Abstract
Background
It is challenging to select the most appropriate biologic treatment for patients with moderate-to-severe plaque psoriasis.
Objective
To compare speed of onset and level of skin improvement between the interleukin (IL)-17A antagonist ixekizumab and the IL-23 p19 inhibitors guselkumab, tildrakizumab, and risankizumab in patients with moderate-to-severe plaque psoriasis.
Methods
Using data from controlled clinical trials, both adjusted indirect comparisons (AICs) and matching adjusted indirect comparisons (MAICs) were performed to determine the risk difference (RD) between ixekizumab and each IL-23 p19 inhibitor for the proportion of patients with ≥75%/90%/100% improvement compared with baseline in Psoriasis Area and Severity Index (PASI 75/90/100) up to week 12. Placebo, etanercept, or ustekinumab were used as the comparator bridge.
Results
In all (M)AICs, RDs generally significantly favored ixekizumab over guselkumab (placebo bridge), tildrakizumab (placebo or etanercept bridge), and risankizumab (placebo or ustekinumab bridge) from the earliest assessment time (≥ week 2) to week 12 when considering PASI 75/90/100 responses.
Conclusion
Ixekizumab provides a faster onset of effect and earlier clinical benefits than guselkumab, tildrakizumab, or risankizumab in patients with moderate-to-severe psoriasis, as reflected by higher levels of skin improvement than with these IL-23 p19 inhibitors up to week 12.
Introduction
The number of biologic agents available for the treatment of patients with moderate-to-severe psoriasis has greatly increased in recent years, thus expanding therapeutic options. Biologic agents have generally proved efficacious, with acceptable tolerability, when compared with placebo (Citation1,Citation2).
Currently, there are limited numbers of head-to-head trials of recently approved biologic agents available for the treatment of psoriasis, making it challenging to select the most appropriate treatment for patients with moderate-to-severe plaque psoriasis. To date, the interleukin (IL)-17A antagonists ixekizumab and secukinumab have each been directly compared with etanercept and the IL-12/IL-23 inhibitor ustekinumab (Citation3–7). Ustekinumab has also been directly compared with both etanercept (Citation8) and the IL-17 receptor antagonist brodalumab (Citation9), whereas the IL-23 p19 inhibitors risankizumab, guselkumab, and tildrakizumab have been compared with ustekinumab, adalimumab, and etanercept, respectively (Citation10–12). Two trials of direct comparisons between an IL-17A antagonist and an IL-23 p19 inhibitor in patients with moderate-to-severe plaque psoriasis have also recently been published; the first compared secukinumab with guselkumab (Citation13) and the second compared ixekizumab with guselkumab (IXORA-R) (Citation14). The latter trial demonstrated the superiority of ixekizumab over guselkumab with respect to rapid and complete skin clearance at week 12 (Citation14).
In the absence of head-to-head randomized controlled trials, providing indirect evidence using network meta-analyses (NMAs) or indirect comparisons (ICs) is recommended (Citation15–17). While NMA enables the simultaneous analysis of networks containing multiple treatments, IC requires a smaller number of statistical assumptions and can provide suitable estimates for the comparison of two treatments based on a common comparator. Hence, the classic adjusted IC (AIC) (Citation18) uses combined outcomes from single direct comparisons to determine an indirect effect estimate via a common comparator, as illustrated in . However, if there is an imbalance in treatment effect modifiers across studies (for example, specific patient characteristics when inclusion/exclusion criteria differ), accuracy can be improved by a method such as matching-AIC (MAIC) (Citation19), as suggested by the United Kingdom National Institute for Health and Care Excellence (NICE) (Citation20). MAICs match individual patient-level data so that the trial populations have similar baseline characteristics before the AIC is performed. This matching reduces the risk of bias introduced by differences in patient characteristics across trials and helps reduce imbalances from different trials that could influence outcomes (Citation19); however, some limitations remain (Citation20).
