Abstract
Purpose: Apremilast, an oral phosphodiesterase 4 inhibitor, was effective in clinical trials in patients with moderate plaque psoriasis (affected body surface area [BSA] 5% to 10%). However, findings from real-world clinical practice are limited.
Materials and methods: An online survey and chart review was conducted among US dermatologists during October 2015 to identify clinical characteristics and 6-month treatment outcomes among patients with moderate psoriasis treated with apremilast.
Results: A total of 83 dermatologists provided patient chart information at the initial and 6-month follow-up time points for 70 patients with moderate plaque psoriasis initially receiving apremilast, of whom 65 were receiving it as their primary therapy (mean age: 47.3 years; 45% were men). Among apremilast-treated patients, 91% (64 of 70) remained on apremilast and 54% were rated as having improved to mild psoriasis at follow-up; mean BSA decreased from 9.9% at initial chart review to 4.9%. There were 8 of 66 (12%) patients who experienced ≥1 side effect, including diarrhea (7.6%), nausea (4.5%), headache (1.5%), and abdominal pain (4.5%). Most dermatologists (68%) stated that apremilast exceeded or met their expectations.
Conclusions: Most patients with moderate psoriasis receiving apremilast had improved at 6-month follow-up. Safety and tolerability were consistent with the safety profile of apremilast.
Introduction
Current treatment options for mild to moderate and moderate to severe psoriasis include topical formulations, oral small-molecule formulations, and systemic or biologic agents. Among these is apremilast, the first oral nonbiologic medication approved in the United States in the past 20 years for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy (Citation1,Citation2). Apremilast, an oral phosphodiesterase 4 inhibitor, was effective and safe in the phase III Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) randomized clinical trial program (ESTEEM 1 and ESTEEM 2), which enrolled patients who were candidates for phototherapy or systemic therapy with moderate to severe psoriasis, defined as Psoriasis Area and Severity Index (PASI) score ≥12, affected body surface area (BSA) ≥ 10%, and static Physician Global Assessment (sPGA) score ≥3 (Citation3,Citation4). Despite the availability of apremilast and other systemic therapies, many patients with moderate disease [defined by the American Academy of Dermatology, AAD, as disease involving ≥5% but <10% of BSA (Citation5)] may be undertreated with topical therapy or no treatment (Citation6–8).
The recent trial of apremilast, Evaluating Apremilast in a Phase IV Trial of Efficacy and Safety in Patients With Moderate Plaque Psoriasis (UNVEIL), was the first study of the efficacy and safety of a systemic treatment specifically in patients with moderate plaque psoriasis, defined as BSA 5% to 10% and sPGA score of 3, who were naive to systemic or biologic therapy (Citation9). Patients with moderate plaque psoriasis had significantly greater improvements on indicators of efficacy and quality of life at week 16 compared with placebo (Citation9) that were sustained for up to 52 weeks with apremilast treatment (Citation10).
In the ESTEEM and UNVEIL trials, apremilast was generally well tolerated, with a favorable risk-benefit profile (Citation3,Citation4,Citation9). Apremilast does not require routine laboratory monitoring (Citation1), potentially making it attractive to healthcare providers as a treatment option for patients with moderate psoriasis who have concerns about the safety and monitoring burden associated with conventional systemic therapies and biologics. The objectives of the present study were to describe real-world clinical characteristics and 6-month treatment outcomes among patients with moderate plaque psoriasis treated with apremilast. The current analysis describes the chart findings of dermatologists identified in the previously published dermatologic survey (Citation11); data are from the subset of patients treated with apremilast who had ≥1 follow-up visit during the 6-month follow-up period.
