Abstract
Objectives
To identify meaningful treatment attributes and quantify patient preferences for attributes of systemic atopic dermatitis (AD) treatments.
Materials and methods
Qualitative interviews were conducted with adults with moderate-to-severe AD (N = 21) to identify AD treatment attributes that patients consider most important and inform attribute selection for an online discrete-choice experiment (DCE) survey administered to patients in the United States with moderate-to-severe AD. Participants identified probability of clear/almost clear skin at 16 weeks, time to itch relief, mode of administration, and safety risks as very important. DCE data were analyzed using a random-parameters logit model to estimate the relative importance of treatment attributes and maximum acceptable risk.
Results
A total of 320 respondents completed the DCE survey (74% female; mean age, 35 years). Annual risk of malignancy was the most important attribute, followed by mode of administration, probability of clear skin at 16 weeks, and time to onset of itch relief. Respondents preferred daily oral treatment over injectable treatment. Respondents were willing to accept increases in adverse event risks for improvements in efficacy and mode of administration.
Conclusion
The findings of this study can help inform joint patient-physician decision making in managing moderate-to-severe AD.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease that is characterized by intense itching (pruritus), dry skin, redness, exudation, and pain (Citation1–5). AD is common, affecting up to approximately 20% of children and adolescents and approximately 5–10% of adults (Citation6–9), and is associated with a substantial economic and quality-of-life burden (Citation10,Citation11).
Treatments for AD include emollients (e.g. creams, lotions, ointments), topical corticosteroids (e.g. hydrocortisone, triamcinolone acetonide), topical calcineurin inhibitors (e.g. tacrolimus, pimecrolimus), phosphodiesterase-4 inhibitors (crisaborole), systemic oral and injectable treatments, and phototherapy (Citation1,Citation12–14). Despite conventional systemic immunomodulators being recommended for the management of moderate-to-severe AD, only a few are licensed for this indication (i.e. systemic corticosteroids in the United States and cyclosporine in Europe) (Citation1,Citation15,Citation16). Dupilumab, an interleukin (IL)-4 receptor alpha antagonist (Citation1), is a relatively new systemic therapy (licensed by the US Food and Drug Administration in March 2017 and the European Medicines Agency in September 2017) available as a subcutaneous injection. Several systemic treatments are currently being developed to expand the armamentarium for moderate-to-severe AD, including oral Janus kinase (JAK) inhibitors (i.e. abrocitinib, baricitinib, and upadacitinib) and injectable anti-IL-13 antibodies (i.e. tralokinumab and lebrikizumab) that have shown promise in earlyphase clinical studies (Citation17–22).
With this potential influx of additional systemic treatment options, it is important to understand how and to what degree the attributes of systemic treatments are valued by patients to improve patient-physician decision making. Unfortunately, no data exist on patient preferences for systemic AD treatments in the United States and Europe. One study was conducted in Japan, which reported that the top 3 attributes for the patients are risk of mild side effects, time until response, and efficacy of reducing itching; however, this study focused more on the differences between the top attributes for patients and physicians for injection treatments (Citation23).
This study, the first of its kind in the United States and the United Kingdom, was designed to address this gap in the literature with 2 objectives. The first objective was to conduct qualitative interviews to identify the AD treatment attributes that patients with moderate-to-severe AD consider most important when making treatment decisions. The second objective was to quantify patient preferences for the systemic AD treatment attributes that emerged from these qualitative interviews and differentiate between systemic treatments using a discrete-choice experiment (DCE).
Materials and methods
Survey development
Qualitative interviews
In-depth face-to-face qualitative interviews were conducted with adult (≥18 years) patients with AD (N = 21). A mix of moderate to very severe disease severity levels, as measured by the Patient-Oriented Eczema Measure (POEM) (Citation24), was represented in the recruitment (16 moderate, 5 severe (inclusive of very severe)). Participants were identified by medical recruiters at qualitative research firms in the United States (L&E Research, Raleigh, NC) and the United Kingdom (Acumen, Manchester, England). Screening criteria for patients were as follows: (1) self-reported diagnosis of AD, (2) diagnosed ≥3 months prior to screening, and (3) experience with prescription or over-the-counter treatment for AD. Research materials were reviewed and approved by Research Triangle Institute’s institutional review board (STUDY00020631); all patients provided informed consent prior to their participation.
