Abstract
Background: Pruritus is a prevalent and bothersome symptom of scalp psoriasis. Validated scales assessing scalp itch are needed to evaluate treatment efficacy.
Objective: To evaluate comprehensibility and reproducibility of the Scalp Itch Numeric Rating Scale (NRS), a novel scale being used in a phase 3 study of apremilast.
Methods: The Scalp Itch NRS, Modified Whole Body Itch NRS, Global Assessment of Psoriasis Severity-Scalp (GAPS-S), and Global Impression of Change-Scalp Itch (GIC-SI) were assessed among patients with moderate to severe scalp psoriasis. Convergent validity and test–retest reliability between two visits (7 ± 3 days apart) were assessed using intra-class and Spearman’s correlations.
Results: Patients found the Scalp Itch NRS easy to use and understand. Convergent validity (Modified Whole Body Itch NRS Visit 1: rs = 0.71, Visit 2: rs = 0.92, p< .0001; GAPS-S Visit 1: rs = 0.62, Visit 2: rs = 0.63, p< .0001), and consistency with changes (Modified Whole Body Itch NRS: rs = 0.69, p< .0001; GAPS-S: rs = 0.42, p = .0029) were demonstrated. The Scalp Itch NRS showed strong test–retest reliability (intra-class correlation coefficient = 0.87; rs = 0.89). Change scores on the Scalp Itch NRS were consistent with change scores on the GIC-SI.
Conclusions: The Scalp Itch NRS is a valid and reproducible measure of scalp itch in patients with moderate to severe scalp psoriasis.
ClinicalTrials.gov: NCT03123471.
Introduction
Psoriasis is a chronic, systemic inflammatory disease with a prevalence ranging from 1% to 4% of the population worldwide (Citation1–3). Plaque psoriasis, the most common form of the disease, is characterized by red, scaly, and itchy patches of inflamed skin that arise from overproliferation of keratinocytes related to abnormal cytokine production (Citation4–7). Pruritus, which affects more than 80% of patients with moderate to severe chronic plaque psoriasis, is a bothersome symptom that substantially impairs health-related quality of life (HRQL) and functioning (Citation7–12). Many patients with psoriasis report that they are most bothered by symptoms such as pruritus in highly visible and difficult-to-treat areas such as the scalp (Citation7,Citation9,Citation13,Citation14).
Valid and reliable measures of scalp psoriasis are critical to assessing the efficacy of treatments in clinical trials and monitoring disease outcomes in clinical practice (Citation15), but there are currently no validated scales designed to assess the severity of scalp itch due to scalp psoriasis. The Scalp Itch Numeric Rating Scale (NRS), a single-item measure to evaluate the severity of scalp itch related to psoriasis, is being used in a clinical trial evaluating the safety and efficacy of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe scalp psoriasis (ClinicalTrials.gov: NCT03123471). Apremilast demonstrated efficacy and safety in the treatment of moderate to severe plaque psoriasis in the ESTEEM phase 3 and LIBERATE phase 3b trials and improved scalp psoriasis in the subset of patients with baseline scalp involvement (Citation16–18).
The aim of the current study was to examine the content validity, comprehensibility, and ease of use of the Scalp Itch NRS and other patient-reported outcome measures of pruritus being administered in an ongoing phase 3 study of apremilast in patients with moderate to severe scalp psoriasis, including the Modified Whole Body Itch NRS (based on a similar measure, the Worst Itch NRS [WI-NRS]), the Global Assessment of Psoriasis Severity-Scalp (GAPS-S), and the Global Impression of Change-Scalp Itch (GIC-SI). Additionally, this study was designed to assess the reproducibility of the Scalp Itch NRS.