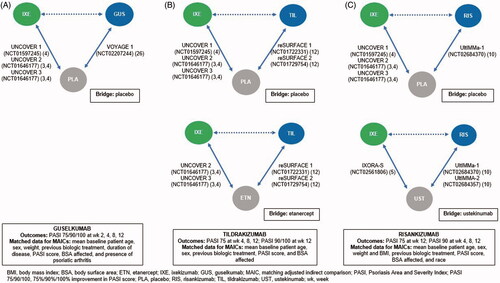
Figure 1. Study design showing the direct (solid lines) and indirect (dashed lines) relationship between ixekizumab and (a) guselkumab, (b) tildrakizumab 200 or 100 mg, and (c) risankizumab, with placebo or active comparator used as the bridge.
NMAs have provided information regarding the relative efficacies of different biologic agents, the most comprehensive of which included conventional systemic agents and small molecules; tumor necrosis factor (TNF) inhibitors; ustekinumab; the IL-17A antagonists ixekizumab and secukinumab; the IL-17 receptor antagonist brodalumab; the IL-23 p19 inhibitors guselkumab and tildrakizumab; and the anti-CD6 immunoglobulin G monoclonal antibody itolizumab (Citation1). In that NMA, Sbidian et al. (Citation1) concluded that, compared with placebo at week 12 to 16, ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab, and ustekinumab offered the best choices for achieving Psoriasis Area and Severity Index (PASI) improvement of at least 90% (PASI 90) in patients with moderate-to-severe psoriasis. The most efficacious drug was ixekizumab (risk ratio [RR] 32.45; 95% confidence interval [CI]: 23.61, 44.60). Sbidian et al. (Citation1) also concluded that additional data comparing a number of new biologic classes are needed, including IL-17A versus IL-23 antagonists.
In addition to the NMA by Sbidian et al. (Citation1), ICs have analyzed the relative efficacies of ustekinumab and TNF inhibitors (Citation21), secukinumab and adalimumab (Citation22,Citation23), and ixekizumab and secukinumab (Citation24). However, there is special interest in the relative efficacies of newly approved IL-23 p19 and IL-17 antagonists, including ixekizumab.
To address this paucity of data and to support physicians when choosing the most appropriate psoriasis treatment, we conducted an AIC and two MAICs to compare the short-term level of skin improvement and speed of onset for potentially the most efficacious IL-17A antagonist ixekizumab (Citation24), with each of the IL-23 p19 inhibitors guselkumab, tildrakizumab, and risankizumab, focusing on the initial double-blind, placebo-controlled 12-week treatment period (the period for which data suitable for IC were available).
Materials and methods
Baseline patient information and efficacy outcome data up to week 12 were extracted from the phase III/IIIb randomized controlled clinical trials for each biologic: UNCOVER 1, 2, and 3 and IXORA-S for ixekizumab (NCT01597245, NCT01646177, NCT01646177, and NCT02561806, respectively) (Citation3–5), VOYAGE 1 for guselkumab (NCT02207244) (Citation25), reSURFACE 1 and 2 for tildrakizumab (NCT01722331 and NCT01729754, respectively) (Citation12), and UltIMMa-1 and −2 for risankizumab (NCT02684370 and NCT02684357, respectively) (Citation10). Inclusion of these studies in the IC was based on knowledge of the respective phase III/IIIb clinical trial programs for each biologic, and no systematic literature search was performed to identify further potential studies.
All studies included patients aged ≥18 years, with a diagnosis of moderate-to-severe chronic plaque psoriasis ≥6 months prior to randomization. Analyses in these studies used non-responder imputation (NRI) and determined the risk difference (RD) between ixekizumab and each IL-23 p19 inhibitor for the proportion of patients with ≥75%/90%/100% improvement compared with baseline in PASI (PASI 75/90/100) up to week 12 relative to a common comparator. Primary endpoints were evaluated at week 16 in some studies (Citation10,Citation25), but week 16 data were not consistently available and were therefore not considered in the current analyses. All studies were approved by an institutional review board or ethics committee at participating sites, and written informed consent was provided by all patients before starting their respective study.