All interviews were conducted using the same semistructured interview guide. To elicit a comprehensive list of treatment attributes that influence preference, participants were first asked about their experiences with AD and its treatments. Participants were then asked what they liked and did not like about current and previous treatments, as well as which factors would influence their decision to try a new treatment. Important concepts and dominant trends were identified across interviews to generate themes through a thematic analysis method (Citation25). Results from the qualitative interviews were used to develop a set of treatment attributes that (1) reflect the priorities of adult patients with moderate-to-severe AD and (2) potentially differentiate between systemic AD treatments. During the qualitative work it emerged that the most important attributes when selecting a new treatment were, in no particular order: (1) time to onset of itch relief, (2) probability of skin clearance, (3) frequency or ease of administration (convenience), and (4) safety. These 4 attributes were among the most frequently reported and included in the top 5 attributes affecting patient preference.
Selection of attribute levels
Both efficacy measures (time to onset of itch relief and probability of skin clearance) were included in the DCE, and published clinical data on available/investigational moderate-to-severe AD treatments were used to guide the selection of ranges for the levels of these attributes (Citation17–19,Citation26).
For safety, the study focused on long-term adverse events that are characteristic of systemic immunosuppressants and the overall JAK class, including the risk of serious infections, risk of venous thromboembolism, and risk of malignancy. Because the ranges for these events among a moderate-to-severe AD population using systemic therapies were not available at the time of this study, we used published data from patients with inflammatory disease states (e.g. rheumatoid arthritis) treated with similar systemic treatment options (Citation27–29). For frequency of administration, we included the 2 most common administration options: a biweekly injection or daily oral pill.
Pretest interviews
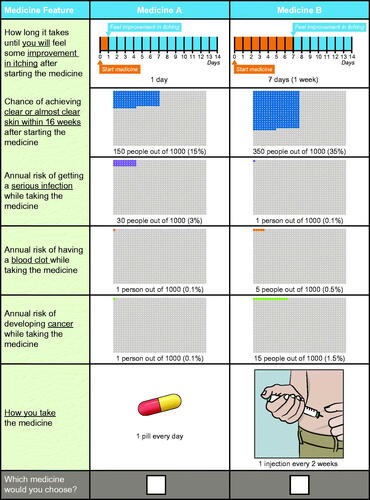
The draft survey instrument (inclusive of the DCE) was pretested in one-on-one qualitative interviews with a convenience sample of 15 participants with AD in Raleigh, NC. The pretest interviews confirmed that the attribute descriptions included in the DCE survey instrument were easily understood, that the overall survey length was appropriate, and that participants were willing to make tradeoffs among treatment attributes and levels and accepted the hypothetical scenario used to contextualize the survey instrument. Participants in the pretest interviews expressed difficulty making tradeoffs between two adverse events (venous thromboembolism and malignancy risks) and efficacy because the levels of these risks were perceived to be too low to affect the treatment decision. To ensure that the range of levels of risk was wide enough to facilitate tradeoffs, the upper bound of the risk ranges for each of these attributes was increased from 1.0% (based on the literature (Citation28,Citation29)) to 1.5%. No other changes to the list of attributes or the levels of each attribute were made. The final attributes and levels are shown in , and an example of a choice question from the DCE is shown in .
Table 1. Attributes and levels included in the discrete-choice experiment questions.
Final survey administration
The final survey included basic demographic and health history questions along with the DCE. The DCE methodology, which follows good research practices, is considered the most suited to quantify preferences and has been widely employed to quantify respondents’ preferences and the tradeoffs they are willing to accept between the benefits and risks of treatments (Citation30–33). Because there is a limit to the number of choice questions each respondent can reasonably answer before becoming fatigued, the DCE experimental design was split into equally sized blocks, each with 12 unique choice questions. The survey was programmed and hosted online, and respondents were randomly assigned to 1 block of 12 choice questions randomized to avoid ordering effects.
