Abstract
Background
Biologic psoriasis treatments are differentiated by efficacy, side effects, and other attributes.
Objective
Determine attributes of biologic psoriasis treatments that drive patients’ treatment choices.
Methods
Respondents (USA: n = 300; Germany: n = 300) with moderate-to-severe psoriasis completed a discrete-choice–experiment survey, choosing between hypothetical treatments characterized by attributes with varying levels: chance of clear skin after 1 year, number of first-year treatments, first-year risks of mild-to-moderate injection site reaction (ISR) and serious infection, and years of proven efficacy/safety.
Results
U.S. respondents most valued clear skin (conditional relative importance, 1.88; p < .05). While other attributes were of generally equivalent importance, ISR risk outweighed serious-infection risk (1.06 vs. 0.70; p < .05). German respondents placed greatest importance on ISR risk (1.61; p < .05) and clear skin (1.49; p < .05).
Limitations
Respondents evaluated hypothetical treatments and were recruited from web panels.
Conclusions
Clear skin and ISR risk are stronger drivers of treatment choice than injection frequency and infection risk.
Introduction
Psoriasis is a heterogeneous, chronic inflammatory disease. Although the most common form is plaque psoriasis, which appears on the skin as thick, red, scaly patches, there are multiple manifestations, some of which can affect the nails and joints. Moderate-to-severe psoriasis has a significant negative impact on quality of life and requires lifelong treatment. According to the joint American Academy of Dermatology–National Psoriasis Foundation guideline, psoriasis treatments should be tailored to each patient’s situation and preferences (Citation1). Various injectable biologics, with generally good safety and efficacy profiles, are licensed for moderate-to-severe plaque psoriasis. These biologics are differentiated by efficacy (in terms of skin clearance), side effects, and other important attributes.
Previous research has explored patient and physician preferences for the attributes of biologic treatments for psoriasis, including attributes of efficacy, safety, and mode of administration (Citation2–8). Studies focusing on preferences in a multicountry population have been limited (Citation9,Citation10), and to our knowledge, no studies to date have explored preferences for treatment attributes beyond those related to efficacy, safety, and mode of administration, such as a treatment’s effectiveness in improving multiple manifestations of psoriasis or the extent of the clinical evidence available.
A greater understanding of patient preferences for psoriasis treatment can facilitate shared decision-making and, by extension, potentially improve patients’ satisfaction with and adherence to treatment (Citation8). The primary objective of this study was to conduct a discrete-choice experiment (DCE) study to measure preferences for treatment features of biologic treatments for moderate-to-severe plaque psoriasis in the USA and Germany, both major markets for psoriasis treatments. A secondary objective was to explore whether preferences varied systematically among patient subgroups defined by disease severity and experience with systemic or biologic treatments, psoriatic arthritis (PsA), or injection site reactions (ISRs).
Materials and methods
Study design
In the DCE, respondents chose between pairs of hypothetical treatment profiles in a series of questions. Each treatment profile was defined by attributes with varying levels; profiles and pairs were determined by an experimental design. Through the pattern of respondents’ choices, the relative importance of attributes could be determined.
Survey development
A cross-sectional DCE survey was developed following best practices (Citation11). The attributes and levels included in the DCE reflect selected differences in the biologic treatments approved to treat psoriasis at the time of the survey because the goal of the study was to understand patients’ preferences for this set of differences. Attributes were also refined based on the published literature (Citation2–6,Citation12–17), input from U.S. and German medical experts, and input from a patient advocate with moderate-to-severe psoriasis to ensure that the attributes and descriptions were patient-centered and clinically relevant. Selection of attribute levels was informed by the prescribing information and recent clinical evidence (Citation18–31). The final set of attributes included chance of clear or almost-clear skin (representing a 90% reduction in the Psoriasis Area and Severity Index [PASI 90]), risk of side effects (defined as risk of serious infections), annual dosing frequency, risk of mild-to-moderate ISRs, and number of years of efficacy and safety data the treatment has from large clinical trials ().
Table 1. Treatment attributes.
A statistically efficient experimental design determined the combinations of attribute levels in each hypothetical treatment alternative and how alternatives were paired in the choice questions. The resulting partial-factorial design included 36 unique choice pairs, divided into four blocks of nine choice pairs each. Each respondent was randomly assigned a block of questions, each asking the respondent to choose between two hypothetical alternatives. In a series of exploratory questions, patients also ranked the importance of the DCE attributes, plus five additional patient-relevant psoriasis treatment attributes ().
