Abstract
Background: There is limited understanding on patterns of systemic treatment in adults with moderate-to-severe atopic dermatitis (AD) in the UK.
Objective: To characterize treatment patterns in adult AD patients prescribed immunosuppressants (IMMs) in the primary care setting.
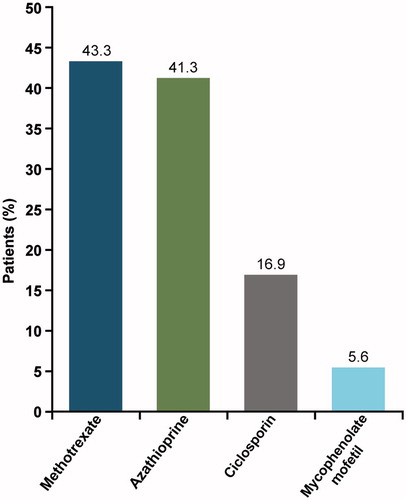
Results: Six hundred and fifty-six patients with AD (6.6%) were prescribed IMM in the analysis (mean age 52.1 years; 59.1% female; age-adjusted Charlson comorbidity index 1.4). Most prevalent (>5%) conditions at baseline were depression (10.8%), contact dermatitis (10.7%), rheumatological disease (7.9%), skin/subcutaneous tissue disorders (6.4%), upper respiratory disease (5.8%), and psoriasis (5.2%). At baseline, up to 50% of patients were prescribed ≥1 IMM. During follow-up, 42.7% of patients were prescribed oral corticosteroids (OCSs), increasing in line with IMM exposure. The most commonly prescribed IMM was methotrexate (43.3%). Ciclosporin, the only approved IMM for AD, was prescribed to 16.9% of patients.
Conclusions: The prevalence of comorbidities and high rate of IMM prescriptions demonstrate the impact of AD on quality of life. The frequency of OCS prescribing in AD patients treated with IMMs suggests a lack of disease control with existing therapies, and an unmet need for safe and effective targeted agents for long-term disease control.
Introduction
Atopic dermatitis (AD) is a chronic skin disorder characterized by immune-mediated inflammation, intense itching, and eczematous lesions (Citation1). Most cases of AD first present in infants and children up to 5 years of age (Citation2), and tend to follow a recurrent, relapsing course that often resolves by puberty (Citation3). However, in ∼50% of cases, AD persists into adulthood and becomes a chronic, lifelong condition. Moderate-to-severe AD is characterized by a multi-dimensional burden that includes persistent itch, pain, sleep disturbances, impaired mental health, and reductions in productivity and health-related quality of life (HRQoL) (Citation4–7).
Adult patients with moderate-to-severe AD, whose symptoms are generally not well controlled with topical therapy, can be treated with phototherapy, or systemically with oral corticosteroids (OCSs) or immunosuppressants (IMMs) (Citation8–11). OCSs are indicated for short-term treatment (up to 3 weeks) of acute flares, but are not recommended for long-term use due to an unfavorable benefit:risk profile (Citation8–11). Diabetes, hypertension, gastric ulcers, osteoporosis, glaucoma, and Cushing’s syndrome have been associated with long-term use of OCSs (Citation8–12).
Immunosuppressive agents commonly prescribed for the treatment of moderate or severe AD include ciclosporin, azathioprine, methotrexate, and mycophenolate mofetil (Citation13). Currently, in several European countries, including the United Kingdom (UK), ciclosporin is the only systemic IMM drug approved for the management of severe AD in adults (Citation14). However, as ciclosporin has a narrow therapeutic index, its use requires regular monitoring of renal function and blood pressure (Citation15), and it has also been associated with an increased risk of squamous cell carcinoma (Citation16). For these reasons, ciclosporin is only approved for a limited treatment period of 1 year (Citation14). Meanwhile, the other immunosuppressive agents are used off-label (Citation14), despite limited evidence supporting their efficacy and safety (Citation17). By establishing the prescribing patterns of these agents, a better understanding could be gained of the clinical management of AD in adult patients and their unmet treatment needs.
The objective of this study was to characterize treatment patterns in adult patients with AD prescribed systemic IMMs in the UK primary care setting.