The survey was administered to adults in the US recruited through an online patient panel (Kantar; New York, NY). Inclusion criteria were as follows: (1) age ≥18 years, (2) self-reported physician diagnosis of AD, and (3) moderate-to-severe AD based on either self-reported treatment history (i.e. currently taking an immunosuppressant or a biologic for AD) or a POEM total score ≥8.
Statistical analyses
Demographic and health history variables were reported descriptively. Choice data from the DCE exercise were analyzed using a random-parameters logit (RPL) model. The RPL model relates treatment choices from each respondent to the attribute levels of each treatment profile in the sequence of choice questions. Using an RPL model is consistent with good research practices and prior precedence for regulatory decision making (Citation31); it accommodates unobserved preference heterogeneity, avoiding estimation bias from unobserved variation in preferences across the sample and within-sample correlation in the series of choices of each respondent. All variables were effects-coded; hence, the mean effect for each attribute was normalized at zero.
The RPL model results in a preference weight for each attribute level. The conditional relative importance of each attribute was calculated as the difference between the attribute level with the highest preference weight and the one with the lowest preference weight, to allow for comparisons across attributes. Preference weights were used to calculate the maximum acceptable percentage-point increase in the risks of adverse events respondents would trade off for each of the changes in each of the remaining attributes. In other words, this analysis was used to determine the increase in risk that exactly offsets an improvement in a specific benefit, all else being equal between alternative treatments. This was computed as the negative ratio of the difference in utility for the positive change in two levels of an attribute to the disutility generated by a unit change in the risk. Since risk was coded as categorical, the disutility between two levels was assumed to be linear between each pair of risk levels included in the survey instrument.
RPL subgroup models were used to assess whether preferences varied as a function of prespecified subgroups (i.e. eczema severity, treatment experience, itch severity, serious infection history, blood clot history, blood thinner use, years since first AD diagnoses, sex, and ethnicity). For each subgroup model, a subgroup-specific, binary variable was interacted with the variables in the main RPL model. A statistically significant p-value from a chi-square test of the joint significance of the interaction terms indicates whether preferences between subgroup pairs are statistically significantly different (Citation34).
Results
Respondent characteristics
A total of 325 respondents completed the online survey; however, 5 respondents were excluded from the analyses because their choices did not show any variability (i.e. they always selected either ‘Medicine A’ or ‘Medicine B’). The final sample of 320 was young (mean age, 35 years), predominantly female (73.8%), and predominantly white (74.7%) (). The median age at diagnosis of AD was 17 years.
Table 2. Demographic characteristics of the sample.
Preference weights
Parameter estimates from the RPL model are presented in (Citation35) and graphically depicted in to facilitate the interpretation of the preference weights. Preference weights for efficacy and risk attributes were ordered as expected, with better outcomes being preferred to worse outcomes. On average, respondents preferred faster time to onset of itch relief; a higher probability of skin clearance at 16 weeks; and lower annual risks of serious infection, venous thromboembolism, and malignancy. Respondents also preferred a daily pill to an injection every 2 weeks.
Figure 2. Random-parameters logit model estimates: preference weights (N = 320). Attributes are presented in the order in which they appeared in the discrete-choice experiment questions. The vertical bars around each mean preference weight (PW) represent the 95% confidence interval around the point estimate. Within each attribute, a higher PW indicates that a level is more preferred. For example, on average, respondents preferred a 50% probability of skin clearance at 16 weeks (PW = 0.642) more than a 35% probability (PW = 0.101). The change in utility associated with a change in the levels of each attribute is represented by the vertical distance between the PWs for any 2 levels of that attribute. Larger differences between PWs indicate that respondents viewed the change as having a relatively greater effect on overall utility. For example, reducing the time to onset of itch relief from 7 days to 1 day is preferable to reducing the probability of a serious infection from 1% to 0.1% because it has nearly 2 times (0.63/0.35) more impact on utility, all else being constant. Within each attribute, the sum of the PW equals 0.