Respondents also ranked the importance of an expanded list of treatment attributes in an exploratory, direct-ranking exercise that included five attributes not included in the DCE but considered relevant to understanding patient preferences for psoriasis treatment. We asked respondents to select the most important and least important features from a list of ten medicine features. After removing these two features from the list, the respondents selected the most and least important from the remaining eight medicine features. This process was repeated five times until a full ranking of the ten features was elicited.
The survey was drafted in U.S. English and translated and culturally adapted for Germany. Both survey instruments were refined based on qualitative pretesting interviews with convenience samples of 15 native speakers in each country.
Study population
The online survey panel and sampling organization Survey Sampling International (SSI) recruited patients aged ≥18 years in the USA and Germany by e-mail from opt-in web panels of individuals willing to participate in health-related research. Eligible respondents reported having been told by a physician that they had moderate-to-severe plaque psoriasis (but could have mild-to-moderate psoriasis at the time of the survey due to a fluctuating disease course or the effects of treatment). Respondents assessed psoriasis severity at the time of the survey with a self-assessed Psoriasis Area Severity Index (SAPASI) score (moderate-to-severe psoriasis: > 10 or of 5–9.9 with plaques in visible/sensitive areas). Respondents in the USA and Germany completed the survey online in January 2018 and June 2018, respectively.
Although minimum sample size calculations are rarely conducted for DCE studies, most published choice experiments have a sample size of 100–300 (Citation32,Citation33). A sample size of 300 respondents per country was considered sufficient to generate preference weights with acceptable precision for this study (Citation34). Once these targets were reached, data collection was discontinued. The study was deemed exempt from full review by the RTI International institutional review board.
Statistical analyses
To estimate the relative importance of the attributes, the data from the DCE were analyzed using a random-parameters logit (RPL) model estimated using 200 Halton draws in Nlogit 5.0. The model yielded preference weights for the levels of each attribute (representing the strength of patients’ preferences for attribute levels). These results were used to compute the perceived importance to respondents of changes in attribute levels; the importance of the attributes relative to one another, conditional on the range of attributes and levels included in the study (i.e. conditional relative attribute importance); and the predicted probability that patients would choose one of three fixed multiattribute treatment profiles ().
Analyses were conducted separately for U.S. and German respondents. A Wald test was used to explore systematic differences in preferences among prespecified subgroups defined by respondents’ disease severity, prior experience with biologic treatments, prior experience with conventional systemic or biologic treatments (in Germany only), self-report of physician diagnosed PsA, or experience with ISRs.
Results
Respondent characteristics
Among 14,636 U.S. individuals invited to be screened for eligibility, 300 respondents completed the survey. Average age was 46 years, and 39% of respondents were male (); 44% had moderate-to-severe psoriasis defined by SAPASI at the time of the survey; 50% were biologic experienced, 48% had previously experienced an ISR, and 49% had self-reported PsA. Among 36,818 German individuals invited to be screened for eligibility, 300 respondents completed the survey. Average age was 47 years, and 58% of respondents were male (); 40% had moderate-to-severe psoriasis, 33% had experience with biologic treatments, 35% had previously experienced an ISR, and 61% had self-reported PsA.
Table 2. Respondent characteristics.
Relative preference weights and conditional relative attribute importance
Among U.S. and German respondents, the estimated preference weights for all attributes were consistent with the natural ordering of the levels ().
Figure 1. Preference weights and conditional relative importance of treatment attributes. (A) Preference weights: overall U.S. sample. (B) Preference weights: overall German sample. Preference weights, shown in dark blue, can be interpreted as weights indicating the relative strength of preference for each attribute level. The vertical bars surrounding each mean preference weight denote the 95% confidence interval about the point estimate. For both the U.S. and German samples, the estimated preference weights for all attributes were consistent with the natural ordering of the levels; that is, better outcomes were preferred to worse outcomes or features. For example, an 80% chance of clear or almost-clear skin after 1 year was preferred to a 60% chance, which was preferred to 50%. Although not directly comparable for the U.S. and German samples, the relative preference weights showed a similar and consistent trend. The conditional relative importance score for an attribute, shown in light blue, is the difference between the highest preference weight for any level of that feature and the lowest preference weight for any level of that feature. (C) Conditional relative attribute importance: biologic-experienced subgroups in the USA. (D) Conditional relative attribute importance: psoriatic arthritis subgroups in the USA. (E) Conditional relative attribute importance: moderate-to-severe injection site reactions subgroups in the USA. (F) Conditional relative attribute importance: moderate-to-severe injection site reactions subgroups in Germany. The conditional relative importance is the difference between the highest preference weight for any level of that feature and the lowest preference weight for any level of that feature. Conditional relative importance can be interpreted as the ranking of overall importance of the treatment attributes included in the DCE questions, given the range of attribute levels included in the DCE. DCE: discrete-choice experiment.