Methods
Patients
Eligible patients were adults aged ≥18 years with moderate to severe scalp psoriasis, defined as Scalp Physician Global Assessment (ScPGA) score ≥3 and scalp surface area (SSA) involvement ≥20% at screening. Patients also had clinical signs of psoriasis on the trunk and/or limbs, had been treated with one or more topical therapies for scalp psoriasis, and were candidates for phototherapy and/or systemic therapy for scalp or body psoriasis lesions. Patients were required to be on a stable treatment course or no treatment for plaque psoriasis for 30 days before study enrollment and to have no plans to change treatment during the study. Key exclusion criteria included a history of any clinically significant cardiac, endocrinologic, pulmonary, neurologic, psychiatric, hepatic, renal, hematologic, or immunologic disease, or other major uncontrolled disease (other than psoriasis) as determined by the investigator. Patients were recruited from six clinical sites in the United States in accordance with current Health Insurance Portability and Accountability Act regulations and were required to provide written informed consent. Study sites were approved by an institutional review board (IRB) and adhered to the IRB-approved protocol.
Patient-reported outcomes
The Modified Whole Body Itch NRS is a single-item scale that asks patients to rate the severity of their whole body itching due to psoriasis on an 11-point NRS ranging from 0 (no itching) to 10 (worst itch imaginable). Patients were asked to consider their worst level of itching over the past 24 h. This scale is adapted from the WI-NRS, which was validated in patients with moderate to severe plaque psoriasis (Citation19,Citation20).
The Scalp Itch NRS, which closely mirrors the Modified Whole Body Itch NRS, is a single-item scale that asks patients to rate the severity of their scalp itching due to psoriasis by considering their worst level of itching over the past 24 h. Similar to the Modified Whole Body Itch NRS, the 11-point Scalp Itch NRS ranges from 0 (no scalp itch) to 10 (worst scalp itch imaginable).
Patients also completed the single-item GAPS-S measure, which served as an anchor-based assessment of scalp psoriasis symptoms for the NRS measures. Using the GAPS-S, patients rated the severity of their scalp psoriasis symptoms overall over the past 24 h using a four-point Likert scale ranging from none (no symptoms) to severe.
The GIC-SI is a single-item measure that asks patients to indicate if there has been any change in scalp itching since they started their study treatment using a seven-point Likert scale with responses ranging from ‘very much better’ to ‘very much worse.’ A description of the rationale for including these scales in the study is presented in .
Table 1. Rationale for including scales in the study.
Study design
This cross-sectional study was conducted in two phases, a qualitative interview phase and a quantitative phase to assess reproducibility (). In the qualitative interview phase, patients participated in a two-part interview consisting of concept elicitation and cognitive interviewing. During concept elicitation, trained interviewers used open-ended questions and probing to identify concepts that were important to patients with regard to their scalp psoriasis. During the cognitive interview, patients completed a series of patient-reported assessments (Modified Whole Body Itch NRS, Scalp Itch NRS, GAPS-S, and GIC-SI) and answered structured questions designed to: (Citation1) assess the relevance, ease of use, and understanding of these measures; (Citation2) identify the patient-reported threshold for meaningful change on the Scalp Psoriasis Itch NRS; and (Citation3) verify that patients could distinguish between the Modified Whole Body Itch NRS and the Scalp Itch NRS.
Figure 1. Study design. *Although the Scalp Itch NRS, Modified Whole Body Itch NRS, and GIC-SI were revised based on feedback from the first eight interviews, an additional four patients completed the original versions of the scales (without revisions). †A total of 49 patients were included in the reproducibility phase because two patients were lost to follow-up (one patient from the ‘interview + follow-up visit’ sample and one from the ‘no interviews’ sample) GAPS-S: Global Assessment of Psoriasis Severity-Scalp; GIC-SI: Global Impression of Change-Scalp Itch; NRS: Numeric Rating Scale.
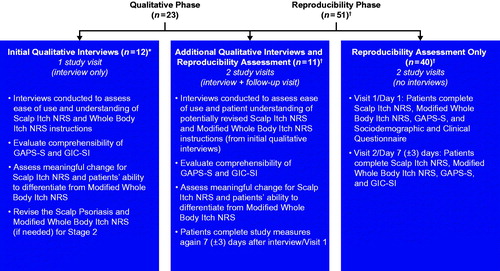
Analyses of the concept elicitation and cognitive interviews were performed in an ongoing and iterative manner to assess whether specific issues with the measurements needed to be addressed before completing the qualitative interviews and to allow for minor revisions that were made to the interview probes and scales based on patient feedback. Concept saturation was evaluated throughout the initial interviews and documented using a saturation grid; after concept saturation was achieved, no additional revisions were made to the scales. For the remainder of the study, additional patients completed and discussed the revised versions of the scales.