Data extraction and analysis
Patient-level data were available for all of the ixekizumab studies, whereas for the three IL-23 p19 inhibitors, relevant patient baseline and week 12 outcomes data were extracted from published literature. Digitation of images, using ‘xyscan’ software, was necessary for some data extraction. The main outcomes of interest in the ICs were PASI 75, PASI 90 and PASI 100 responses up to week 12 of treatment. No PASI 100 data to week 12 were available in the published literature for risankizumab (Citation10).
AICs using the methods of Bucher et al. (Citation18) and two modified MAICs (Citation19) were performed to determine response rates up to week 12 in patients receiving ixekizumab (160 mg at week 0 then 80 mg every 2 weeks thereafter) and those receiving each of the three IL-23 p19 inhibitors (guselkumab 100 mg at weeks 0, 4, and 12; tildrakizumab 200 or 100 mg at weeks 0 and 4; and risankizumab 150 mg at weeks 0 and 4) relative to a common comparator (comparator bridge: placebo, etanercept, or ustekinumab; see for further details).
Similar to propensity score methods, the MAICs calculated weights for patients treated with ixekizumab to match for mean baseline characteristics and/or treatment effect modifiers reported for patients treated with each IL-23 p19 inhibitor, in accordance with NICE guidance (Citation20). The data that were weighted included baseline age, sex, weight/body mass index (BMI), previous treatments, duration of disease, PASI score, body surface area (BSA) affected, presence of psoriatic arthritis, and race (see for specific matching used in each comparison). In the next step, the calculated weights were used to recalculate the initial outcomes of interest, i.e. the outcome of a single patient was multiplied by the weight. Finally, the weighted outcomes for patients treated with ixekizumab were used along with those from patients treated with each IL-23 p19 inhibitor for calculation of an AIC. Data were matched overall (MAIC [overall]) or per treatment arm separately (MAIC [per treatment arm]). Bootstrap estimates with 1000 iterations were used to calculate the variance of the weighted estimator in the MAIC. This variance was used to calculate P-values and CIs. The RD between ixekizumab and each IL-23 p19 inhibitor was calculated with associated 95% CIs. All statistical analyses were conducted using R version 3.2.4.
Results
Ixekizumab versus guselkumab
Relevant baseline patient characteristics for ixekizumab and guselkumab are summarized in Supplementary Table 1, both before and after matching.
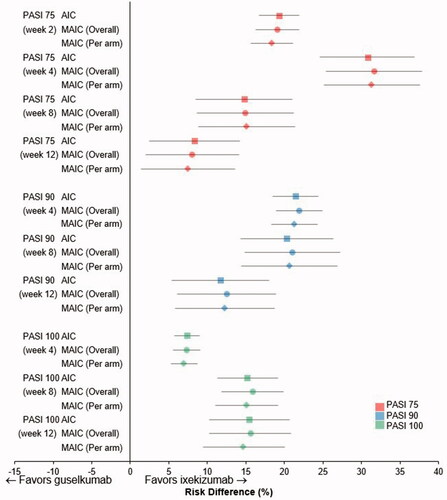
Using AIC with placebo as the comparator bridge (), the PASI 75 response RD favored ixekizumab over guselkumab from week 2 through to week 12 (p < .006 at weeks 2 and 4; and Supplementary Table 2). Similarly, PASI 90 and PASI 100 response RDs significantly favored ixekizumab over guselkumab at week 4, the earliest time at which these endpoints could be evaluated, and at weeks 8 and 12 (p < .001 for all). Results using both MAICs were generally consistent with these findings ( and Supplementary Table 2).
Figure 2. Risk difference for Psoriasis Area Severity Index (PASI) 75% improvement (PASI 75), 90% improvement (PASI 90), and 100% improvement (PASI 100) up to week 12. Calculated using adjusted indirect comparison (AIC) (Citation20) or matching AIC (MAIC), with adjustment overall or per treatment arm separately (Citation21) for ixekizumab versus guselkumab, with placebo as the comparator bridge. Data shown are mean risk differences with 95% confidence intervals.