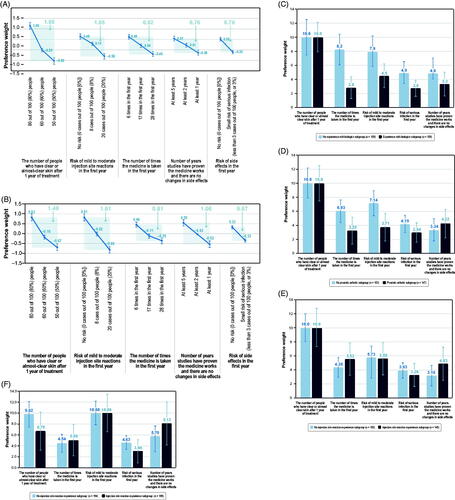
Among U.S. respondents, the most important attribute was clear skin (conditional relative importance score, 1.88; p < .05) (). The remaining attributes were similarly important to one another (p ≥ .05 for all), except that the risk of ISR was more important than the risk of serious infection (conditional relative importance scores, 1.06 vs. 0.70; p < .05).
Among German respondents, the most important attribute was the risk of mild-to-moderate ISRs (conditional relative importance score, 1.61), followed by clear skin (1.49) (). The conditional relative importance scores of these two attributes were not statistically significantly different from each other, but they were both greater than each of the other attributes (p < .05). The other attributes had similar importance (p ≥ .05 for all), except that the number of years of available efficacy and safety data were more important than risk of serious infections (p < .05).
Subgroup analyses
Subgroup analyses revealed systematically different preferences between biologic-experienced and biologic-naive respondents in the USA (chi-square statistic = 20.43; p = .02) (), between respondents with PsA and without PsA in the USA (chi-square statistic = 27.24; p < .01) (), and between patients with and without prior experience with mild-to-moderate ISRs in both the USA (chi-square statistic = 19.58; p = .02) and Germany (chi-square statistic = 27.27; p < .01) (). In both countries, preferences of respondents with moderate-to-severe psoriasis at the time of the survey were not statistically different from the preferences of respondents with mild psoriasis at the time of the survey (as defined by SAPASI).
Among the U.S. sample, respondents experienced with biologics prioritized clear skin over other attributes, which had similar importance to one another. Respondents without biologic experience prioritized clear skin, injection frequency, and risk of ISRs more than risk of infection and number of years of evidence. Respondents with prior experience with biologics placed more importance on clear skin relative to dosing frequency than respondents without biologic experience. Among respondents with PsA, clear skin was prioritized over the other attributes, which had similar importance to one another. Respondents without PsA prioritized clear skin, risk of ISR, and injection frequency more than risk of infection and long-term evidence. Respondents with PsA placed less importance on number of treatments per year, risk of mild-to-moderate ISR, and risk of serious infection than respondents without PsA. The two subgroups valued efficacy and years of evidence similarly.
Among the German sample, there was no statistically significant difference in preferences between biologic-experienced and biologic-naive respondents or between respondents with PsA and those without PsA.
In both U.S. and German samples, the preferences of respondents with ISR experience were statistically significantly different from those of respondents without such experience. In the U.S. sample, the number of people with clear or almost-clear skin after 1 year of treatment was the most important attribute for respondents in both subgroups. The least important attribute for respondents with ISR experience was risk of serious infection. For respondents with no ISR experience, the least important attribute was the number of years of evidence. In the German sample, the risk of mild-to-moderate ISR in the first year was the most important attribute for respondents in both subgroups. Whereas risk of ISR was approximately as important as clear skin for respondents with no ISR experience, it was approximately 1.5 times more important than clear skin to respondents with ISR experience. The least important attribute for respondents with ISR experience was risk of serious infection and for respondents with no such experience was the number times the medicine is taken in the first year.