Ten patients who completed the revised scales during the qualitative interview phase also participated in a second visit for the reproducibility phase of the study. An additional 39 patients were recruited to take part in the reproducibility phase of the study. The reproducibility phase consisted of two study visits. During Visit 1, patients completed the Modified Whole Body Itch NRS, the Scalp Itch NRS, and the GAPS-S (and no qualitative interview). During Visit 2 (7 ± 3 days later), patients completed the same series of scales that were administered during Visit 1, as well as the GIC-SI. Responses on the GIC-SI were used to classify patients as improved, stable, or worsened for the reproducibility analyses. Patients who responded that their scalp itch was ‘about the same’ since their last visit were considered stable and included in reproducibility analyses comparing responses on Visit 1 and Visit 2.
Data analysis
Audio files from the interviews were transcribed and analyzed using ATLAS.ti (version 7.5.12) and a coding dictionary. Participant responses were then coded to examine comprehension, relevance to patients’ experiences, and the ease or difficulty of selecting a response on each item of the measures. A saturation grid was developed to document the saturation of themes. Responses were organized in spreadsheets and categorized using qualitative and quantitative summary tables. Sociodemographic data, self-reported disease characteristics, and clinical characteristics were summarized using descriptive statistics.
Convergent validity for the Scalp Itch NRS with the Modified Whole Body Itch NRS and GAPS-S scores was assessed at both study visits using Spearman’s rank correlation coefficients. For the correlation analysis, GAPS-S response categories were assigned numerical values (none [no symptoms] = 0; mild = 1; moderate = 2; severe = 3). Mean Scalp Itch NRS and mean Modified Whole Body Itch NRS scores were then presented by GAPS-S response group and compared using a general linear model. A post hoc analysis was performed to examine consistency of change between the Scalp Itch NRS, Modified Whole Body Itch NRS, and GAPS-S in which change scores for each measure were correlated using Spearman’s rank correlation coefficients. Known groups validity was assessed by categorizing mean change scores for the Scalp Itch NRS and Modified Whole Body Itch NRS by GIC-SI scores at Visit 2.
To evaluate test–retest reliability of the Scalp Itch NRS, intra-class correlation coefficients (ICCs) and Spearman’s correlation coefficients were calculated for the subgroup of patients who indicated no change in their scalp itch between study visits (i.e. those who answered ‘About the same’ on the GIC-SI). A sensitivity analysis was performed among patients who reported ‘a little bit better,’ ‘about the same,’ or ‘a little worse’ on the GIC-SI. ICC values greater than 0.70 were considered acceptable for establishing test–retest reliability. Paired t-tests were used to evaluate the statistical significance of change scores between visits.
Results
A total of 23 patients (across four of the six clinical sites) were interviewed for the qualitative phase of the study; of these, 22 patients completed a full interview (). The remaining patient completed a majority of the interview, but the interviewer did not engage the participant on the final section of the discussion guide (related to the GIC-SI) due to time constraints for the patient.
A total of 51 patients took part in the reproducibility phase of the study across the six clinical sites; however, two patients were lost to follow-up (i.e. did not return for a second visit), thus only 49 patients were included in the final test–retest reliability analyses ().
Demographics and baseline clinical characteristics were generally comparable for patients enrolled in the qualitative and reproducibility phases of the study (). In the qualitative and reproducibility phases, respectively, mean (SD) age was 49.1 (17.5) years and 50.3 (15.3) years, the majority of participants were female (69.6% [16/23] and 70.6% [36/51]) and white (65.2% [15/23] and 76.5% [39/51]), and mean (SD) duration of treatment for moderate to severe psoriasis was 14.1 (12.2) years and 14.6 (11.7) years. Patients had moderate to severe scalp psoriasis, with mean (SD) ScPGA score and SSA percentage, respectively, of 3.1 (0.5) and 40.0% (15.4) for patients in the qualitative phase and 3.0 (0.4) and 37.8% (19.1) for patients in the reproducibility phase.