Ixekizumab versus tildrakizumab
Relevant baseline patient characteristics for ixekizumab and tildrakizumab are summarized in Supplementary Table 3, both before and after matching.
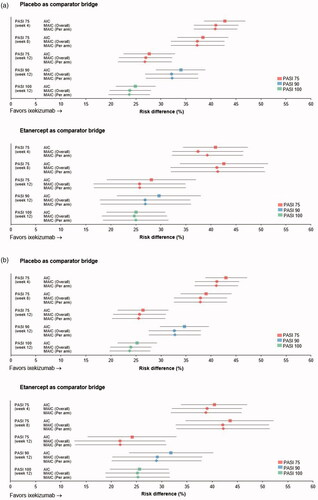
Using AIC with placebo as the comparator bridge (), the PASI 75 response RD significantly favored ixekizumab over both doses of tildrakizumab (100 and 200 mg) from week 4 through to week 12 (p < .001 at all timepoints evaluated; and Supplementary Table 2). PASI 90 and PASI 100 response RDs at week 12 also significantly favored ixekizumab over both doses of tildrakizumab. Findings were similar when the AIC was conducted using etanercept as the comparator bridge (), with PASI 75, PASI 90, and PASI 100 response RDs all significantly favoring ixekizumab over both doses of tildrakizumab (p < .001 for all; and Supplementary Table 2). Results using both MAICs were consistent with the AIC findings ( and Supplementary Table 2).
Figure 3. Risk difference for Psoriasis Area Severity Index (PASI) 75% improvement (PASI 75), 90% improvement (PASI 90), and 100% improvement (PASI 100) up to week 12. Calculated using adjusted indirect comparison (AIC) (Citation20) or matching AIC (MAIC), with adjustment overall or per treatment arm separately (Citation21) for ixekizumab versus tildrakizumab (a) 100 mg or (b) 200 mg, with placebo or etanercept as the comparator bridge. Data shown are mean risk differences with 95% confidence intervals.
Ixekizumab versus risankizumab
Relevant baseline patient characteristics for ixekizumab and risankizumab are summarized in Supplementary Table 4, both before and after matching. Ixekizumab patient numbers in the comparisons using ustekinumab as the comparator bridge were lower than those in the other analyses.
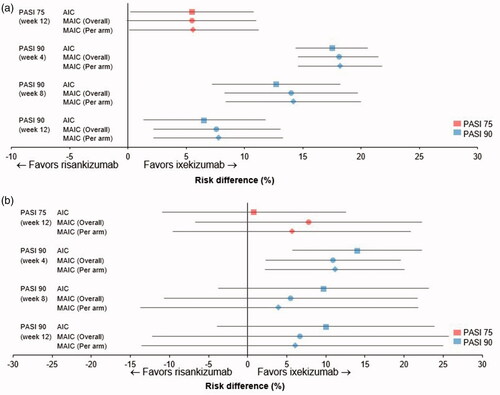
Using AIC with placebo as the comparator bridge (), the PASI 75 response RD significantly favored ixekizumab over risankizumab at week 12 (p < .05), as did PASI 90 response RDs at weeks 4 (p < .001), 8 (p < .001), and 12 (p < .05; and Supplementary Table 2). The choice of comparator bridge had little effect on the observed PASI response RD trends ( and Supplementary Table 2), although significance was not achieved for most PASI response RDs when ustekinumab was used as the comparator bridge. However, treatment effects were similar irrespective of whether placebo or ustekinumab was used as the comparator bridge. Results using both MAICs were generally consistent with the AIC findings ( and Supplementary Table 2).
Figure 4. Risk difference for Psoriasis Area Severity Index (PASI) 75% improvement (PASI 75), 90% improvement (PASI 90), and 100% improvement (PASI 100) up to week 12. Calculated using adjusted indirect comparison (AIC) (Citation20) or matching AIC (MAIC), with adjustment overall or per treatment arm separately (Citation21) for ixekizumab versus risankizumab, with (a) placebo or (b) ustekinumab as the comparator bridge. Data shown are mean risk differences with 95% confidence intervals; PASI 100 data were not available for risankizumab.