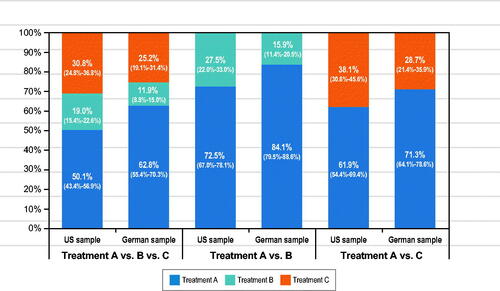
Predicted choice probabilities
Consistently among U.S. and German respondents, Medicine A was likely to be preferred to Medicines B and C in 2-way and 3-way comparisons (). Medicine A was preferred to Medicine C despite more frequent dosing with Medicine A. More frequent dosing with Medicine A was outweighed by no ISR and 5 years of proven efficacy and safety.
Medicine feature ranking
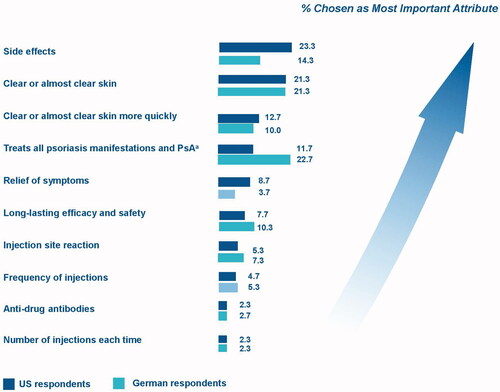
In the exploratory ranking exercise, U.S. respondents most frequently chose side effects, clear skin, time to achieve clear skin, and the ability of the treatment to treat all manifestations of psoriasis and PsA as the most important attributes (). German respondents – 61% of whom had self-reported PsA – most frequently chose a treatment’s ability to treat all manifestations of psoriasis and PsA, followed by clear skin, side effects, and duration of evidence (). In both samples, number of injections per dose and antidrug antibodies were ranked least important.
Figure 3. Summary of most important medicine features from expanded list. PsA: psoriatic arthritis. Note: Each respondent was asked to select the most important and least important feature over the course of five questions until a full ranking of features (1–10) was elicited for each respondent. The figure presents the percentage of respondents who chose each feature as the most important feature out of all 10 medicine features. Treatment’s ability to work on psoriatic arthritis and other manifestations of psoriasis (e.g., nail, palmoplantar, scalp).
Discussion
Consistent with previous preference studies (Citation5,Citation6,Citation17,Citation35), achieving clear or almost-clear skin was important to patient respondents in this study. However, less commonly assessed attributes (e.g. risk of ISR; ability to address manifestations of psoriasis other than skin plaques, including PsA; and years of proven efficacy and safety in large clinical studies) were also important. PsA was self-reported by proportionally more patients in our samples (49% of U.S. respondents and 61% of German respondents) than is observed in the general psoriasis population, approximately one-third of which has PsA (Citation36,Citation37). That proportionally more German respondents than U.S. respondents self-reported PsA may also explain why German patients more frequently ranked a medication’s ability to treat all manifestations of psoriasis and PsA as most important in the direct-ranking exercise.
Patients and physicians value both efficacy and safety and prioritize clear skin over safety (Citation2–7). However, the efficacy and safety attributes in our study are unique among DCEs of injectable psoriasis treatments in that efficacy was defined as probability of improvement (vs. severity/location of plaques, percentage of body area affected, time to improvement, or visual or nonvisual effects of treatment). Further, there is little to no information in the literature on the risk of ISRs or years of evidence as attributes.
PsA and psoriasis manifestations other than skin plaques were important drivers of preferences in this study. PsA is a common comorbidity in psoriasis and can lead to impaired functioning and disability if untreated (Citation37); thus, there is a need to educate patients and health care providers about the importance of managing PsA. In another study exploring the relative importance of psoriatic disease attributes, patients and physicians ranked the relative bother of 20 psoriatic symptoms differently in a best-worst scaling study (Citation38). Whereas physicians considered joint pain to be considerably more bothersome than painful skin, patients considered painful skin more bothersome than joint pain. These results emphasize that all manifestations of psoriatic disease should be considered in treatment decisions and that attributes beyond clear skin are drivers of preferences. As psoriasis is chronic and requires lifelong treatment, pain at injection and long-term data were key preferences for patients, as expected. Taken together, these results provide information on patients’ preferences and experiences from a real-world setting and can inform shared decision-making between patients and physicians. Further, such information can complement data on factors influencing patients’ satisfaction with treatment to characterize patients’ experience of inflammatory diseases (Citation39).