Materials and methods
Dermatologist survey
The survey was previously described in detail (Citation11). Briefly, the survey was conducted among US dermatologists treating patients with psoriasis during October 2015. These dermatologists were randomly selected from a database that used partner panels. Eligible survey participants were US dermatologists who had been in practice for 2 to 30 years and had treated ≥20 adult patients with plaque psoriasis per month, ≥1% of whom must have been considered to have moderate psoriasis. To participate, dermatologists had to spend ≥40% of their practice time in medical dermatology or ≥70% in medical and surgical dermatology. Survey participants also had to spend >75% of their practice time in direct patient care. Potentially eligible dermatologists were invited to complete an online survey that assessed their approach to identifying and managing patients with moderate plaque psoriasis.
Findings from the online survey were previously reported (Citation11). The current analysis describes the chart findings from the subset of patients treated with apremilast who had ≥1 follow-up visit during the 6-month follow-up period.
Prospective 6-Month patient chart review
Surveyed dermatologists were also asked to provide data from the charts of their four most recently seen (i.e., in the last month) patients with a diagnosis of moderate plaque psoriasis, including ≥1 patient treated with apremilast; 6 months later they were invited to provide follow-up information. Information gathered included patient demographic characteristics, clinical disease characteristics, treatment patterns (including treatment switches and discontinuations), treatment effectiveness, safety, and tolerability. Surveyed dermatologists were also asked to choose their top three reasons for treatment selection for the patients included in the chart review. Answer options included favorable safety profile, overall efficacy, mechanism of action, low risk of infections, patient has contraindications to other treatments, cost/insurance, comfort/familiarity with the therapy, patient’s goal of therapy (their desire/need for complete clearance), route of administration, as an add-on to primary treatment, rapid speed of onset, comprehensive clinical trial data, low risk of malignancy, routine laboratory monitoring not required, indicated for psoriasis and psoriatic arthritis, for an “as needed” treatment, formulary coverage, and other.
To be considered for the chart review, patients were required to meet the following inclusion criteria: adult patient (≥18 years of age) diagnosed with plaque psoriasis and currently living, moderate disease severity (as determined by the surveyed, treating dermatologist), seen by the dermatologist within the month before the dermatologist took the survey, were using prescription topical or systemic medication for the treatment of moderate plaque psoriasis at the time of the survey, and were seen at least once during the 6-month follow-up period.
Results
Respondent dermatologists
Among the 173 dermatologists who were contacted, 23 did not fit the inclusion criteria for participation. The remaining 150 dermatologists responded to the survey (Citation11); 83 responded at both time points and provided 6-month follow-up information for patients with moderate psoriasis who were receiving treatment with apremilast at the time of the initial survey. The dermatologists who participated in the chart review (n = 83) had an average of 14 years in practice. Most (87%) currently worked in a single-physician or multiphysician dermatology specialty practice, 10% worked in a multispecialty office group, and the remainder worked in a hospital-based practice. Dermatologists saw an average of 66 patients with psoriasis per month; 42% of the patients with psoriasis seen each month were considered to have moderate plaque psoriasis.
Patient disposition, demographics, and clinical features
At the time of the initial survey, participating dermatologists identified 600 charts for patients with moderate plaque psoriasis, of which 270 had ≥1 visit within the 6-month follow-up period. Among these, 70 patients who had ≥1 follow-up visit (mean visits: 2) within the 6-month follow-up period were initially receiving apremilast; 91% (64 of 70) of these patients remained on apremilast at the end of the follow-up period (). Most patients treated with apremilast (79%) received apremilast 30 mg twice daily. The top three reasons that surveyed dermatologists chose for selecting apremilast were favorable safety profile (66%), route of administration (49%), and routine laboratory monitoring not required (28%). Apremilast discontinuation occurred in six patients (some patients discontinued for more than one reason); reasons for discontinuation included loss of efficacy (n = 2), poor tolerability (n = 3), and noncompliance (n = 2). Sixty-five patients were receiving apremilast as their primary therapy. There were also three patients who had been treated with other therapies and were switched to apremilast during the follow-up period ().
Figure 1. 6-Month disposition of apremilast-treated patients. *Includes one patient switched from biologic plus topical steroid and two patients who added apremilast to topical therapy.
