Abstract
Background
Itch, skin pain, and sleep disturbance are burdensome symptoms in atopic dermatitis (AD) that negatively influence a patient’s quality of life (QoL).
Objective
To evaluate the impact of baricitinib on patient-reported outcomes (PROs) in adult patients with moderate-to-severe AD, and explore the association between improvement in key signs and symptoms of AD with improvements in QoL and patient’s assessment of disease severity.
Methods
Data were analyzed from two phase III monotherapy trials (BREEZE-AD1/BREEZE-AD2) in which patients were randomized 2:1:1:1 to once-daily placebo, baricitinib 1-mg, 2-mg, or 4-mg for 16 weeks and assessed using PRO measures.
Results
At week 16, baricitinib 4-mg and 2-mg significantly reduced itch severity (Itch Numeric Rating Scale (NRS) (BREEZE-AD1: percent change from baseline −36.6% and −29.4% vs. placebo (–12.0%), p≤.001 and p≤.05; BREEZE-AD2: −47.2% and −46.9% vs. placebo (–16.6%), p≤.001). Baricitinib significantly reduced SCORing AD (SCORAD) pruritus (4-mg in BREEZE-AD1 and 2-mg in BREEZE-AD2) and Patient Oriented Eczema Measure (POEM) itch (both doses). Improvements in skin pain severity and sleep disturbance were also observed. Improvements in AD symptoms showed higher correlations with patients’ assessment of AD severity and QoL than improvements in skin inflammation.
Conclusions
Baricitinib significantly improved symptoms in patients with moderate-to-severe AD.
ClinicalTrials.gov identifiers
NCT03334396 (BREEZE-AD1) and NCT03334422 (BREEZE-AD2).
Introduction
Atopic dermatitis (AD) is a common, chronic, relapsing, and highly symptomatic inflammatory skin disease (Citation1). AD presents with eczematous lesions with typical morphology and distribution (Citation2). Patients with moderate-to-severe AD experience multiple, intense, debilitating symptoms that can profoundly affect their quality of life (QoL) (Citation3). AD is considered the most burdensome skin disorder globally (Citation4).
Intense, unrelenting itch is a hallmark of the disease, and can lead to sleep disturbances (Citation5,Citation6). Patients commonly complain of skin pain (due to itch, scratching, skin erosions or contact, such as topical products or washing) and describe their symptoms using terms resembling neuropathic pain (Citation7,Citation8). Itch, sleep disturbances, and skin pain contribute considerably to the burden of AD. Recent studies showed a high prevalence of skin pain, anxiety, and depression among patients with moderate-to-severe AD, all of which decreased QoL (Citation9–11). Patients with AD also experience: (1) a greater likelihood of comorbidities (e.g. bronchial asthma and food allergies (Citation12)); (2) skin infections (Citation13); (3) increased school and/or work absenteeism (Citation14); (4) impaired mental health (Citation15); and (5) impaired social interaction (Citation3).
AD also presents a substantial socioeconomic burden to caregivers, healthcare providers, and payers (Citation16), including increased healthcare resource utilization (Citation17,Citation18) and costs (Citation19,Citation20). An estimated $5 billion is accrued annually in the United States (US) from direct and indirect costs associated with AD (Citation19).
Topical corticosteroids and topical calcineurin inhibitors (Citation21), and broad systemic immunosuppressants like cyclosporine, azathioprine, and methotrexate (Citation22), are commonly used to treat moderate-to-severe AD. Many new therapies have been developed such as biologics like dupilumab, an interleukin 4 receptor α antagonist (Citation23), and Janus kinase (JAK) inhibitors, a novel class of small molecules. The use of baricitinib, an oral selective JAK 1 and 2 inhibitor, recently showed improvement in signs and symptoms of AD in two global phase III trials (Citation24).
