT is associated with less discomfort and possibly increased efficiency of PDT effects (Citation20–22). One way to deliver very low irradiance PDT is by the use of daylight to activate PpIX. It has been demonstrated that for patients with AKs and in particular field AKs, PDT using daylight can have a similar efficacy to c-PDT, is almost pain free and produces excellent cosmetic results (Citation22–30). However, it is important to note that in these studies, the efficacy of d-PDT was only followed up over 3–6 months, although with high efficacy rates still apparent at six months (Citation31,Citation32) and this is also typical of the follow-up interval used in most studies of most treatments for AK.
Daylight PDT (d-PDT)
The wavelengths of light known to activate PpIX in the skin fall within the visible spectrum of daylight (Citation33). This use of an abundant natural resource provides greater flexibility as well as making it easier to treat larger areas of AKs, while being almost pain free. However, the lower irradiance of daylight means that a longer exposure time is required to achieve the same levels of PpIX activation (Citation33). Pre-illumination time, in contrast, is shorter (<30 min) because the absorption of pro-drug can continue during daylight exposure and PpIX is continuously being produced and activated. One explanation for the reduction in pain intensity during d-PDT is that there is no significant PpIX accumulation before illumination.
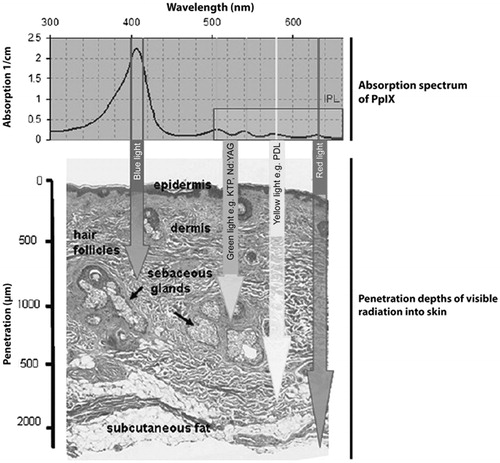
Thus, as can be seen from (34), there is maximal absorption of PpIX in the blue light wavelengths of the solar spectrum and most of the effects of d-PDT are therefore attributed to blue light PDT.
Figure 1. Wavelength-dependent penetration of blue, green, yellow and red light in PDT. Arrows indicate the approximate 50% optical penetration depth in human skin. Reproduced with permission (Citation34).
Clinical experience with d-PDT
Initial efficacy and safety studies of d-PDT with MAL were conducted in Copenhagen (Citation22–26). Subsequently, studies were conducted in central and southern Europe, Brazil and Australia (Citation27,Citation29–31). Patients in these studies mostly had mild-to-moderate AKs on the face and scalp and duration of exposure varied between studies (90–240 min). This varies from the now licensed exposure period of 2 h. Some patients remained in the hospital grounds during the exposure period while others were allowed to go home after pro-drug application. The two phase III studies performed in Australia and Europe both used a continuous 2 h of daylight exposure.
Despite the variation in exposure times, response rates have been shown to be similar in various countries. In Europe, the mean rate of clearance of AKs was 75–83% after one treatment for patients with Grade I/II AKs (Citation22–27,Citation29). In Australia and Brazil, the response was greater, at 89% and 88%, respectively, for patients with mild AKs (Citation30,Citation31). Pain scores were generally low with some studies demonstrating that d-PDT was almost pain free (Citation24,Citation27). Individual patients who reported higher discomfort levels had either increased the period between pro-drug application and sunlight exposure or had covered breaks during the exposure period, both of which result in increased time for intracellular concentrations of PpIX to increase (Citation27,Citation33).
A small study also compared the thickness of pro-drug application and PpIX fluorescence to determine the optimal thickness for d-PDT. No statistical difference was observed in PpIX fluorescence with 0.1–1 mm thickness of cream after 3 h incubation (Citation35).