Table 2. Patient sample, demographics, and clinical characteristics.
Concept elicitation results
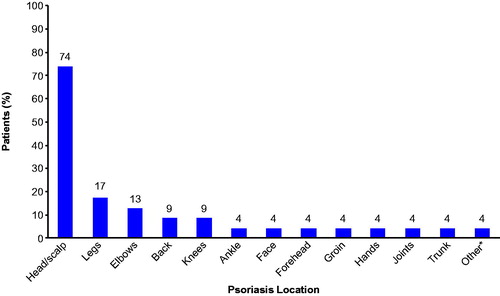
All 23 patients participated in concept elicitation interviews. When asked to describe the term ‘scalp,’ all patients (n = 23, 100%) provided similar interpretations of the term and most (n = 21, 91.3%) preferred this term to describe this psoriasis location (two patients [8.7%] preferred the term ‘head’). While all study participants had scalp psoriasis symptoms, other commonly reported psoriasis locations included arms or elbows (n = 16, 69.6%), legs or knees (n = 14, 60.9%), and back (n = 11, 47.8%). When asked which psoriasis location was most bothersome to them, the majority of patients (n = 17, 73.9%) reported that their scalp (or head) psoriasis was the most bothersome (). Other locations identified as most bothersome by more than 5% of patients included the legs or knees, elbows, and back.
Figure 2. Most bothersome psoriasis locations reported during concept elicitation (N = 23). *Actual response was ‘physically, all equal; psychologically, face.’ Some patients (n = 8) reported >1 bothersome location; therefore, cumulative percentage exceeds 100.
All patients (n = 23, 100%) reported experiencing itching as a psoriasis symptom. Other reported psoriasis symptoms included flaking (n = 14, 60.9%); pain, soreness, or burning (n = 11, 47.8%); bleeding (n = 5, 21.7%); dryness (n = 4, 17.4%); redness (n = 4, 17.4%); scaling (n = 2, 8.7%); red bumps or spots (n = 2, 8.7%); pus or ‘leaking’ (n = 2, 8.7%); inflammation (n = 1, 4.3%); and hair loss (n = 1, 4.3%).
Most patients (n = 20, 87.0%) mentioned that scalp psoriasis had an impact on their lives and reported feeling embarrassed or self-conscious due to visible flakes or scales on their clothing, face, or hair. When describing the emotional impact of their scalp psoriasis, patients used terms such as ‘upsetting,’ ‘embarrassing,’ and ‘depressing.’ Some patients reported experiencing a negative social impact from misconceptions about scalp psoriasis (i.e. perceived as head lice, belief that psoriasis is contagious; n = 4, 17.4%). Likewise, some patients described the need to cover their hair in public or avoid dark clothing to help conceal their psoriasis (n = 8, 34.8%) or stated they avoided physical activity as a result of their scalp psoriasis symptoms (n = 2, 8.7%).
Cognitive interview results
Revisions made to the scales
After reviewing the feedback from the first eight interviews, minor revisions were made to the scales. The Modified Whole Body Itch NRS and Scalp Itch NRS initially contained images of the body and scalp, respectively. These images were removed because patients indicated they were not necessary for understanding and responding to the scales. Among seven patients who initially completed the GIC-SI, six patients suggested removing ‘very much better’ and ‘very much worse’ as response options. Based on this feedback, the number of GIC-SI response options was reduced from seven options to five options ranging from ‘much worse’ to ‘much better.’ No changes were made to the GAPS-S measure. The revised measures, including the final version of the scalp itch NRS (), were administered thereafter.
Psoriasis Itch NRS measures
Almost all patients reported that the Modified Whole Body Itch NRS was easy to understand (n = 22, 95.7%) and demonstrated that they correctly interpreted the instructions (n = 20, 87.0%). In general, patients understood the 0–10 response scale (n = 22, 95.7%) and the 24-h recall period (n = 22, 95.7%), and most were able to select a response without difficulty (n = 20, 95.2%) and think about the intensity of their itch in the previous 24 h when responding (n = 21, 91.3%). Similarly, all patients (n = 23, 100%) correctly interpreted the instructions to the Scalp Itch NRS and were able to select a response based on the 24-h recall period.