Unlike psoriasis, AD is a disease where symptoms are invasive and often difficult for clinicians to assess; therefore, patient-reported symptoms are equally important in evaluating disease severity (Citation3,Citation25). Most primary endpoints in clinical trials are clinician-reported outcome measures (due to requirements for regulatory approval) that focus on improvement in skin inflammation. The Harmonizing Outcome Measures for Eczema initiative defined four core outcome domains as the minimum components to be measured in all AD clinical trials and in routine practice: clinical signs, patient-reported symptoms, QoL, and long-term control (Citation26).
The multidimensional burden of moderate-to-severe AD is not fully captured by clinician-reported outcome assessments. To obtain further insight into baricitinib efficacy, we evaluated its impact on the patient-reported outcomes (PROs) of AD, including itch, sleep disturbance, and skin pain, and analyzed the correlation of PROs and clinician-reported outcomes to improvements in QoL and patient assessment of disease severity in two phase III placebo-controlled trials.
Patients and methods
Study design
BREEZE-AD1 (NCT03334396) and BREEZE-AD2 (NCT03334422) were identical 16-week randomized, double-blind, parallel-group, placebo-controlled trials (Citation24). Eligible patients (≥18 years old with moderate-to-severe AD (validated Investigator Global Assessment AD (vIGA-AD) ≥3; Eczema Area and Severity Index (EASI) ≥16)) were randomized 2:1:1:1 to once-daily placebo, baricitinib 1-mg, 2-mg, or 4-mg. Prior to randomization, treatments for AD were washed out for four weeks for systemic treatments and two weeks for topical therapies. Both studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the institutional review board or ethics committee at each participating site. All eligible patients provided written informed consent.
PRO measures
Select PROs were assessed in BREEZE-AD1 and BREEZE-AD2. Patient assessment of severity was evaluated by the Patient Oriented Eczema Measure (POEM) (Citation27) and Patient’s Global Impression of Disease Severity (PGI-S-AD). The PGI-S-AD is a global patient assessment to evaluate severity of AD at a specific point in time on a single-item, five-point scale ranging from (0) no symptoms to (4) severe. Itch and skin pain severity were assessed by an 11-point Numeric Rating Scale (NRS; 0 = no itch/pain; 10 = worst itch/pain imaginable) (Citation28) measuring the worst itch and worst pain daily, while the SCORing AD (SCORAD) subjective assessments of itch and sleep loss by a 0–10 cm visual analogue scale (VAS; 0 = no itching/trouble sleeping; 10 = unbearable itching/a lot of trouble sleeping) assessed the average severity over past three days (Citation29,Citation30).
Additional PROs were investigated here to better understand the effect size of baricitinib on key signs and symptoms of AD and their association with patient-reported severity and QoL.
Sleep disturbance due to itch was evaluated by the Atopic Dermatitis Sleep Scale (ADSS) (Citation31). The ADSS is a three-item questionnaire that assesses the impact of itch on sleep including difficulty falling asleep (0 = not at all; 4 = very difficult), number of night time awakenings due to itch (0–29), and difficulty getting back to sleep (0 = not at all; 4 = very difficult).
Work Productivity and Activity Impairment (WPAI)-AD measured overall work productivity and impairment during the past seven days. Scores were calculated as percentages of impairment; higher scores indicated greater impairment and less productivity (Citation32).
Health-related QoL (HRQoL) was evaluated using the Dermatology Life Quality Index (DLQI) and EuroQoL 5-Dimensions (EQ-5D). DLQI scores range from 0 to 30 with higher scores indicating greater impairment of QoL (Citation33,Citation34). The EQ-5D health state profile consists of a descriptive system of the respondent’s health and a rating of their current health state (0–100 mm VAS) (Citation35).
The Hospital Anxiety and Depression Scale (HADS) evaluated symptoms of anxiety and depression (Citation36,Citation37). Scores range from 0 to 21; higher scores indicate a greater probability of anxiety or depression.
Statistical analysis
The intent-to-treat (ITT) population analysis set included all randomized patients in each study. Comparisons of categorical endpoints were made within the framework of a logistic regression model with effects for treatment, region, baseline disease severity (vIGA-AD) and baseline value. Non-responder imputation was used for visits where patients were discontinued or rescued with topical corticosteroids (any potency) or systemic therapies. Treatment comparisons of continuous endpoints were made within the framework of a mixed-model repeated measures (MMRM) analysis with effects for treatment, region, baseline disease severity (vIGA-AD), visit, and treatment-by-visit interaction as fixed categorical effects; and baseline and baseline-by-visit interaction as fixed continuous effects. The treatment comparisons are not adjusted for multiplicity.