The study results should be interpreted in view of some strengths and limitations. The study was designed according to best practices for DCEs (Citation11,Citation40,41) and explored new attributes, including ISRs and the availability of long-term evidence. A ranking exercise tested the potential importance of additional attributes not included in the DCE, although discrepancies between exploratory direct-ranking and DCE results may be due to differences in attribute descriptions and question context. Furthermore, the study explored only a subset of the attributes of psoriasis treatment; additional attributes may be relevant to treatment choice (e.g. cost) but were not included to reduce the complexity of the design. Patients are often insulated from the true cost of drug, making this dimension less critical. The selected attributes and levels reflect differences in the available treatments, allowing discrimination among them in the preference analyses, and did not represent the actual treatment environment or all possible attributes. Further, because respondents evaluated hypothetical treatments, their choices among these treatments do not have the same significance as choices involving actual treatment decisions. Use of a web panel to recruit people with moderate-to-severe psoriasis may limit the representativeness of the sample and the generalizability of the results. Finally, all data were self-reported.
Conclusions
Although achieving clear or almost-clear skin was important to patients in this study, other attributes, including treating all manifestations of psoriasis including PsA and avoiding ISR, are also important. These findings may help patients and physicians make treatment decisions for moderate-to-severe psoriasis and highlight the importance of PsA management as a driver for treatment selection.
Acknowledgements
Kimberly Moon of RTI Health Solutions provided overall project management for this study. Caitriona O’Neill, Yvonne Geissbuehler, Becky Germino, Adriana Guana, and Nima Melzer of Novartis contributed to the study design and/or interpretation of the study results. Kate Lothman of RTI Health Solutions provided medical writing services, which were funded by Novartis.
Disclosure statement
This study was performed under a research contract between RTI Health Solutions and Novartis Pharma A/G and was funded by Novartis Pharma A/G. IG and KB are salaried employees of Novartis. CP, BM, and MB are salaried employees of RTI Health Solutions. MA has received consulting or lecturing fees and/or research grants from AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene Corporation, Centocor, Eli Lilly, GSK, Janssen-Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB, and XenoPort. MG has received consulting fees and/or travel expenses from Astellas Pharma, Lilly and Novartis Pharma. SF has received research, speaking, and/or consulting support from a variety of companies, including Novartis, Galderma, GSK/Stiefel, Leo Pharma, Baxter, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Valeant, AbbVie, Cosmederm, Anacor, Astellas, Janssen, Lilly, Merck, Merz, Qurient, National Biological Corporation, Caremark, Advance Medical, Suncare Research, Informa, UpToDate, and the National Psoriasis Foundation. He is founder and majority owner of www.DrScore.com, a patient satisfaction survey service, and is a founder and part owner of Causa Research, a company working to enhance patients’ adherence to treatment.
Additional information
Funding
References
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029–1072.
- Fairchild AO, Reed SD, Johnson FR, Anglin G, et al. What is clearance worth? Patients' stated risk tolerance for psoriasis treatments. J Dermatolog Treat. 2017;28:709–715.
- Ashcroft DM, Seston E, Griffiths CE. Trade-offs between the benefits and risks of drug treatment for psoriasis: a discrete choice experiment with U.K. dermatologists. Br J Dermatol. 2006;155:1236–1241.
- Kjaer T, Bech M, Gyrd-Hansen D, et al. Ordering effect and price sensitivity in discrete choice experiments: need we worry? Health Econ. 2006;15:1217–1228.
- Kauf TL, Yang JC, Kimball AB, et al. Psoriasis patients' willingness to accept side-effect risks for improved treatment efficacy. J Dermatolog Treat. 2015;26:507–513.
- Gonzalez JM, Johnson FR, McAteer H, et al. Comparing preferences for outcomes of psoriasis treatments among patients and dermatologists in the U.K.: results from a discrete-choice experiment. Br J Dermatol. 2017;176:777–785.
- Schaarschmidt ML, Herr R, Gutknecht M, et al. Patients' and physicians' preferences for systemic psoriasis treatments: a nationwide comparative discrete choice experiment (PsoCompare). Acta Derm Venereol. 2018;98:200–205.
- Sain N, Willems D, Charokopou M, et al. The importance of understanding patient and physician preferences for psoriasis treatment characteristics: a systematic review of discrete-choice experiments. Curr Med Res Opin. 2020;36;1257–1275.
- Boeri M, Saure D, Schacht A, et al. Modeling heterogeneity in patients' preferences for psoriasis treatments in a multicountry study: a comparison between random-parameters logit and latent class approaches. Pharmacoeconomics. 2020;38:593–606.
- Christophers E, Griffiths CE, Gaitanis G, et al. The unmet treatment need for moderate to severe psoriasis: results of a survey and chart review. J Eur Acad Dermatol Venereol. 2006;20:921–925.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health-a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–413.