Most patients (n = 13, 56.5%) stated that an improvement of three to four points on the Scalp Itch NRS would represent a meaningful improvement in their scalp itch, compared with six patients (26.1%) who indicated a one- to two-point change would be meaningful and four patients (17.4%) who said they would consider a five-point change to be meaningful. Similar results were obtained when patients were further queried to consider what Scalp Itch NRS rating they would need to feel scalp itch had improved in a meaningful way (change of one to two points, n = 8 [34.8%]; three to four points, n = 12 [52.2%]; five points, n = 2 [8.7%]; eight points, n = 1 [4.3%]).
Most patients (n = 15, 65.2%) reported different responses for the Modified Whole Body Itch NRS and the Scalp Itch NRS, indicating they understood that the measures assessed distinct concepts. Among the patients who marked different responses, 80% (n = 12) had a one- or two-point difference in severity between the two scales while the remaining 20% (n = 3) had a three- or four-point difference; the majority (n = 11, 73.3%) indicated that their scalp itch was more severe than their whole body itch.
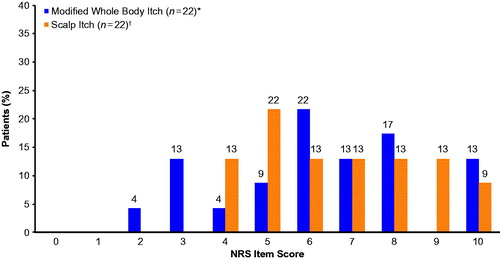
Mean (SD) scores were similar for the Modified Whole Body Itch NRS (6.3 [2.3]; range 2.0–10.0) and Scalp Itch NRS (6.7 [2.0]; range 4.0–10.0), and there was no evidence of a floor or ceiling effect for either measure. The most frequently selected NRS score was 6 for the Modified Whole Body Itch NRS measure and 5 for the Scalp Itch NRS measure ().
Figure 4. Frequency distributions for the Modified Whole Body Itch NRS and Scalp Itch NRS during the qualitative interview phase. Patients rated the severity of their whole body or scalp itching due to psoriasis by considering their worst level of itching over the past 24 h on an 11-point NRS ranging from 0 (no itching) to 10 (worst itch imaginable). *One participant marked two responses (5 and 6) and was marked as missing. †One participant marked two responses (6 and 7) and was marked as missing. NRS: Numeric Rating Scale.
Global Assessment of Psoriasis Severity-Scalp
All patients (n = 23, 100%) understood the instructions of the GAPS-S, found the response scale to be clear and easy to understand, and reported experiencing the symptoms listed on the measure. When asked about the recall period, the majority (n = 21, 91.3%) reported correctly following the instructions to think back to the past 24 h.
The mean (SD) GAPS-S score was 2.1 (0.6), with most patients indicating they had moderate (n = 13, 56.5%) or severe (n = 6, 26.1%) scalp psoriasis symptoms (vs. three patients [13.0%] with mild symptoms and zero patients with no symptoms); one patient marked two responses (moderate and severe) and was recorded as missing.
Global Impression of Change- Scalp Itch
Among the 22 patients who were interviewed about the GIC-SI, all patients demonstrated they were able to understand the instructions and the response options. Of the 21 patients who were asked, the majority (n = 20, 95.2%) indicated they would be able to use this scale to evaluate changes in scalp itching after initiating treatment.