Discussion
In the absence of head-to-head comparisons, clinicians must rely on alternative methods to determine the relative clinical benefit of the available treatment options. ICs, such as those reported here, are generally accepted by payers on a national level, for example, NICE in the United Kingdom (Citation20) and the Pharmaceutical Benefits Advisory Committee (Australian Government) in Australia (Citation26). Results of the current ICs of 12-week data indicate that, for induction therapy, the IL-17A antagonist ixekizumab showed a faster onset of action and greater early efficacy than the IL-23 p19 inhibitors guselkumab, tildrakizumab, and risankizumab in most studied scenarios. The advantages of ixekizumab were observed across a range of PASI response cutoffs, including the stringent PASI 100 response, using three analysis methods, and, in some instances, using multiple comparator bridges. In general, results were more robust when placebo was used as the comparator bridge instead of an active comparator, possibly due to the larger sample size for the former analyses. Our findings are clinically meaningful given the importance of these endpoints to patients: patients with moderate-to-severe psoriasis have indicated that rapid and/or complete resolution of skin lesions are important treatment goals (Citation27,Citation28). Additionally, a rapid onset of treatment effect improves patient confidence in therapy, another important patient-relevant treatment goal (Citation27).
In clinical trials, patients with moderate-to-severe psoriasis treated with ixekizumab (Citation3,Citation4), guselkumab (Citation25), tildrakizumab (Citation12), or risankizumab (Citation10) achieved high and persistent response rates at weeks 12–16, measured using PASI 75, PASI 90, and PASI 100 responses. In all studies, the efficacy of ixekizumab (Citation3,Citation4) and the IL-23 p19 inhibitors (Citation10,Citation12,Citation25) was significantly greater than that of placebo and, when evaluated, an active comparator (etanercept or ustekinumab). The recently published results of a direct comparison of ixekizumab with guselkumab support and lend credibility to our findings, as they show that patients treated with ixekizumab had significantly higher PASI response rates at all evaluated times (including PASI 75 at week 2, PASI 90 at weeks 4 and 8, and PASI 100 at weeks 4, 8 and 12) (Citation14). Results of a 48-week trial comparing guselkumab with secukinumab have also recently been published. At week 12, numerically higher PASI response rates were reported for patients receiving secukinumab compared with guselkumab (Citation13), in accord with our findings.
We chose the IL-17A antagonist ixekizumab as the main comparator in our analyses because patients with moderate-to-severe psoriasis treated with this biologic achieved faster skin clearance (higher PASI 90 response rates at week 4), that was maintained at week 12 of treatment (PASI 90 and PASI 100 responses), than those treated with another IL-17A antagonist, secukinumab (Citation24). These findings of Warren et al. (Citation24) were also based on (M)AIC, using similar methodology (Citation18,Citation19) to that in the current analyses, and an additional meta-analysis of available evidence. Using the results of all trials that allowed an active comparator bridge, RDs at week 12 for ixekizumab versus secukinumab were significantly different (p < .05) for PASI 90 response (10.0–12.6%) and PASI 100 response (11.7–13.1%) across all analyses (Citation24). Guselkumab, tildrakizumab, and risankizumab were selected as comparators because these agents have recently become available for patients with psoriasis.
The current analyses did not include safety because of potential differences in the criteria used to collect, define, or analyze such events as well as variation in the investigated timepoints in the individual studies included. In addition, relevant differences in safety between treatments are often not observed in the short term, making long-term data necessary. Nonetheless, given that calculation of PASI responses used NRI (i.e. all patients who discontinued for any reason, including adverse events, were considered as nonresponders), indicates that at least an indirect element of tolerability was taken into account.