Studies by Wiegell and colleagues have concluded that measurement of absolute light doses are not required in d-PDT (Citation33); however, the procedure does require daylight to reach a critical threshold, above which efficacy appears to be independent of light irradiance. This threshold was estimated to be 8 J/cm2 and the conservative threshold for treatment has been applied at 130 W/m2 (24). The temperature must also be warm enough (judged as >10 °C) for the patient to comfortably sit outside for two hours. The requirement for the patient to be outside also rules out treatment on rainy days unless suitable indoor space is available with diffuse natural daylight (e.g. a conservatory) and the day is not too overcast. Indirect sunlight is sufficient for successful d-PDT, but if the skies are heavily overcast, with no apparent daylight then we would not advise d-PDT. In practice, it would only be these extreme conditions that would be limiting and most of the time it is entirely feasible to undertake effective d-PDT. For these reasons, while d-PDT can be conducted year-round in southern Europe, in northern Europe, it must be restricted to Spring, Summer and early Autumn (Citation36).
The European consensus set out guidelines on the use of d-PDT, providing a useful overview. However, in order that this protocol is appropriate for use in the varying climatic conditions throughout Europe, more specific guidelines are required. From a UK perspective, seasonal variation in daylight hours and adverse weather conditions may limit the usage of d-PDT seasonally and at certain times of the day. The development of a UK-specific consensus statement is therefore crucial in maximising the efficacy of this treatment.
d-PDT in the UK
Recently, a group of experts met at the invitation of Galderma to discuss the consensus protocol in the light of their own experience with d-PDT in the UK, and to make recommendations for UK patients and healthcare professionals. The objectives of the meeting were to investigate which types of patients may be most suitable for d-PDT, to establish clarity on the treatment protocol during and after treatment, to consider the information that may be required for patients and to investigate the extent to which patients could potentially independently complete their treatment/daylight exposure.
Daylight in the UK
Information on UK weather conditions (1986–2005) provided by METEOTEST (Bern, Switzerland) using the meteonorm software (www.meteonorm.com) was assessed by the group of experts to determine when d-PDT can be undertaken (see and ; personal communication).
Figure 2. Meteorological data for the UK, showing the daily average light irradiances throughout the year between 09:00 and 18:00 (Panel a) and the days with >1.0 mm precipitation (Panel b; personal communication). The threshold above which efficacy appears to be independent of light irradiance (130 W/m2) (Citation24) is indicated by the dashed line.
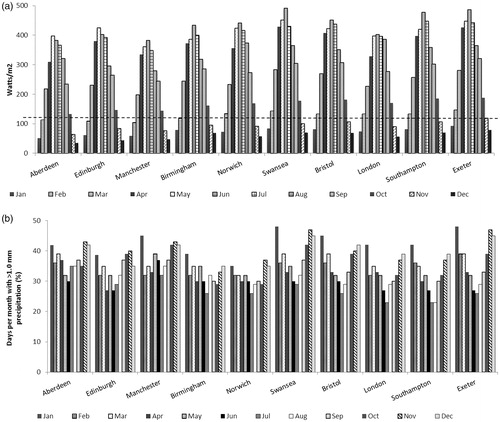
Table 1. Information on average UK weather conditions 1986–2005 (personal communication).
In the UK, patterns of daylight and rainfall mean that in areas south of Nottingham, d-PDT is restricted to between March and October, and in areas north of Nottingham it is restricted to between March/April and September (when temperatures are >10 °C; personal communication). Overall it was agreed that if d-PDT in the UK takes place within these seasonal boundaries it can be assumed that there is sufficient light between 9:00 and 18:00, and there is no need to take light measurements. It has recently been demonstrated that artificial light sources may also be used, such as halogen and LED lamps in an indoor “daylight room”. It was also shown that greenhouses are beneficial for d-PDT in weather conditions which might be uncomfortable, such as wind and rain (Citation37).