Reproducibility results
Modified Whole Body Itch NRS and Scalp Itch NRS scores
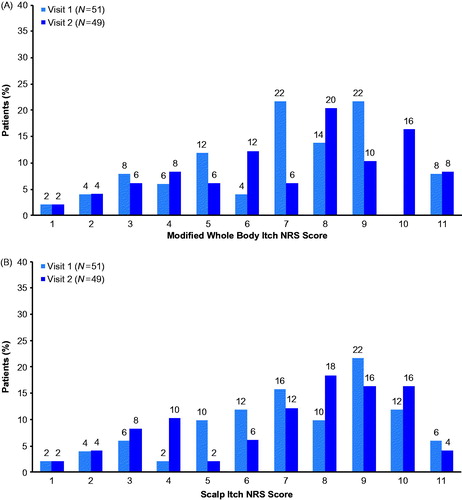
Among the 51 patients who participated in the reproducibility assessment, mean (SD) score was 5.8 (2.5) for the Modified Whole Body Itch NRS and 6.2 (2.5) for the Scalp Itch NRS at Visit 1, with similar scores observed at Visit 2 (Modified Whole Body Itch NRS: 6.2 [2.7]; Scalp Itch NRS: 6.1 [2.7]). Responses ranged from 0.0 to 10.0 for both scales at Visits 1 and 2 with no notable floor or ceiling effects observed for either measure. The frequency distribution of scores for the Modified Whole Body Itch NRS and Scalp Itch NRS at Visits 1 and 2 are presented in , respectively. For both itch NRS measures, most patients rated their itch severity with a score of 6 or higher at Visit 1 (n = 33, 64.7%) and 7 or higher at Visit 2 (n = 27, 55.1%).
Figure 5. Frequency distributions for the (A) Modified Whole Body Itch NRS and (B) Scalp Itch NRS during the reproducibility phase. Patients rated the severity of their whole body or scalp itching due to psoriasis by considering their worst level of itching over the past 24 h on an 11-point NRS ranging from 0 (no itching) to 10 (worst itch imaginable). NRS: Numeric Rating Scale.
Scalp Itch NRS validity
The Scalp Itch NRS demonstrated convergent validity, as shown by significant and strong Spearman’s correlations with the Modified Whole Body Itch NRS, as well as significant and moderate correlations with GAPS-S scores, at both visits (). Consistency in change was also observed between change scores for the Scalp Itch NRS and the GAPS-S and Modified Whole Body Itch NRS ().
Table 3. Spearman’s correlations of the Scalp Itch NRS with the Modified Whole Body Itch NRS and GAPS-S.
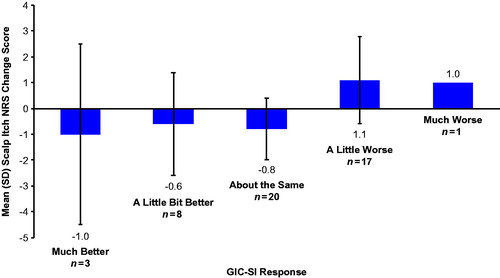
Mean change scores on the Scalp Itch NRS were consistent with change scores on the GIC-SI (). Scalp Itch NRS scores increased from Visit 1 to Visit 2 among patients who indicated on the GIC-SI that their scalp itch had worsened, with the greatest worsening occurring among those who reported their scalp psoriasis was ‘much worse.’ Similarly, patients who indicated that their scalp itch had improved had decreased scores on the Scalp Itch NRS at Visit 2 vs. Visit 1.
Scalp Itch NRS reliability in patients who remained stable
The Scalp Itch NRS demonstrated strong reproducibility in the subgroup of patients who remained stable (i.e. those who responded ‘about the same’ at Visit 2; n = 20) on the GIC-SI (). Paired t-tests evaluating the difference between mean scores on the Scalp Itch NRS at Visit 1 and Visit 2 among patients who remained stable on the GIC-SI found a small, statistically significant difference (mean difference of 0.8; possible range of scores: 0 [no scalp itch] to 10 [worse scalp itch imaginable]; ). A sensitivity analysis that included patients who responded ‘about the same,’ ‘a little bit better,’ or ‘a little worse’ on the GIC-SI did not detect a statistically significant difference between Visits 1 and 2 on the Scalp Itch NRS ().
Table 4. Reproducibility of the Scalp Itch NRS between Visit 1 and Visit 2 based on the GIC-SI response at Visit 2.
Discussion
This study represents one of the first analyses of an itch NRS designed specifically to evaluate scalp itch in patients with moderate to severe scalp psoriasis. Our findings demonstrate the validity and reproducibility of the Scalp Itch NRS for assessing a patient’s experience with scalp itch associated with moderate to severe plaque psoriasis.