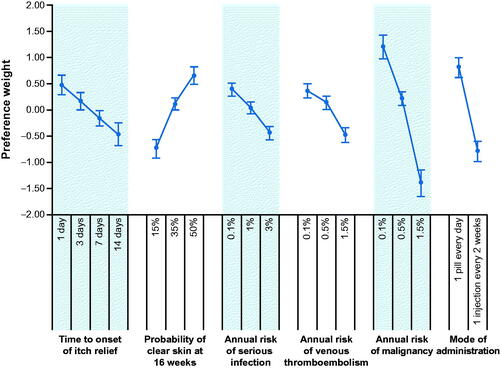
Table 3. Random-parameters logit model estimates: preference weights (N = 320).
The annual risk of malignancy was the most important attribute given the ranges of attribute levels included in the study; indeed, a change in the levels of this attribute from the lowest (0.1%) to the highest risk (1.5%) yielded the largest change in utility. The next most important changes (moving from an injection every 2 weeks to 1 pill every day and improving the probability of clear skin at 16 weeks from 15 to 50%) were approximately equally important, as the confidence intervals indicated that the estimated differences are not statistically different from each other. Reducing the time to onset of itch relief from 14 days to 1 day and reducing the annual risks of serious infection and venous thromboembolism were also approximately equally important given the ranges of onset of action and risks included in the study. It is important to highlight that these measurements of importance are conditional on the ranges of levels included in the DCE (e.g. smaller ranges across levels of a particular attribute would likely lead that attribute to be viewed as less important relative to others).
Maximum acceptable increases in risk
The results from the RPL model were also used to assess the respondents’ maximum acceptable increases in the risk of serious infection, venous thromboembolism, and malignancy for improvements in each of the efficacy attributes and preferred mode of administration (). On average, respondents were willing to accept more than the largest possible percentage-point increase in risk of serious infection presented in the DCE (2.9 percentage-points; i.e. the difference between the lower bound risk of 0.1% and the upper bound risk of 3%) to reduce the time to onset of itch relief from 14 days to 1 day, improve the probability of skin clearance at 16 weeks from 15% to 35% or 50%, or use a daily pill instead of an injectable medication.
Table 4. Maximum acceptable risk of serious infection, venous thromboembolism, and malignancy (N = 320).
Additionally, on average, respondents were willing to accept more than the largest possible percentage-point increase in risk of venous thromboembolism presented in the DCE (1.4 percentage-points; i.e. the difference between the lower bound risk of 0.1% and the upper bound risk of 1.5%) for either reducing the time to onset of itch relief from 14 days to 1 day, improving the probability of skin clearance at 16 weeks from 15 to 50%, or using a daily pill over an injectable medication (). On the other hand, respondents demonstrated a smaller risk tolerance for malignancy. Respondents were willing to accept increases in risk of malignancy of 0.38, 0.65, and 0.78 percentage points for reducing the time to onset of itch relief from 14 days to 1 day, improving the probability of skin clearance at 16 weeks from 15% to 50%, and using a daily pill over an injectable, respectively ().
Subgroup analyses
The standard deviations of the normal distribution associated with each attribute level in the RPL indicated that preferences varied among respondents. A subgroup analysis measured the differences in patient preferences between prespecified groups, and among 9 subgroups tested, those based on prior serious infection and current blood thinner use showed differences in preferences (). Reducing the risk of malignancy was more important among those without a history of serious infections (n = 215) compared with respondents with prior serious infections (n = 105) (). There were no notable differences in preferences between the two groups for annual risk of serious infections; furthermore, respondents with prior serious infections did not differentiate between 7 and 14 days for onset of itch relief, 35 and 50% for probability of skin clearance at 16 weeks, 0.1 and 1% for risk of serious infection, and 0.1 and 0.5% for risk of venous thromboembolism (). The preferences of respondents who reported using blood thinners (n = 33) were also different compared with those who do not (n = 287), although caution should be applied given the small sample size, large confidence intervals, and disordering of the preference weights (; ).