Strengths and limitations
Strengths of these analyses include the robust and well-established methodology, which is underscored by the consistency of the results obtained using either AIC or MAIC, and also the consistency of the ixekizumab versus guselkumab comparison with the results of a recent head-to-head comparison (Citation14). AIC (Citation18) is appropriate in situations where results from trials comparing each treatment option with the same comparator (e.g. placebo) are available (Citation29). In MAIC, baseline differences in clinical characteristics and treatment effect modifiers between studies were adjusted for (Citation19), thereby reducing the risk of bias. In general, including active comparators (etanercept or ustekinumab) as comparator bridges was more informative than use of placebo only, because placebo response rates (especially for PASI 90 or PASI 100) were very low and therefore could be considered noninformative with respect to variations in study design.
However, several factors should be considered when interpreting our results. Our analyses were not head-to-head comparisons between ixekizumab and the IL-23 p19 inhibitors, and the same limitations associated with all MAIC apply, including the assumptions that treatment effects are population dependent and that all treatment effect modifiers and prognostic factors were taken into account (Citation20). In addition, matching baseline characteristics and/or effect modifiers may not remove all confounders, and differences in trial populations, designs, and conduct may remain to influence treatment effects. Matching also reduced the numbers of patients available for inclusion in the MAICs, although trials conducted in patients with psoriasis generally have similar inclusion and exclusion criteria (as seen by the similarities in populations in Supplementary Tables 1–3), and report similar baseline characteristics and treatment effects, thus representing a good basis for performing meaningful and reproducible AICs and MAICs.
The analyses also considered published results to week 12 only, reflecting the limited long-term data from randomized controlled trials and the duration of common comparator data for all drugs in the analyses. Additionally, the included studies were, as planned, the initial published Phase III trials of the biologics of interest, and no systematic literature review for additional studies was performed. Finally, statistical difference was not found to confirm the trend for differences between ixekizumab and risankizumab when ustekinumab was used as the comparator bridge, likely because of the small sample size in IXORA-S. In addition, IXORA-S had wider inclusion criteria: patients with baseline PASI ≥10 were included; patients with baseline PASI <12 were therefore excluded during matching, further reducing the size of the available study population. Future research could include an NMA of published data and, depending on data availability, longer-term analyses.
In conclusion, our results suggest that ixekizumab can provide clinical benefits over the IL-23 p19 inhibitors guselkumab, tildrakizumab, and risankizumab in terms of faster onset of treatment effect and higher levels of early skin improvement up to week 12 in patients with moderate-to-severe psoriasis. Findings were generally consistent across the different analysis methods (AIC vs. MAIC), outcomes (PASI 75, 90, or 100 responses), and bridge comparators (placebo and other biologics). However, additional head-to-head trials, including long-term investigations, should be considered to confirm the relative efficacies of these agents and to compare their safety profiles.
Supplemental Material
Download PDF (205.4 KB)Acknowledgments
This study was sponsored by Eli Lilly & Company. Medical writing services were provided by Caroline Spencer and Dr Sue Chambers (Rx Communications, Mold, UK) and were funded by Eli Lilly & Company.
Disclosure statement
AG is a consultant/advisory board member for AbbVie, Allergan, Avotres Therapeutics, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corp., Dermira, Eli Lilly & Company, Incyte, Janssen Inc., Leo, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB, Valeant, and Xbiotech and has received research/educational grants from Boehringer Ingelheim, Incyte, Janssen Inc., Novartis, UCB, and Xbiotech. DS, SW, MD, and CS are employees of Eli Lilly & Company; DS, SW, and MD are also stockholders. SS has been an advisor for and/or received speaking fees from and/or served as an investigator in clinical trials for AbbVie, Bristol Myers Squibb, Eli Lilly & Company, Janssen-Cilag, Novartis, Pfizer, Sanofi Genzyme, and UCB. YR has received speaker honoraria and consultancy fees from Pfizer, AbbVie, Novartis, Janssen, and Eli Lilly & Company. DT has been a consultant and advisor for and/or received speaking fees and/or grants from and/or served as an investigator in clinical trials for AbbVie, Almirall, Amgen, Asana, Bioskin, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermira, DS-Biopharma, Eli Lilly & Company, Galapagos, Galderma, LEO Pharma, Janssen-Cilag, Kymab, Merck Sharp & Dohme, Morphosis, Novartis, Pfizer, Regeneron, Roche, Sandoz, Sanofi, Samsung, and UCB.