All patients in the qualitative phase of the study reported experiencing moderate to severe scalp and whole body itching and confirmed that itch associated with scalp psoriasis was extremely bothersome. These findings are in agreement with previous research indicating that itch is among the most bothersome psoriasis symptoms and is highly prevalent among psoriasis patients with scalp involvement (Citation7,Citation9,Citation13). Furthermore, patients described social and emotional impacts of scalp psoriasis consistent with previous findings on the effects of pruritus on HRQL and psychosocial well-being (e.g. depressive symptoms, distress) (Citation10,Citation11,Citation14).
Although patients rated similar mean scores on the Modified Whole Body Itch NRS and the Scalp Itch NRS, they were able to distinguish that the two measures were assessing different concepts, suggesting that the Scalp Itch NRS is a relevant measure to specifically address pruritus due to scalp psoriasis. The cognitive interviews also showed that the Scalp Itch NRS, as well as the Modified Whole Body Itch NRS, GAPS-S, and GIC-SI, was easy for patients to use and understand. These findings are consistent with patient-reported ease of use and understanding in a prior analysis of the WI-NRS measure, which served as the framework for the development of the Modified Whole Body Itch NRS and the Scalp Itch NRS (Citation19).
Most patients indicated that a change of three to four points on the Scalp Itch NRS would represent a meaningful change in their scalp itch due to psoriasis, consistent with prior research demonstrating that a four-point improvement on the WI-NRS was clinically meaningful and predictive of static Physician Global Assessment (sPGA) response (i.e. sPGA rating of 0 or one or ≥ two-point improvement from baseline) (Citation20).
Prior studies have shown that scales using numeric rating systems to measure itch severity are valid and reliable for the assessment of overall pruritus in patients with moderate to severe psoriasis or chronic pruritus of any origin (Citation20,Citation21). The current study shows that an NRS is a valid and reliable way to measure scalp itch in patients with moderate to severe psoriasis. The novel Scalp Itch NRS demonstrated convergent validity, was responsive to changes in psoriasis symptoms, and demonstrated strong reproducibility. Although reproducibility analyses detected a significant difference in Scalp Itch NRS scores between Visit 1 and Visit 2, this difference was not clinically meaningful (mean difference of 0.8) based on the finding that patients considered a two- to four-point change on the Scalp Itch NRS to be clinically meaningful and results of a prior analysis that identified a four-point change in the WI-NRS as clinically meaningful (Citation20). Kimball et al. also found that WI-NRS scores correlated with changes in HRQL in patients with psoriasis (as evaluated using the Dermatology Life Quality Index Symptoms and Feelings domain) (Citation20). Considering that scalp itch can contribute substantially to impaired HRQL (Citation13), measures that specifically address scalp itch are needed to help inform researchers and clinicians about the effects of treatments for scalp itch on patients’ lives.
Other itch scales have been used in clinical trials that included patients with moderate to severe plaque psoriasis of the scalp, such as pruritus visual analog scales (VASs) and the numeric Psoriasis Symptom Diary (PSD) (Citation16–18,Citation22,Citation23). In an analysis of various patient-reported measures of pruritus intensity, an 11-point NRS showed high reliability and concurrent validity with a widely used VAS and a verbal rating scale (VRS) in patients with chronic pruritus. The NRS, however, had a higher completion rate compared with the VAS and VRS, suggesting that the NRS was simpler and easier to use for patients with chronic pruritus (Citation21). By incorporating the easy-to-use 11-point NRS system into a scalp-specific measure, the Scalp Itch NRS has the potential to complement traditional scales used to assess itch throughout the body. The PSD is another validated measure that uses an 11-point NRS to assess 16 items related to psoriasis symptoms, including itching (Citation23). Although the PSD also demonstrated strong reliability, validity, and responsiveness to changes in the severity of psoriasis symptoms (Citation23), a scale dedicated to scalp itch may be more relevant when evaluating the efficacy of treatments for scalp psoriasis. Therefore, the Scalp Itch NRS will be useful to researchers in clinical trials or clinicians who commonly encounter patients with scalp itch as a prominent symptom of psoriasis.
Limitations
The small sample size for the qualitative and reproducibility phases may limit the generalizability of the findings. The sample was restricted to patients with moderate to severe scalp psoriasis and thus did not assess the Scalp Itch NRS in patients with mild scalp psoriasis.