Figure 3. Random-parameters logit model estimates for the (A) serious infection subgroup and (B) blood thinner subgroup (N = 320). (A) Attributes are presented in the order in which they appeared in the discrete-choice experiment questions. The vertical bars around each mean preference weight (PW) represent the 95% confidence interval around the point estimate. Within each attribute, a higher PW indicates that a level is more preferred. For example, on average, respondents without serious infection experience preferred a 50% probability of skin clearance at 16 weeks (PW = 0.837) more than a 35% probability (PW = 0.008). The change in utility associated with a change in the levels of each attribute is represented by the vertical distance between the PW for any 2 levels of that attribute. Larger differences between PWs indicate that respondents viewed the change as having a relatively greater effect on overall utility. For example, among those without serious infection experience, reducing the annual risk of malignancy from 1.5% to 0.1% (change in PW = ΔPW = 1.47 − [−1.746] = 3.216) is preferable to reducing the annual risk of venous thromboembolism from 1.5% to 0.1% (ΔPW = 0.431 − [−0.541] = 0.972) because it has approximately 3 times (3.216/0.972) more impact on utility, all else being constant. (B) Within each attribute, a higher PW indicates that a level is more preferred. For example, on average, respondents not currently taking a blood thinner preferred a 0.1% annual risk of malignancy (PW = 1.330) more than a 1.5% annual risk of malignancy (PW = −1.529). The change in utility associated with a change in the levels of each attribute is represented by the vertical distance between the PWs for any 2 levels of that attribute. Larger differences between PWs indicate that respondents viewed the change as having a relatively greater effect on overall utility. For example, among those not currently taking a blood thinner, reducing the annual risk of malignancy from 1.5% to 0.1% (ΔPW = 1.33 − [−1.529] = 2.859) is preferable to reducing the annual risk of venous thromboembolism from 1.5% to 0.1% (ΔPW = 0.364 − [−0.502] = 0.866) because it has approximately 3 times (2.859/0.866) more impact on utility, all else being constant.
![Figure 3. Random-parameters logit model estimates for the (A) serious infection subgroup and (B) blood thinner subgroup (N = 320). (A) Attributes are presented in the order in which they appeared in the discrete-choice experiment questions. The vertical bars around each mean preference weight (PW) represent the 95% confidence interval around the point estimate. Within each attribute, a higher PW indicates that a level is more preferred. For example, on average, respondents without serious infection experience preferred a 50% probability of skin clearance at 16 weeks (PW = 0.837) more than a 35% probability (PW = 0.008). The change in utility associated with a change in the levels of each attribute is represented by the vertical distance between the PW for any 2 levels of that attribute. Larger differences between PWs indicate that respondents viewed the change as having a relatively greater effect on overall utility. For example, among those without serious infection experience, reducing the annual risk of malignancy from 1.5% to 0.1% (change in PW = ΔPW = 1.47 − [−1.746] = 3.216) is preferable to reducing the annual risk of venous thromboembolism from 1.5% to 0.1% (ΔPW = 0.431 − [−0.541] = 0.972) because it has approximately 3 times (3.216/0.972) more impact on utility, all else being constant. (B) Within each attribute, a higher PW indicates that a level is more preferred. For example, on average, respondents not currently taking a blood thinner preferred a 0.1% annual risk of malignancy (PW = 1.330) more than a 1.5% annual risk of malignancy (PW = −1.529). The change in utility associated with a change in the levels of each attribute is represented by the vertical distance between the PWs for any 2 levels of that attribute. Larger differences between PWs indicate that respondents viewed the change as having a relatively greater effect on overall utility. For example, among those not currently taking a blood thinner, reducing the annual risk of malignancy from 1.5% to 0.1% (ΔPW = 1.33 − [−1.529] = 2.859) is preferable to reducing the annual risk of venous thromboembolism from 1.5% to 0.1% (ΔPW = 0.364 − [−0.502] = 0.866) because it has approximately 3 times (2.859/0.866) more impact on utility, all else being constant.](/cms/asset/63faa536-98d0-4883-b40e-ed6a0fe03504/ijdt_a_1832185_f0003_c.jpg)
Table 5. Discrete-choice experiment subgroup analysis: Subgroup description and results of the test of joint significance of the interaction terms (N = 320).