Data availability
Data are not publicly available.
Additional information
Funding
References
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD011535.
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5–9.
- Griffiths CE, Reich K, Lebwohl M, et al. UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551.
- Gordon KB, Blauvelt A, Papp KA, et al. UNCOVER-1 study group; UNCOVER-2 study group; UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356.
- Reich K, Pinter A, Lacour JP, et al.; on behalf of the IXORA-S investigators. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4):1014–1023.
- Langley RG, Elewski BE, Lebwohl M, et al. ERASURE study group; FIXTURE study group. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409.
- Young MS, Horn EJ, Cather JC. The ACCEPT study: ustekinumab versus etanercept in moderate-to-severe psoriasis patients. Expert Rev Clin Immunol. 2011;7(1):9–13.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288. Erratum in: Lancet. 2017;390(10091):230.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–839.
- Blauvelt A, Papp K, Gottlieb A, et al. On behalf of the IXORA-R Investigators. A head-to-head comparison of ixekizumab versus guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety, and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2019. [Epub ahead of print]. DOI:https://doi.org/10.1111/bjd.188511323
- Haute Autorité de Santé. Indirect comparisons. Methods and validity. July 2009. [cited 2018 Oct 11]. Available from: https://www.has-sante.fr/portail/upload/docs/application/pdf/2011-02/summary_report__indirect_comparisons_methods_and_validity_january_2011_2.pdf.
- US Preventive Services Task Force. Section 6. Methods for arriving at a recommendation. U.S. Preventive Services Task Force. July 2017. [cited 2018 Oct 11]. Available from: https://www.uspreventiveservicestaskforce.org/Page/Name/section-6-methods-for-arriving-at-a-recommendation.
- Laws A, Kendall R, Hawkins N. A comparison of national guidelines for network meta-analysis. Value Health. 2014;17(5):642–654.
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
- Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28(10):935–945.
- Phillippo DM, Ades Ae Dias S, Dias S, et al. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. Report by the Decision Support Unit. December 2016. [cited 2018 Oct 11]. Available from: http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2017/05/Population-adjustment-TSD-FINAL.pdf
- Reich K, Burden AD, Eaton JN, et al. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta-analysis of randomized controlled trials. Br J Dermatol. 2012;166(1):179–188.
- Nash P, McInnes IB, Mease PJ, et al. Secukinumab versus adalimumab for psoriatic arthritis: Comparative effectiveness up to 48 weeks using a matching-adjusted indirect comparison. Rheumatol Ther. 2018;5(1):99–122.
- Strand V, Betts KA, Mittal M, et al. Comparative effectiveness of adalimumab versus secukinumab for the treatment of psoriatic arthritis: a matching-adjusted indirect comparison. Rheumatol Ther. 2017;4(2):349–362.
- Warren RB, Brnabic A, Saure D, et al. Matching-adjusted indirect comparison of efficacy in patients with moderate-to-severe plaque psoriasis treated with ixekizumab vs. secukinumab. Br J Dermatol. 2018;178(5):1064–1071.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417.
- Australian Government Department of Health. Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee (version 5.0). 2016. Sep [cited 2019 Jan 27]. Available from: https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf
- Blome C, Gosau R, Radtke MA, et al. Patient-relevant treatment goals in psoriasis. Arch Dermatol Res. 2016;308(2):69–78.
- Kromer C, Schaarschmidt ML, Schmieder A, et al. Patient preferences for treatment of psoriasis with biologicals: a discrete choice experiment. PLoS One. 2015;10(6):e0129120.
- Kiefer C, Sturtz S, Bender R. Indirect comparisons and network meta-analyses. Dtsch Arztebl Int. 2015;112(47):803–808.