- Schaarschmidt ML, Schmieder A, Umar N, et al. Patient preferences for psoriasis treatments: process characteristics can outweigh outcome attributes. Arch Dermatol. 2011;147:1285–1294.
- Schaarschmidt ML, Umar N, Schmieder A, et al. Patient preferences for psoriasis treatments: impact of treatment experience. J Eur Acad Dermatol Venereol. 2013;27:187–198.
- Schaarschmidt ML, Kromer C, Herr R, et al. Patient preferences for biologicals in psoriasis: top priority of safety for cardiovascular patients. PLoS One. 2015;10:e0144335.
- Kromer C, Schaarschmidt ML, Schmieder A, et al. Patient preferences for treatment of psoriasis with biologicals: a discrete choice experiment. PLoS One. 2015;10:e0129120.
- Hauber AB, Gonzalez JM, Schenkel B, et al. The value to patients of reducing lesion severity in plaque psoriasis. J Dermatolog Treat. 2011;22:266–275.
- Feldman SR, Moeller AH, Erntoft Idemyr ST, et al. Relative importance of mode of administration in treatment preferences among plaque psoriasis patients in the United States. JHEOR. 2016;4:141–157.
- Blauvelt A, Gooderham M, Iverson L, et al. Efficacy and safety of ixekizumab for the treatment of moderate-to-severe plaque psoriasis: results through 108 weeks of a randomized, phase III clinical trial (UNCOVER-3). J Am Acad Dermatol. 2017a;76:AB112.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017b;76:60–69.e9.
- Langley RG, Elewski BE, Lebwohl M, et al. ERASURE Study Group; FIXTURE Study Group. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326–338.
- Langley RG, Lebwohl M, Krueger GG, et al.; PHOENIX 2 Investigators. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. Br J Dermatol. 2015;172:1371–1383.
- Gordon K, Leonardi C, Braun D, et al. Results after at least 52 weeks of open label treatment with ixekizumab, an anti-IL-17A monoclonal antibody, in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2014;70:AB183.
- Gordon KB, Blauvelt A, Papp KA, et al. UNCOVER-1 Study Group; UNCOVER-2 Study Group; UNCOVER-3 Study Group. phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345–356.
- Kimball AB, Papp KA, Wasfi Y, et al.; PHOENIX 1 Investigators. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535–1545.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961–969.
- Griffiths CE, Reich K, Lebwohl M, et al. UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541–551.
- Menter A, Thaçi D, Papp KA, et al. Five-year analysis from the ESPRIT 10-year postmarketing surveillance registry of adalimumab treatment for moderate to severe psoriasis. J Am Acad Dermatol. 2015;73:410–419.e6.
- Secukinumab FDA prescribing information. 2018 [accessed 2019 Jul 2]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125504s001s002lbl.pdf
- Ixekizumab FDA prescribing information. 2018 [accessed 2019 Jul 2]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125521s000lbl.pdf
- Ustekinumab FDA prescribing information. 2018 [accessed 2019 Jul 2]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761044lbl.pdf
- Adalimumab FDA prescribing information. 2018 [accessed 2019 Jul 2]. Available from: https://www.fda.gov/ohrms/dockets/ac/03/briefing/3930B1_02_B-Abbott-Humira%20Prescribing%20Info.pdf
- De Bekker-Grob EW, Donkers B, Jonker MF, et al. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8:373–384.
- Soekhai V, de Bekker-Grob EW, Ellis AR, et al. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37:201–226.
- Yang JC, Johnson FR, Kilambi V, et al. Sample size and utility-difference precision in discrete-choice experiments: a meta-simulation approach. J Choice Model. 2015;16:50–57.
- Neidhardt K. Patient-relevant endpoints in psoriasis – a literature review of patient preference studies. Value Health. 2016;19:a571.
- Eder L, Polachek A, Rosen CF, et al. The development of psoriatic arthritis in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms: a prospective cohort study. Arthritis Rheumatol. 2017;69:622–629.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2019;24:1–18.
- Husni ME, Fernandez A, Hauber B, et al. Comparison of US patient, rheumatologist, and dermatologist perceptions of psoriatic disease symptoms: results from the DISCONNECT study. Arthritis Care Res (Hoboken). 2018; 20:102.
- Tischer B, Mehl A. Patients' and nurses' preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Prefer Adherence. 2018;12:1413–1424.
- Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16:3–13.
- Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CGM, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19:300–315.