Spearman’s correlation analysis by study was used to analyze correlations (rs) at week 4 and week 16 between the change from baseline (CFB) in PGI-S-AD and DLQI and Itch NRS, Skin Pain NRS, ADSS Item 2, and EASI total score. Missing data were imputed using a last observation carried forward method for the most recent non-missing value prior to censoring.
Results
A total of 624 and 615 patients were enrolled in BREEZE-AD1 and BREEZE-AD2, respectively. Baricitinib met the primary endpoint of both trials. Baricitinib 2-mg and 4-mg achieved significant improvement vs. placebo for the proportion of patients achieving vIGA-AD (0,1) at week 16. Significant improvement for patients treated with baricitinib 4-mg and 2-mg was observed as early as weeks 1–2 for all PROs.
Patient global index of severity – atopic dermatitis and Dermatology Life Quality Index
Significant improvement in PGI-S-AD was observed at week 16 for baricitinib 4-mg with a least square mean (LSM) CFB of −0.8 and −1.0 for baricitinib 4-mg and −0.3 for placebo in both studies (p<.001) (). In BREEZE-AD2, significant improvement in PGI-S-AD was observed at week 16 for baricitinib 2-mg with an LSM CFB of −0.9 (p<.001).
Table 1. Summary of patient-reported outcomes (BREEZE-AD1 and BREEZE-AD2) at week 16.
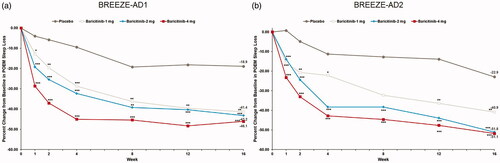
Significant improvements in DLQI were seen through week 16 for patients achieving a DLQI total score of 0–1, indicating no effect on HRQoL (Citation24). At week 16, baricitinib 4-mg and 2-mg significantly improved the proportion of patients achieving DLQI of 0–1 compared to placebo (BREEZE-AD1: 16.8% (4-mg) and 11.4% (2-mg) vs. 4.8%, p≤.001 and p≤.05; BREEZE-AD2: 15.4% (4-mg) and 11.4% (2-mg) vs. 2.9%, p≤.001 and p≤.01). Baricitinib 1-mg showed significant improvement at week 16 in BREEZE-AD2 only (9.6% vs. 2.9% reduction, p≤.01) (Citation24).
Relative to placebo, the proportion of patients with DLQI total score ≤5 (representing no to small effect on QoL) was significantly higher with baricitinib treatment. At week 16, baricitinib 4-mg showed significant improvement in DLQI ≤5 (BREEZE-AD1: 40.9% (4-mg) vs. 9.7%, p<.001 and BREEZE-AD2: 27.0% (4-mg) and 22.1 (2-mg) vs. 6.8%, p<.001) ().
Figure 1. DLQI total score ≤5 response rate in BREEZE-AD1 (a) and in BREEZE-AD2 (b). DLQI: Dermatology Life Quality Index; N: number of patients in the analysis population; NRS: Numeric Rating Scale. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo. For continuous endpoints, LS means are from MMRM analyses. For categorical endpoints, a nonresponder imputation was applied at censoring.
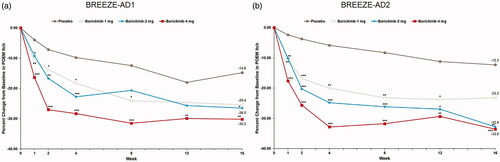
Itch
At week 16, significant reductions in LSM percent CFB Itch NRS were observed with baricitinib 4-mg and 2-mg treatment (BREEZE-AD1: −36.6% and −29.4% vs. placebo (–12.0%), p≤.001 and p≤.05; BREEZE-AD2: −47.2% and −46.9% vs. placebo (–16.6%), p≤.001), while a significant reduction of −31.3% (p≤.01) was observed with baricitinib 1-mg treatment in BREEZE-AD1 ().