Methods
Data source
This retrospective healthcare study utilized data from The UK Health Improvement Network (THIN), a large primary care database (Citation18) of anonymized medical records of more than 13 million patients (3.5 million active patients) from 587 general practices across England, Wales, Scotland, and Northern Ireland. Of note, the data from the THIN database are representative of general practitioners and not specialists in dermatology. The study protocol was reviewed and approved by the UK Independent Scientific Review Committee, and the study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Study design
The first recorded diagnosis of AD during the study period was the index diagnosis between January 1 2007 and December 31 2009. The index date was defined as the date of the first systemic IMM prescription after the index diagnosis. Baseline assessment was 12 months prior to the index date, and follow-up was 12 months after the index date. Systemic IMM prescriptions that were screened included methotrexate, azathioprine, mycophenolate mofetil, and ciclosporin.
Population
The THIN database contains anonymized records of more than 11 million patients from more than 500 general practitioner practices in the UK (Citation19,Citation20). The analysis population comprised a cohort of eligible adults (≥18 years of age) with a diagnosis of AD recorded during the study period, validated by at least one medical-claim evidence of AD diagnosis. Patients were eligible if they received at least one prescription for a systemic IMM within 12 months of the first AD diagnosis. The cohort identified by this approach was therefore deemed to be representative of those cases with moderate-to-severe AD. Patients who died during the study period or who received an organ transplantation, bone marrow transplantation, or stem cell transplantation before or during the study period were excluded.
Comorbid conditions
Baseline data on patient Charlson Comorbidity Index (CCI) score (Citation21), atopic comorbidities (allergic rhinitis and asthma), comorbid skin conditions (all skin conditions, skin and subcutaneous tissue disorders/inflammatory conditions, and psoriasis), and mood disorders (anxiety, depression, and sleep disorders) were evaluated, and the most common comorbidities (>5%) were reported.
Prescribing patterns
Prescribing data were evaluated during the follow-up period. The mean number of prescriptions and the prevalence of at least one prescription for topical corticosteroids, topical calcineurin inhibitors, phototherapy, OCSs, and IMMs were reported. In addition, the relationship between IMM exposure and OCS prescribing was explored. Systemic IMM exposure was defined as the number of once-daily tablets prescribed during the 1-year follow-up period divided by 365 days. To determine the quartile of IMM exposure, concomitant OCS prescribing was stratified according to IMM exposure (≤25% vs. >25%; ≤50% vs. >50%; and ≤75% vs. >75%).
Statistical analyses
The Chi-squared test was used to compare categorical variables between groups, with the two-tailed significance level (α) set at 0.05. Data analyses were performed using SAS software version 9.2 (Cary, NC).
Results
Patient demographics
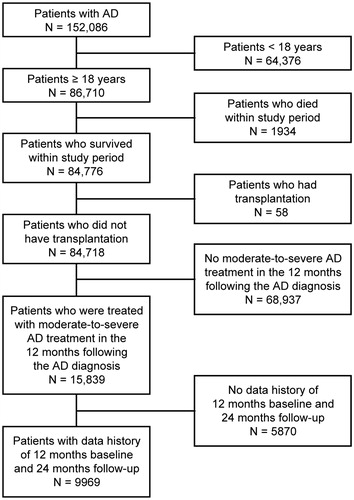
Of 152,086 eligible adult patients with AD identified in the UK THIN database, 9969 had adequate historical data and comprised the population of analysis (). A total of 656 patients (6.6%) with a mean age of 52.1 (standard deviation (SD) 17.9) years met the inclusion criteria; 59.1% of patients were female ().
Table 1. Baseline demographic characteristics of the analytic population.
Comorbidities
The most prevalent (>5%) comorbidities were depression (10.8%), contact dermatitis (10.7%), rheumatological disease (7.9%), skin and subcutaneous tissue disorders not inclusive of codes for vasculitis and collagen diseases (6.4%), upper respiratory disease not inclusive of asthma (5.8%), and psoriasis (5.2%) ().
Table 2. Most common comorbidities (>5%) at baseline among UK patients with AD treated with immunosuppressants.
Prescription patterns during the follow-up period
Prescribing during follow-up is shown in ; most patients were prescribed topical corticosteroids (61.9%) and/or emollients (49.4%).
Table 3. Overall prescribing patterns at follow-up among adult patients with AD who received ≥1 systemic immunosuppressant prescription.
Overall, 42.7% of patients were prescribed an OCS, with a mean of 6.3 (SD 5.6) prescriptions per year. The most commonly prescribed IMMs were methotrexate (43.3%) and azathioprine (41.3%). Ciclosporin was prescribed in 16.9% of patients (). The mean number of prescriptions per year for any IMM was 9.2 (SD 6.8) ().