Conclusions
This analysis of the Scalp Itch NRS, a newly developed patient-reported outcome measure for evaluating scalp itch, demonstrated the ease of use and reproducibility of this measure to evaluate the severity of scalp itch in patients with moderate to severe scalp psoriasis. The Scalp Itch NRS will be useful for assessing clinically meaningful improvements in scalp itch in clinical trials and may assist healthcare providers with interpreting treatment efficacy in their practices.
Acknowledgements
The authors received editorial support in the preparation of this manuscript from Amy Shaberman, PhD, of Peloton Advantage, LLC, Parsippany, NJ, USA, sponsored by Celgene Corporation, Summit, NJ, USA. The authors, however, directed and are fully responsible for all content and editorial decisions for this manuscript.
Data availability statement
Celgene is committed to responsible and transparent sharing of clinical trial data with patients, healthcare practitioners, and independent researchers for the purpose of improving scientific and medical knowledge as well as fostering innovative treatment approaches. For more information, please visit: https://www.celgene.com/research-development/clinical-trials/clinical-trials-data-sharing.
Disclosure statement
Y.W.: Celgene Corporation – employment. K.C.: Evidera – employment; Celgene Corporation – research funding to Evidera. H.S.: Celgene Corporation, Janssen, Lilly, AbbVie, Bristol-Myers Squibb, and Dermavant – investigator and consultant. N.S.: no conflicts of interest to disclose. B.C.: Evidera – employment; Celgene Corporation – research funding to Evidera. Z.Z.: Celgene Corporation – employment. K.N.: Celgene Corporation – employment at the time of study conduct.
Additional information
Funding
References
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003–2006 and 2009–2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37–45.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–516.
- Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine. 2015;73:342–350.
- Bata-Csorgo Z, Hammerberg C, Voorhees JJ, et al. Flow cytometric identification of proliferative subpopulations within normal human epidermis and the localization of the primary hyperproliferative population in psoriasis. J Exp Med. 1993;178:1271–1281.
- Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin. 2015;33:13–23.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9–13.
- Mrowietz U, Chouela EN, Mallbris L, et al. Pruritus and quality of life in moderate-to-severe plaque psoriasis: post hoc explorative analysis from the PRISTINE study. J Eur Acad Dermatol Venereol. 2015;29:1114–1120.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Am Acad Dermatol. 2014;70:871–881.
- Reich A, Hrehorow E, Szepietowski JC. Pruritus is an important factor negatively influencing the well-being of psoriatic patients. Acta Derm Venerol. 2010;90:257–263.
- Zachariae R, Zachariae CO, Lei U, et al. Affective and sensory dimensions of pruritus severity: associations with psychological symptoms and quality of life in psoriasis patients. Acta Derm Venereol. 2008;88:121–127.
- Yosipovitch G, Goon A, Wee J, et al. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000;143:969–973.
- Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes. 2009;7:62.
- Kim TW, Shim WH, Kim JM, et al. Clinical characteristics of pruritus in patients with scalp psoriasis and their relation with intraepidermal nerve fiber density. Ann Dermatol. 2014;26:727–732.
- U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Rockville (MD): U.S. Department of Health and Human Services; 2009.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]). J Am Acad Dermatol. 2015;73:37–49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387–1399.
- Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept, and placebo, in patients with moderate to severe plaque psoriasis: 52-week results from a phase 3b, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31:507–517.
- Naegeli AN, Flood E, Tucker J, et al. The Worst Itch Numeric Rating Scale for patients with moderate to severe plaque psoriasis or psoriatic arthritis. Int J Dermatol. 2015;54:715–722.
- Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157–162.
- Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92:502–507.
- Strober B, Sigurgeirsson B, Popp G, et al. Secukinumab improves patient-reported psoriasis symptoms of itching, pain, and scaling: results of two phase 3, randomized, placebo-controlled clinical trials. Int J Dermatol. 2016;55:401–407.
- Strober B, Zhao Y, Tran MH, et al. Psychometric validation of the Psoriasis Symptom Diary using Phase III study data from patients with chronic plaque psoriasis. Int J Dermatol. 2016;55:e147–e155.