Figure 2. Percent change from baseline in Itch NRS severity in BREEZE-AD1 (a) and BREEZE-AD2 (b). N: number of patients in the analysis population; NRS: Numeric Rating Scale. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo. For continuous endpoints, LS means are from MMRM analyses. For categorical endpoints, a non-responder imputation was applied at censoring.
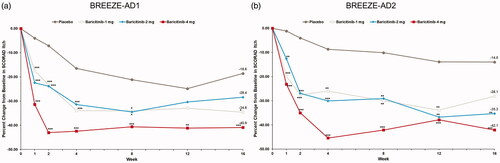
A consistent reduction from baseline in SCORAD pruritus VAS at week 16 was observed with baricitinib 1-mg treatment vs. placebo across both trials with a mean percent CFB at week 16 of −40.9% vs. −18.6% (p<.001) in BREEZE-AD1 and −42.1% vs. −14.0% (<.001) in BREEZE-AD2 (; ). Significant improvements at week 16 in BREEZE-AD2 (mean percentage CFB of −35.3% vs. −14.0% (p=.009) at week 16) were observed with baricitinib 1-mg treatment.
Figure 3. Percent change from baseline in SCORAD Itch in BREEZE-AD1 (a) and in BREEZE-AD2 (b). SCORAD: SCORing Atopic Dermatitis. Data reported as % change in LS means from MMRM analyses. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo.
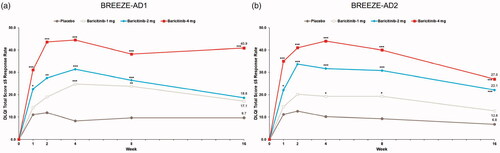
At week 16, significant reductions in LSM percent CFB POEM Itch were seen with baricitinib 4-mg and 2-mg treatment (BREEZE-AD1: −30.2% and −26.5% vs. placebo (–14.8%), p=.0034 and p=.0379; BREEZE-AD2: −33.6% and −32.8% vs. placebo (–12.3%), p=.0009 and p=.0025) (; ).
Skin pain
Significant reductions from baseline in Skin Pain NRS were observed with baricitinib 4-mg and 2-mg relative to placebo at week 16 in BREEZE-AD1. The LSM percent CFB in Skin Pain NRS at week 16 for baricitinib 4-mg, 2- mg, and 1-mg was −33.2%, −27.2%, and −33.1% vs. −14.5% (p=.002, p=.051, and p=.005, respectively). In BREEZE-AD2, the LSM percent CFB in Skin Pain NRS for baricitinib 4-mg and 2-mg was −41.1% and −43.1% vs. −14.2% (p<.001) (; ). Skin Pain NRS ≥4 improvement response rates for patients in both trials are shown in .
Figure 5. Percent change from baseline in Skin Pain NRS in BREEZE-AD1 (a) and in BREEZE-AD2 (b). NRS: Numeric Rating Scale. Data reported as % change based on the (CFB LSM from MMRM)×100/(pooled baseline mean). *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo.
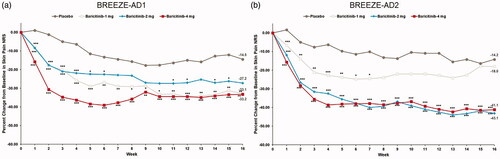
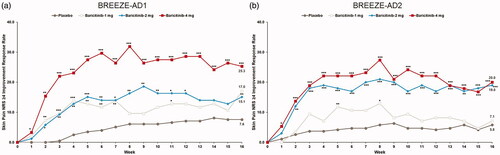
Figure 6. Skin Pain NRS ≥4 improvement response rate in BREEZE-AD1 (a) and in BREEZE-AD2 (b). NRS: Numeric Rating Scale. Primary censoring rule excludes data collected after first rescue therapy date or permanent study drug discontinuation. p Values obtained by logistic regression analysis with treatment, baseline value, region and baseline disease severity (IGA) as factors. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo.