Concomitant oral corticosteroid prescribing by systemic immunosuppressant exposure
The proportion of patients with one or more OCS prescription was numerically higher among patients with greater IMM exposure than with patients with lower IMM exposure; however, the difference attained statistical significance only for IMM exposures of ≤75% versus >75% (p = .0159) (). The mean number of concomitant OCS prescriptions per year was generally similar (5.4–6.6 per year) regardless of systemic IMM exposure ().
Table 4. Concomitant oral corticosteroid prescribing by systemic immunosuppressant exposure.
The mean number of IMM prescriptions per year was 9.2. Heavy IMM users (defined as patients who received more than nine IMM prescriptions per year) had a significantly higher mean number of OCS prescriptions than patients with less than nine IMM prescriptions per year (7.3 vs. 5.6, respectively; p = .0147). When evaluated for specific IMM use, this comparison reached statistical significance for patients receiving mycophenolate mofetil or methotrexate (p = .0497 and p = .0016, respectively) ().
Table 5. Concomitant oral corticosteroid prescribing by number of immunosuppressant prescriptions per year.
Concomitant oral corticosteroid prescribing by number and type of systemic immunosuppressant
Compared with prescriptions for a single systemic IMM, prescriptions for two unique systemic IMMs during the follow-up period were associated with a greater proportion of patients with at least one OCS prescription (44.2% vs. 53.5%) () and a higher mean number of OCS prescriptions per year (6.2 vs. 7.7) (); however, the differences were not statistically significant. OCS use was generally high regardless of the type of systemic IMM used (32.4–64.9%) (). Compared with patients receiving ciclosporin, those receiving methotrexate, azathioprine, and mycophenolate mofetil had significantly higher numbers of OCS prescriptions during the follow-up period ().
Table 6. Concomitant oral corticosteroid prescribing by number and type of systemic immunosuppressant.
Discussion
This retrospective study based on the UK THIN primary care database (Citation18) characterized the real-world treatment patterns in UK adult patients with AD treated with systemic IMMs. In the UK, prescribing of IMMs for AD is initiated by dermatologists in secondary care, but usually prescribing is then continued by primary care physicians under joint supervision.
We showed that in this cohort of 9969 adult patients with AD, 6.6% were prescribed a systemic IMM. The data indicated that ciclosporin, the only approved IMM for AD in the UK, was prescribed to just 16.9% of patients treated with IMMs seen by general practitioners. This relatively low rate of use concurs with the observations of ciclosporin use found through an online survey of UK dermatologists (Citation22), which found that ciclosporin was not the preferred first-line systemic agent, with only 37% of dermatologists prescribing the drug as first line. The prescribing in the UK differs somewhat from studies reporting IMM usage in Dutch (Citation23) and French (Citation24) adult patients with AD, in which ciclosporin was prescribed to 80% of patients. One explanation for the divergence in prescribing rates of ciclosporin between the UK and other European countries may be explained by the consensus among British Dermatologists that ciclosporin should be discontinued after 1–2 years of therapy (Citation23,Citation24). In addition, the British National Formulary recommends that ciclosporin should only be used for short-term treatment of severe AD for a maximum period of 8 weeks (Citation25). These recommendations may have influenced ciclosporin prescribing in patients with chronic, severe disease.
Conversely, methotrexate (43%) and azathioprine (41%) were the most frequently prescribed immunosuppressive agents in this study (). The online survey reported by Taylor et al. (Citation22) pointed to azathioprine as the preferred first-line systemic agent by 47% of dermatologists, ahead of systemic corticosteroids (42%) and ciclosporin (37%); in addition, methotrexate was the most commonly used second-line systemic agent (45%). Despite being off-label, the increased prescribing of azathioprine in the present study and the study by Taylor et al. (Citation22) may be explained by British and European guidance (Citation11,Citation26–28), which recommend it for the treatment of refractory moderate-to-severe AD. Meanwhile, evidence to support the effectiveness of methotrexate compared with azathioprine (Citation29) may explain the increasing trends towards methotrexate prescribing.
More than 40% of patients treated with a systemic IMM were also prescribed an OCS. Concomitant OCS prescribing is suggestive of the treatment of patients with refractory/severe disease, severe flares, or comorbid conditions, such as asthma. Our analyses showed no reduction in OCS use among patients with a higher number of IMM prescriptions. In fact, OCS use generally increased among patients with greater IMM exposure. It is possible that a higher number of IMM prescriptions is correlated with greater disease severity. Therefore, it might be expected that an increase in OCS use would be observed among patients with more IMM prescriptions (greater disease severity).