Selection of patients for d-PDT
Accurate diagnosis of AK is a key factor when treating the condition. As set out in the European consensus statement, d-PDT is most effective for Grades I and II AKs (Citation36), the types of lesions most commonly treated by the UK experts, in addition to areas of field AKs or those with risk factors for developing SCC.
The location of the AKs will also impact the decision to use d-PDT over c-PDT although in practice, d-PDT is likely to be interspersed with c-PDT. d-PDT is particularly suited for treating large field areas and areas that can be readily exposed to illumination, such as the face and scalp. It is particularly suited to patients with large areas of AKs due to the fact that it can be used to treat multiple lesions in a single treatment session. Whilst d-PDT can be used at other sites, which can be exposed to daylight, as the licensed regime is restricted to use on face and scalp, we have limited our discussions to these within license indications. It was recommended to continue to use c-PDT for focussed hyperkeratotic lesions, BCCs, areas affected by Bowen’s disease or sensitive areas (e.g. around the eyes).
Patient preference will also be an important consideration in the choice of therapy. Patients who have previously experienced discomfort, side effects or treatment failure with c-PDT or other topical treatment modalities, want a quicker one-off appointment and those who are prepared to be outdoors for 2 h may prefer d-PDT and be willing to comply with the protocol, even if inconvenienced by rescheduling due to rain or the need for a second treatment if there has been an incomplete response. If rain interrupts the procedure within 30 min, the MAL should be removed and the procedure rescheduled () however, if they have received >30 min exposure, an assessment can be made based on the exposure time.
Table 2. d-PDT Protocol considerations.
Within the public sector in the UK, the high number of patients presenting with AKs who might benefit from treatment results in consideration of all contributory costs by the National Health Service (NHS). Under current NHS arrangements, GPs are most likely to refer patients with large areas of sun-damaged skin to a consultant for a definitive diagnosis and to formulate a management plan. In the future, however, intermediate care with a general practitioner with specialised interest (GPSI) in dermatology could be a suitable setting for d-PDT, with emphasis placed on establishing an accurate diagnosis in the first instance.
d-PDT treatment protocol
Recently, Morton and colleagues published a European consensus on the treatment of AKs, which details the d-PDT treatment protocol based on the results of recent clinical studies (Citation36). summarises the protocol and how it varies from the protocol for c-PDT.
Figure 3. European consensus protocol for daylight photodynamic therapy (d-PDT) compared with protocol for conventional PDT (c-PDT). Adapted from (Citation11,Citation36).
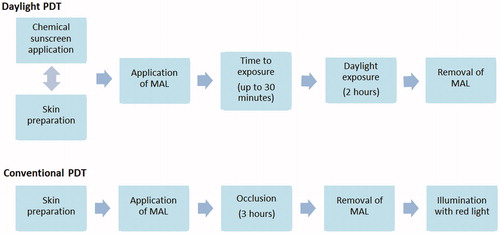
Morton and colleagues not only considered the order and duration of the different steps in the procedure, but provided detailed information on each step of the protocol (Citation36). As with the UK experts, they stress the simplicity and flexibility of d-PDT, and it was agreed that it is likely to be robust enough to accommodate slight variations in the protocol (e.g. whether sunscreen is applied before or after skin preparation). The UK licence recommends the application of sunscreen beforehand.
In hospitals in the UK, lesion diagnosis is undertaken by the doctor, but trained nurses or technicians will be primarily responsible for the procedure and will probably prefer more specific guidance on the items summarised in .
Patient considerations, information and understanding
It is essential that patients understand the reasons for their treatment, how the treatment works and the procedure itself. Information sheets should include pictorial diagrams of the area(s) to be treated and the schedule and duration of each step in the procedure. summarises the key information and protocol considerations that patients must understand prior to treatment.
Table 3. Patient understanding for treatment with d-PDT.
Reception staff at the hospital should also have this information. As they will be involved in booking appointments, it is important for them to understand the weather implications and possible need to reschedule. This means that patients will need a human interface for making appointments – secondary care booking systems are not appropriate for this approach.