On average, a history of serious infections did not appear to influence the importance of avoiding the risk of future serious infection. Similarly, current usage of blood thinners did not appear to influence the importance of avoiding the risk of venous thromboembolism.
Discussion
This was one of the first studies (first in the United States and the United Kingdom) to our knowledge to assess patient preferences for systemic treatment options for moderate-to-severe AD. A study conducted in Japan, which assessed preferences among patients with moderate-to-severe AD focused more on differences between preferences of patients and physicians, specifically regarding attributes of injection treatments (Citation23). Qualitative interviews from our study indicated that for systematic treatments, respondents value probability of skin clearance, time to onset of itch relief, convenient dosing/administration schedule, and avoidance of safety risks.
The results of the DCE indicated that for efficacy, probability of skin clearance at 16 weeks was the most important attribute, followed closely by time to onset of itch relief. Respondents preferred daily oral treatments to biweekly injections. From a safety perspective, the avoidance of annual risk of malignancy was most important followed by avoidance of annual risk of serious infections and venous thromboembolism, which were equally important. Higher risks, such as a 1.5% annual risk of malignancy, were significantly associated with reduced preference.
Although malignancy risk was considered the most important across all attributes (followed by probability of skin clearance at 16 weeks, mode of administration, time to onset of itch relief, serious infection risk, and venous thromboembolism risk, in that order), it is necessary to note that this result is conditional on the ranges assessed by the design of this DCE. Because data on the rates of adverse events among the moderate-to-severe AD population are lacking, the ranges were largely derived from published data for patients with other the immunological conditions, such as rheumatoid arthritis (Citation27–29), who have a presumably higher background risk for malignancy, serious infection, and venous thromboembolism. Assuming that the different patient groups have the same preferences, if background risks of these adverse events among patients with AD are indeed lower, and smaller ranges of these risks were used in the DCE, then the degree of importance respondents placed on these events may have also been lower.
The results suggest that differences in preferences across respondents in the sample were not explained by common demographic and clinical characteristics including sex, ethnicity, and disease severity. Patient preferences did vary by history of serious infection and potentially by prior blood thinner use, suggesting that prior experience with specific events may influence patient preferences. However, on average preferences regarding the attributes for a risk of serious infection or a risk of venous thromboembolism did not vary between their respective subgroups (i.e. history of serious infection and current use of blood thinners). Other preferences did vary across these groups but in generally subtle ways; if anything, patients with these experiences were less risk averse, but further research is needed to confirm this.
Although the intent of this study was not to establish a preference for a particular treatment over another, the results could be viewed in the context of the various profiles of systemic treatment options. In general, oral treatments (e.g. systemic immunosuppressant agents and JAK inhibitors in development) may be preferred over injectable biologics (e.g. dupilumab and other cytokine inhibitors in development) by most patients, all else being equal. Janus kinase 1 (JAK1) selective inhibitors, including abrocitinib and upadacitinib, in particular, have been associated with a particularly rapid reduction in itch as well as generally high probability of skin clearance at weeks 12 and 16 of treatment, respectively (17,19), which suggests these products would be preferred on those grounds.
Dupilumab has not demonstrated increased risks for the long-term safety issues assessed in this study and, therefore, may be preferred over currently available systemic immunosuppressants (e.g. methotrexate, azathioprine) that have established increased malignancy risks (Citation13). There are potential safety concerns with the JAK inhibitor class including serious infections, venous thromboembolism, and malignancy (Citation36). However, the specific frequencies of these risks are still under evaluation in AD. This study showed that the risk of malignancy was highly relevant for patients, but they placed more value on efficacy and convenience (i.e. ease of administration) attributes relative to the risks of serious infection and venous thromboembolism.