Phototherapy/photochemotherapy had a negligible prescription rate in the current study. This is because the THIN database only reports patients seen in general practice, and phototherapy can only be prescribed by a consultant dermatologist or accredited practitioner working under the supervision of a consultant dermatologist (Citation30), and delivered in secondary care as a hospital day case treatment. By contrast, the study by Taylor et al. (Citation22) was conducted among specialists, who can prescribe phototherapy, and in this study phototherapy was the most common therapeutic modality. These results are not unusual, as specialists are more likely to follow treatment guidelines than primary care physicians and use a step-wise treatment approach in doing so (Citation31), whereby phototherapy is prescribed prior to IMMs.
There are some limitations of the THIN data that should be considered when interpreting these results. THIN does not provide data on AD severity, such as SCORing AD (SCORAD) (Citation32) or the Eczema Area and Severity Index (EASI) (Citation33). In the current study, the prescription of an IMM was applied as a surrogate marker of severe disease. It was not possible to assess disease control as THIN does not include data on key AD-specific signs, symptoms, or QoL outcomes. Hence, OCS use was considered a surrogate for disease control, given its use in clinical practice on a short-term basis to treat disease exacerbations. In addition, the number of IMM prescriptions received per year does not necessarily correlate with the length of time a patient spends on treatment, as clinical practice is highly variable and can differ for each IMM. For example, clinicians may provide monthly prescriptions for ciclosporin (equivalent to 12 prescriptions per year) or 3-monthly prescriptions for methotrexate (equivalent to four prescriptions per year). Finally, as the dataset does not allow confirmation of the disease being treated, some of the OCS prescribing may have been directed towards conditions identified here as co-morbidities (e.g. rheumatological disease, respiratory, and non-AD skin disease). However, in the UK, psoriasis is not treated with OCS. For the 7.9% and 5.8% of patients with concomitant rheumatological disease, or upper respiratory disease (including asthma), respectively, OCSs may have been prescribed to treat the comorbidity rather than AD. However, we want to highlight that OCS usage in the ciclosporin- and azathioprine-treated groups, treatments that are only used for AD, was equivalent to that in the methotrexate/mycophenolate groups. Thus, although this does not exclude the possibility that some patients in the cohort had AD requiring IMM treatment and another unrelated condition requiring OCS treatment, we suspect that this potential error in interpretation is small. Despite these limitations, THIN provides a valid (Citation34–36) and generalizable source of data to study treatment patterns in the general UK adult population (Citation37) and has been used extensively in other disease areas to address similar objectives (Citation36,Citation38–41).
In conclusion, this analysis of data from the THIN primary care database of more than 13 million patients in the UK indicates a high prescription rate of systemic IMMs among adults with AD (6.6%). Significant comorbidities, such as depression (10.8%), are associated with this skin condition. The prevalence of concomitant OCS use among UK adult patients with AD prescribed systemic IMMs, suggests inadequate disease control with existing systemic therapies, and a significant unmet need for new therapies that provide safe and effective disease control.
Acknowledgements
Simon Gosset provided input into the study concept and design, and data acquisition and analysis. Medical writing support was provided by Fernando Gibson, PhD, Abby Armitt, MSc, and Michele Damo, PharmD, of Prime, UK, and funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Disclosure statement
LK, CA, RR, and RH are employees and stockholders of Sanofi. AG is an employee of and stockholder in Regeneron Pharmaceuticals, Inc. MA-J is an employee of the University of Southampton, which has received research funding from Sanofi and Regeneron Pharmaceuticals, Inc.
Additional information
Funding
References
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122.
- Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy. 2014;2014:1.
- Garmhausen D, Hagemann T, Bieber T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013;68:498–506.
- Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74:491–498.
- Guttman-Yassky E, Simpson E, Margolis DJ, et al. Patient-reported disease burden in adults with atopic dermatitis: a US cross-sectional study. 25th European Academy of Dermatology and Venereology; 2016 Sep 28–Oct 2; Vienna, Austria; 2016.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77:274–279.
- Girolomoni G, Gadkari A, Auziere S, et al. The patient-reported disease burden in adults with atopic dermatitis: a cross-sectional study in Canada and Europe. Value Health. 2017;20:A807.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116–132.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338–351.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327–349.
- Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis). Part II. J Eur Acad Dermatol Venereol. 2012;26:1176–1193.
- Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429–438.