Setting for d-PDT
Secondary care
In the UK, the consensus group agreed that d-PDT will initially predominantly take place in hospital clinics where c-PDT is currently performed, as initial accurate diagnosis is critical. The patient could either remain at the hospital for the complete procedure, or undergo sunscreen application, lesion preparation and pro-drug application at the hospital and then return home (or go to an appropriate open space nearby) for daylight exposure and treatment completion. Patients who do not remain at the hospital should receive adequate information on the protocol before they leave and also be given a contact number in case they have questions.
If the patient is unable to return home or go to a suitable outdoor space nearby for daylight exposure within 30–45 min of pro-drug application, they will have to receive the full treatment at the hospital. An appropriate risk-assessed designated space (either outdoors or indoors) would be required and a certain level of supervision would be required during treatment. This makes the setting in clinics ideal, where several patients could be treated at the same time to allow for group supervision.
Patients who live in rural settings and have to attend city hospitals for treatment may not have the option to return home to complete their treatment and may be unwilling to sit in a public place for daylight exposure. Two potential scenarios can be envisaged for these (and other) patients in the future: treatment in primary/intermediate care or home-based care.
Primary/intermediate care
The faculty concluded that in the future, patients may have access to a local GPSI in dermatology that could supervise treatment and in some cases diagnose and initiate treatment. It was agreed that it would initially be more likely that the consultant would refer the patient to the GPSI for treatment following diagnosis. For this to become a reality, it would be necessary to establish that there was interest within primary/intermediate care, as currently it is not seen as a key health issue in this setting. Other factors that would need to be addressed are the need for appropriate training, the cost of MAL compared with other potential treatment options, and the need for endorsement from secondary care. One possible solution could be for initial treatment to be administered in hospital and any subsequent treatment to be given in intermediate care, although a shared-care protocol would be required.
Summary and conclusions
Overall, it was agreed that d-PDT offers an effective therapeutic option for suitable patients with field areas of AK on the face and scalp compared with current therapies for AKs, particularly over large areas. d-PDT offers a relatively straightforward, flexible and almost pain free treatment option, which harnesses an abundant natural resource. Indeed, compared with the use of topical treatments or c-PDT for field AKs, this technique could allow a much larger area to be treated in a single session without significant side effects.
The European consensus provided an important baseline from which to develop guidelines for the use of d-PDT in the UK. However, due to the reliance of the treatment on weather conditions, localised guidelines are required in order that they are relevant to the geographical location, taking into account seasonal variation.
This PDT treatment approach also facilitates streamlining and efficacy of PDT clinic services. The lack of specialist equipment required, the option of doing other outdoor activities during illumination and the potential for local or home-based treatment in the future makes d-PDT an exciting new treatment option. The recommendations made by the UK experts are summarised in .
Table 4. Summary of recommendations.
Acknowledgements
The authors thank all of the participants in the meeting and Galderma UK. The authors would like to thank MedSense Ltd., High Wycombe, UK for providing editorial assistance during the preparation of this manuscript, which was funded by Galderma UK.
Disclosure statement
Dr Ibbotson has received honoraria and travel expenses from Galderma, Ambicare and Spirit Healthcare. The Primary Care Dermatology Society receives sponsorship for educational activities from Galderma and almost all pharmaceutical companies involved in dermatology. Dr Kownacki has also received support from Galderma for attendance at the EADV in March 2015. Dr Stones, Dr Bowling, Dr Sivaramakrishnan and Dr Valentine have received honorarium and travel expenses from Galderma UK. Dr Campbell has received honorarium, travel expenses and speaker fees from Galderma UK. Dr Morton has received speaker honoraria from Galderma, Biofrontera, Leo Pharma and Spirit Healthcare, and reimbursed for advisory board attendance from Almirall, Biofrontera, Galderma and Leo Pharma.
Funding
This paper originates from a report of an advisory board on “UK Metvix daylight activated photodynamic therapy” held in Birmingham, UK, 12 November 2014. This meeting was organised and funded by Galderma UK, who selected the participants.