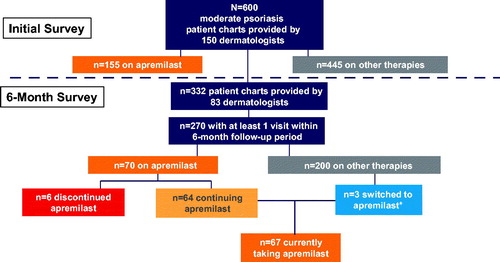
Patients receiving apremilast as their primary therapy were mostly white (92%). Mean age was 47.3 years, 45% were men, and 54% had ≥1 comorbid condition, most commonly a cardiometabolic condition (40%); the most common cardiometabolic condition was hypertension (23%) (). Patients given apremilast had been treated by the responding dermatologists for a mean of 24.8 months, with office visits occurring at a mean of every 2.8 months. The characteristics of the apremilast-treated patients were similar to those of the overall group of patients with moderate psoriasis, except that patients prescribed apremilast had a shorter time since diagnosis (4.5 years) and a lower proportion had comorbid obesity (9%), compared with the overall moderate psoriasis group (time since diagnosis, 5.8 years; obesity, 17%) ().
Table 1. Demographic and clinical characteristics among patients with moderate plaque psoriasis receiving apremilast as primary treatment.
Clinical effectiveness of apremilast treatment for moderate plaque psoriasis
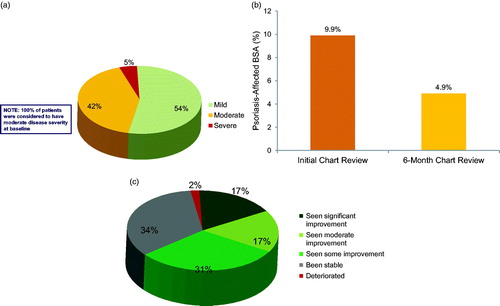
Of patients receiving apremilast as their primary therapy, 54% were rated as having improved from moderate to mild psoriasis at the end of the 6-month chart review period (); mean affected BSA decreased from 9.9% at initial chart review to 4.9% at the 6-month chart review (). Likewise, at the 6-month chart review, 65% of patients receiving apremilast as their primary therapy were rated by dermatologists as improved, and 17% were rated as having improved significantly (). When asked about their clinical experience with apremilast, 68% of dermatologists stated that apremilast exceeded or met their expectations.
Figure 2. (a) Disease severity at 6 months, among patients with moderate psoriasis treated with apremilast. Data reflect dermatologist (n = 150) response to the question: What would you consider to be this patient’s current disease severity level? Percentages may not add to 100 because of rounding. (b) BSA at initial chart review and at 6 months in patients treated with apremilast. (c) Dermatologist assessment of condition change at 6 months in patients treated with apremilast. Data reflect dermatologist (n = 150) response to the question: How has this patient’s condition changed over the past 6 months? Percentages may not add to 100 because of rounding.
Safety and tolerability profile of apremilast
Dermatologists provided tolerability data for 66 patients who were continuing to receive apremilast at the time of 6-month follow-up. Among these, eight (12%) reported side effects during treatment; these included diarrhea (7.6%, n = 5), nausea (4.5%, n = 3), abdominal pain (4.5%, n = 3), and headache (1.5%, n = 1) (). All side effects were considered mild, except one case of diarrhea that was considered moderate; two patients took loperamide to manage diarrhea. On average, side effects resolved within 2 weeks, with the exception of abdominal pain, which persisted for an average of 7 weeks. One patient temporarily discontinued apremilast because of diarrhea.
Table 2. Side effects and their management with apremilast as primary therapy in 66 patients.