- Akhavan A, Rudikoff D. Atopic dermatitis: systemic immunosuppressive therapy. Semin Cutan Med Surg. 2008;27:151–155.
- Bieber T, Straeter B. Off-label prescriptions for atopic dermatitis in Europe. Allergy. 2015;70:6–11.
- Jorga A, Holt DW, Johnston A. Therapeutic drug monitoring of cyclosporine. Transplant Proc. 2004;36:396S–403S.
- Behnam SM, Behnam SE, Koo JY. Review of cyclosporine immunosuppressive safety data in dermatology patients after two decades of use. J Drugs Dermatol. 2005;4:189–194.
- Schmitt J, Schmitt N, Meurer M. Cyclosporin in the treatment of patients with atopic eczema – a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2007;21:606–619.
- OPEN Health. The UK Health Improvement Network (THIN); [cited 2017 Jun 5]. Available from: http://www.openhealth.co.uk/news/2017/thin-data-the-health-improvement-network.aspx
- European Federation of Allergy and Airways Diseases Patients’ Associations. Atopic eczema: itching for life. Quality of life and costs for people with severe atopic eczema in Europe; 2018; [cited 2018 Nov 15]. Available from: http://www.efanet.org/images/2018/EN_-_Itching_for_life_Quality_of_Life_and_costs_for_people_with_severe_atopic_eczema_in_Europe_.pdf
- University of Birmingham. More about THIN; 2019; [cited 2019 Apr 15]. Available from: https://www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PCCS/THIN/more-about-thin/index.aspx
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Taylor K, Swan DJ, Affleck A, et al. Treatment of moderate-to-severe atopic eczema in adults within the U.K.: results of a national survey of dermatologists. Br J Dermatol. 2017;176:1617–1623.
- Tidman MJ, Smith CJ. Principles of systemic therapy. In: Griffiths CE, Barker JN, Bleiker T, et al. editors. Rook's textbook of dermatology. Oxford: John Wiley & Sons; 2015. Available from: http://www.rooksdermatology.com/manual/c19-sec-0201?view=chapter
- Ardern-Jones MR, Hampton P, Vleugels RA. Handbook of dermatology treatments. London: JP Medical Publishers; 2017.
- British National Formulary – National Institute for Health and Care Excellence (NICE). Ciclosporin; [2017 Jun 20]. Available from: https://bnf.nice.org.uk/drug/ciclosporin.html
- Schram ME, Borgonjen RJ, Bik CM, et al. Off-label use of azathioprine in dermatology. Arch Dermatol. 2011;147:474–488.
- Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367:839–846.
- Meggitt SJ, Anstey AV, Mohd Mustapa MF, et al. British Association of Dermatologists' guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol. 2011;165:711–734.
- Schram ME, Roekevisch E, Leeflang MM, et al. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J Allergy Clin Immunol. 2011;128:353–359.
- British Association of Dermatologists (BAD). Phototherapy service guidance 2017; [cited 2018 Mar 21]. Available from: http://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=4151
- Chaplin S. Guide to treatments used for atopic dermatitis in adults. Prescriber. 2016;27:30–39.
- European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31.
- Hanifin JM, Thurston M, Omoto M, et al. The Eczema Area and Severity Index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11–18.
- Lewis JD, Schinnar R, Bilker WB, et al. Validation studies of The Health Improvement Network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401.
- Denburg MR, Haynes K, Shults J, et al. Validation of The Health Improvement Network (THIN) database for epidemiologic studies of chronic kidney disease. Pharmacoepidemiol Drug Saf. 2011;20:1138–1149.
- Martin-Merino E, Fortuny J, Rivero E, et al. Validation of diabetic retinopathy and maculopathy diagnoses recorded in a U.K. primary care database. Diabetes Care. 2012;35:762–767.
- Blak BT, Thompson M, Dattani H, et al. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19:251–255.
- Diwakar L, Cummins C, Ryan R, et al. Prescription rates of adrenaline auto-injectors for children in UK general practice: a retrospective cohort study. Br J Gen Pract. 2017;67:e300–e305.
- Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;6:e010210.
- Khalid JM, Globe G, Fox KM, et al. Treatment and referral patterns for psoriasis in United Kingdom primary care: a retrospective cohort study. BMC Dermatol. 2013;13:9.
- James GD, Petersen I, Nazareth I, et al. Use of long-term antibiotic treatment in COPD patients in the UK: a retrospective cohort study. Prim Care Respir J. 2013;22:271–277.