References
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42:4–7.
- Franceschi S, Levi F, Randimbison L, La Vecchia C. Site distribution of different types of skin cancer: new aetiological clues. Int J Cancer. 1996;67:24–8.
- Dodds A, Chia A, Shumack S. Actinic keratosis: rationale and management. Dermatol Ther (Heidelb). 2014;4:11–31.
- de Berker D, McGregor JM, Hughes BR. British Association of Dermatologists Therapy Guidelines and Audit Subcommittee. Guidelines for the management of actinic keratoses. Br J Dermatol. 2007;156:222–30.
- Ulrich C, Arnold R, Frei U, et al. Skin changes following organ transplantation: an interdisciplinary challenge. Dtsch Arztebl Int. 2014;111:188–94.
- Flohil SC, van der Leest RJ, Dowlatshahi EA, et al. Prevalence of actinic keratosis and its risk factors in the general population: the Rotterdam Study. J Invest Dermatol. 2013;133:1971–8.
- Stockfleth E, Ulrich C, Meyer T, Christophers E. Epithelial malignancies in organ transplant patients: clinical presentation and new methods of treatment. In Dummer PDR, Nestle PDFO, Burg PDG, editors. Cancers of the Skin [Internet]. Berlin Heidelberg: Springer, 2002 [cited 2015 Feb 10]. p 251–8. Available from http://link.springer.com/chapter/10.1007/978-3-642-59410-6_30.
- Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–91.
- Wlodek C, Ali FR, Lear JT. Use of photodynamic therapy for treatment of actinic keratoses in organ transplant recipients. BioMed Res Int [Internet]. 2013;2013. doi:10.1155/2013/349526.
- Morton CA, McKenna KE, Rhodes LE. British Association of Dermatologists Therapy Guidelines and Audit Subcommittee and the British Photodermatology Group. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245–66.
- Metvix SPC [Internet] [cited 2015 May 2]. Available from http://www.medicines.org.uk/emc/medicine/11913.
- Ameluz SPC [Internet] [cited 2015 May 2]. Available from http://www.medicines.org.uk/emc/medicine/27817.
- Lehmann P. Methyl aminolaevulinate-photodynamic therapy: a review of clinical trials in the treatment of actinic keratoses and nonmelanoma skin cancer. Br J Dermatol. 2007;156:793–801.
- NICE. Photodynamic Therapy for Non-Melanoma Skin Tumours (including Premalignant and Primary Non-Metastatic Skin Lesions) – Guidance And Guidelines, 2006. Available from http://www.nice.org.uk/guidance/ipg155. Accessed April 8, 2015.
- Primary Care Dermatology Society. Actinic Keratosis (Solar Keratosis), 2014. Available from http://www.pcds.org.uk/clinical-guidance/actinic-keratosis-syn.-solar-keratosis. Accessed April 8, 2015.
- Rud E, Gederaas O, Høgset A, Berg K. 5-aminolevulinic acid, but not 5-aminolevulinic acid esters, is transported into adenocarcinoma cells by system BETA transporters. Photochem Photobiol. 2000;71:640–7.
- Wiegell SR, Haedersdal M, Wulf HC. Cold water and pauses in illumination reduces pain during photodynamic therapy: a randomized clinical study. Acta Derm Venereol. 2009;89:145–9.
- Halldin CB, Gonzalez H, Wennberg AM, Lepp M. Patients' experiences of pain and pain relief during photodynamic therapy on actinic keratoses: an interview study. Acta Derm Venereol. 2013;93:433–7.
- Arits AH, van de Weert MM, Nelemans PJ, Kelleners-Smeets NW. Pain during topical photodynamic therapy: uncomfortable and unpredictable. J Eur Acad Dermatol Venereol. 2010;24:1452–7.
- Cottrell WJ, Paquette AD, Keymel KR, et al. Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas. Clin Cancer Res. 2008;14:4475–83.