The results should be interpreted within the context of common limitations for this type of study. The sample of respondents is a convenience sample recruited through panels of individuals who self-reported a diagnosis with AD. Patient characteristics and preferences may not reflect the preferences and characteristics of the broader population of patients with AD. Additionally, the treatment profiles and choice pairs presented in the survey are hypothetical and, although attributes and levels were defined on existing or potential future systemic AD treatments, the study was not designed to predict actual choices or present real treatment options in real healthcare settings. DCEs, however, are a well-established method for eliciting patient preferences for treatment features, and this study followed the applicable good practices for such study designs (Citation30,Citation31,Citation33).
Adults with moderate-to-severe AD preferred a higher probability of skin clearance at 16 weeks, faster time to onset of itch relief, oral administration, and lower long-term safety risks. Respondents were willing to accept higher risks of serious infections, venous thromboembolisms, and malignancies to have a once-daily oral medicine over a twice-monthly injectable and to improve the time to onset of itch relief and probability of skin clearance at 16 weeks. This research highlights the patient perspective surrounding the relevant benefits and risks of different AD systemic treatments, which can help inform joint patient-physician decision making.
Acknowledgments
Medical writing support under the guidance of the authors was provided by Irene Park, PhD, and Jennifer Jaworski, MS, at ApotheCom, San Francisco, CA, and was funded by Pfizer Inc., New York, NY, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464).
Disclosure statement
MB, JS, and BH are employees of RTI Health Solutions, which received funds from Pfizer Inc. to conduct this study. JCC, WR, and MD are employees and shareholders of Pfizer Inc.
Additional information
Funding
References
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10–22 e2.
- Newton L, DeLozier AM, Griffiths PC, et al. Exploring content and psychometric validity of newly developed assessment tools for itch and skin pain in atopic dermatitis. J Patient Rep Outcomes. 2019;3(1):42.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Pain is a common and burdensome symptom of atopic dermatitis in United States Adults. J Allergy Clin Immunol Pract. 2019;7(8):2699–2706.e7.
- Suarez-Farinas M, Tintle SJ, Shemer A, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127(4):954–964. e1-4.
- Vakharia PP, Chopra R, Sacotte R, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548–552 e3.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in america study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132(5):1132–1138.
- Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103(1):125–138.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77(2):274–279 e3.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132.
- Sidbury R, Davis DM, Cohen DE, et al.; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–349.
- Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: Section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71(6):1218–1233.
- Drucker AM, Ellis AG, Bohdanowicz M, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol. 2020;156(6):659–667.
- Wollenberg A, Barbarot S, Bieber T, et al.; the European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371–1379.
- Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913–921.e9.
- Guttman-Yassky E, Thaci D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863.
- Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78(5):863–871 e11.
- Wollenberg A, Howell MD, Guttman-Yassky E, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143(1):135–141.
- Okubo Y, Ho KA, Fifer S, et al. Patient and physician preferences for atopic dermatitis injection treatments in Japan. J Dermatol Treat. 2020;31(8):821–830.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513–1519.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348.
- Bechman K, Subesinghe S, Norton S, et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford). 2019;58(10):1755–1766.
- Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology (Oxford). 2019;58(Suppl 1):i34–i42.
- Scott IC, Hider SL, Scott DL. Thromboembolism with janus kinase (JAK) inhibitors for rheumatoid arthritis: how real is the risk? Drug Saf. 2018;41(7):645–653.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health-a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413.
- Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–315.
- Marshall D, Bridges JF, Hauber B, et al. Conjoint analysis applications in health - how are studies being designed and reported?: An update on current practice in the published literature between 2005 and 2008. Patient. 2010;3(4):249–256.
- Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13.
- Greene WH. Econometric analysis. 7th ed. Upper Saddle. River (NJ): Pearson; 2012.
- Hensher DA, Rose JM, Greene WH. Applied choice analysis. 2nd ed. Cambridge (UK): Cambridge University Press; 2005.
- Bechman K, Yates M, Galloway JB. The new entries in the therapeutic armamentarium: The small molecule JAK inhibitors. Pharmacol Res. 2019;147:104392.