Discussion
A variety of treatment options are available for patients with psoriasis who fall within the range of moderate disease severity (i.e., affected BSA 5% to 10%), including apremilast (Citation12). In the current 6-month chart review among patients with moderate plaque psoriasis receiving apremilast as their primary treatment, the large majority (91%) maintained apremilast treatment for 6 months. Mean psoriasis-affected BSA was 9.9% at initial chart review among patients treated with apremilast, which is within the range of the AAD definition of moderate psoriasis (Citation5). In apremilast-treated patients, mean psoriasis-affected BSA decreased by approximately 50% at the 6-month chart review so that most patients were considered to have only mild disease severity at follow-up. Dermatologists had a positive clinical experience with apremilast, with most reporting that apremilast met or exceeded their expectations. Safety findings were consistent with the safety profile of apremilast (Citation3,Citation4). Nausea and diarrhea resolved on average within 2 weeks.
In the present chart review, patients with moderate plaque psoriasis who were treated with apremilast were typical of the broader psoriasis patient population (Citation13) and generally similar to patients in the overall moderate psoriasis patient sample in the current study. Both groups of patients treated with apremilast or not treated with apremilast comprised older, white adults; the majority had ≥1 cardiometabolic comorbidity. Apremilast patients differed from the overall sample in that they had a shorter time since diagnosis and fewer were considered to have comorbid obesity, suggesting that the dermatologists prescribed this therapy earlier in the course of disease than other available treatments.
The findings of benefit in the moderate psoriasis population are consistent with those of the recent UNVEIL trial of apremilast in patients with moderate plaque psoriasis, in which apremilast was effective versus placebo at 16 weeks (Citation9), which was sustained for up to 52 weeks during open-label treatment (Citation10). Analyses using data from real-world clinical settings are important because results from clinical trials may not adequately reflect treatment outcomes in clinical practice. Apremilast was effective and safe in patients with moderate plaque psoriasis in a real-world dermatology setting.
Acknowledgements
The authors received editorial support in the preparation of this report from Amy Shaberman, PhD, of Peloton Advantage, LLC, Parsippany, NJ, USA. The authors, however, directed and are fully responsible for all content and editorial decisions for this manuscript.
Disclosure statement
MLFK: AbbVie, Allergan, Amgen, Celgene Corporation, Eli Lilly, Galderma, Novartis, and Sun Pharma—advisory board member, consultant, and/or speaker. AbbVie, Amgen, Bayer, Biogen, Crown Labs, Eli Lilly, Johnson and Johnson, LEO Pharma, and Medimetriks—investigator. EL: Celgene Corporation—employment. JS: AbbVie, Allergan, Amgen, Celgene Corporation, Eli Lilly, Genentech, Genzum, Janssen, Kadmon, Merz, Pfizer, and Regeneron—advisory board member, investigator, and/or speaker.
Additional information
Funding
References
- Otezla [package insert]. Summit (NJ): Celgene Corporation; June 2017.
- Oral Otezla® (apremilast) approved by the European Commission for the treatment of both patients with psoriasis and psoriatic arthritis [press release]. Celgene Corporation; 2015. Accessed March 14, 2017. Available from: http://files.shareholder.com/downloads/AMDA-262QUJ/3977267429x0x803746/56511ED3-7864-49A1-8BD3-C5C5B9116DE5/CELG_News_2015_1_16_General_Releases.pdf
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]). J Am Acad Dermatol. 2015;73:37–49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387–1399.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65:137–174.
- Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149:1180–1185.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871–881.
- Armstrong AW, Koning JW, Rowse S, et al. Under-treatment of patients with moderate to severe psoriasis in the United States: analysis of medication usage with health plan data. Dermatol Ther (Heidelb). 2017;7:97–109.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801–808.
- Stein Gold L, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis: 52-week results of UNVEIL. J Drugs Dermatol. 2018;17:221–228.
- Knuckles MLF, Levi E, Soung J. Defining and treating moderate plaque psoriasis: a dermatologist survey. J Dermatolog Treat. 2018;29:1–6.
- Richards HL, Fortune DG, Griffiths CE. Adherence to treatment in patients with psoriasis. J Eur Acad Dermatol Venereol. 2006;20:370–379.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003–2006 and 2009–2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37–45.