- Attili SK, Lesar A, McNeill A, et al. An open pilot study of ambulatory photodynamic therapy using a wearable low-irradiance organic light-emitting diode light source in the treatment of nonmelanoma skin cancer. Br J Dermatol. 2009;161:170–3.
- Wiegell SR, Haedersdal M, Philipsen PA, et al. Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study. Br J Dermatol. 2008;158:740–6.
- Wiegell SR, Haedersdal M, Eriksen P, Wulf HC. Photodynamic therapy of actinic keratoses with 8% and 16% methyl aminolaevulinate and home-based daylight exposure: a double-blinded randomized clinical trial. Br J Dermatol. 2009;160:1308–14.
- Wiegell SR, Fabricius S, Stender IM, et al. A randomised, multicentre study of directed daylight exposure times of 1½ vs. 2½ h in daylight-mediated photodynamic therapy with methyl aminolaevulinate in patients with multiple thin actinic keratoses of the face and scalp. Br J Dermatol. 2011;164:1083–90.
- Wiegell SR, Fabricius S, Gniadecka M, et al. Daylight-mediated photodynamic therapy of moderate to thick actinic keratoses of the face and scalp: a randomised multicentre study. Br J Dermatol. 2012;166:1327–32.
- Wiegell SR, Fabricius S, Heydenreich J, et al. Weather conditions and daylight-mediated photodynamic therapy: protoporphyrin IX-weighted daylight doses measured in six geographical locations. Br J Dermatol. 2013;168:186–91.
- Braathen LR. Daylight photodynamic therapy in private practice in Switzerland: gain without pain. Acta Derm Venereol. 2012;92:652–3.
- Pérez-Pérez L, García-Gavín J, Gilaberte Y. Daylight-mediated photodynamic therapy in Spain: advantages and disadvantages. Actas Dermosifiliogr. 2014;105:663–74.
- Fai D, Romano I, Fai C, et al. Daylight photodynamic therapy with methyl aminolaevulinate in patients with actinic keratoses: a preliminary experience in southern Italy. G Ital Dermatol Venereol. 2016;151:154–9.
- Grinblat BM, Festa Neto C, Sanches JA, Jr, et al. Daylight photodynamic therapy for actinic keratoses in São Paulo, Brazil. Photodermatol Photoimmunol Photomed. 2015;31:54–6.
- Rubel DM, Spelman L, Murrell DF, et al. Daylight photodynamic therapy with methyl aminolevulinate cream as a convenient, similarly effective, nearly painless alternative to conventional photodynamic therapy in actinic keratosis treatment: a randomised controlled trial. Br J Dermatol. 2014;171:1164–71.
- Lacour J, Ulrich C, Gilaberte Y, et al. Daylight photodynamic therapy with methyl aminolevulinate cream is effective and nearly painless in treating actinic keratoses: a randomised, investigator-blinded, controlled, phase III study throughout Europe. J Eur Acad Dermatol Venereol. 2015;29:2342–8.
- Wiegell SR, Wulf HC, Szeimies RM, et al. Daylight photodynamic therapy for actinic keratosis: an international consensus: International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:673–9.
- Sakamoto FH, Torezan L, Anderson RR. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practise: Part II. Understanding parameters for acne treatment with photodynamic therapy. J Am Acad Dermatol. 2010;63:195–211.
- Wulf HC. Optimising the amount of Metvix in daylight PDT. Euro-PDT 13th Annual Congress; Madrid, Spain; May 31st–June 1st, 2013.
- Morton CA, Wulf HC, Szeimies RM, et al. Practical approach to the use of daylight photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: a European consensus. J Eur Acad Dermatol Venereol. 2015;29:1718–23.
- Lerche C, Heerfordt I, Heydenreich J, Wulf H. Alternatives to outdoor daylight illumination for photodynamic therapy-use of greenhouses and artificial light sources. Int J Mol Sci. 2016;17:309.