Sleep disturbance
Baricitinib treatment resulted in improvement over placebo in patients with a ≥ 1-point reduction in ADSS Item 1 (difficulty falling asleep due to itch) through week 16. The greatest reductions were seen after baricitinib 4-mg treatment at weeks 2, 4, and 16 vs. placebo across both trials (BREEZE-AD1: 39.8% vs. 10.9% at week 2, p<.001, 43.9% vs. 11.9% at week 4, p<.001 and 31.6% vs. 13.9% reduction at week 16, p<.001 and BREEZE-AD2: 29.2% vs. 8.7% at week 2, p<.001, 30.2% vs. 10.4% at week 4, p<.001 and 28.1% vs. 6.0% at week 16, p<.001).
At week 16, treatment with baricitinib 4-mg resulted in improvement in CFB of ADSS Item 2 (number of night time awakenings due to itch) in BREEZE-AD1 (–1.4 vs. −0.8, p=.006) and treatment with baricitinib 4-mg and 2-mg resulted in improvement in BREEZE-AD2 (–1.1 and −1.0 vs. −0.5, p<.001 and p=.003, respectively) (Citation24). Baricitinib also improved the proportion of patients with a ≥ 1.5-point reduction in ADSS Item 2 through week 16. At week 16, treatment with baricitinib 4-mg improved ADSS Item 2 in BREEZE-AD1 (28.3% vs.15.2%, p=.048) and both baricitinib 4 mg and 2 mg improved the proportion of patients with a ≥ 1.5-point reduction in ADSS Item 2 in BREEZE-AD2 (26.0% and 26.7 vs. 8.3%, p=.005 and p=.003).
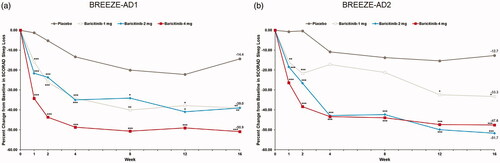
All doses of baricitinib reduced SCORAD sleep loss from baseline compared to placebo at week 16 across both trials; the LSM percent CFB in SCORAD sleep loss VAS at week 16 for baricitinib 4-mg, 2-mg, and 1-mg was −50.9%, −39.0%, and −30.9% vs. −14.4% (p<.001, p=.003, and p=.004, respectively) in BREEZE-AD1 and −51.7%, −47.6%, and −33.3% vs. −12.7% (p<.001, p<.001, and p=.023) in BREEZE-AD2 (; ).
Figure 7. Percent change from baseline in SCORAD Sleep Loss in BREEZE-AD1 (a) and in BREEZE-AD2 (b). SCORAD: SCORing Atopic Dermatitis. Data reported as % change in LS means from MMRM analyses. *p≤.05, **p≤.01, and ***p≤.001 for analyses comparing baricitinib with placebo.
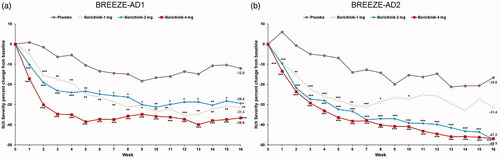
All doses of baricitinib reduced POEM sleep disturbance from baseline compared to placebo at week 16 across both trials; the LSM percent CFB in POEM sleep disturbance at week 16 for baricitinib 4-mg, 2-mg, and 1-mg was −46.1%, −43.2%, and −41.4% vs. −18.9% (p<.0001, p=.0009, and p=.0024, respectively) in BREEZE -AD1 and −51.1%, −51.8%, and −40.9% vs. −22.9% (p<.0001, p=.0002, and p=.0197) in BREEZE-AD2 (; ).
Anxiety and depression
In BREEZE-AD1, significant improvement in HADS total score was observed at week 16 for baricitinib 4-mg and 2-mg with a LSM CFB of −3.6 for baricitinib 4-mg, −3.2 for baricitinib 2-mg, and −1.2 for placebo (p=.001 and p=.008, respectively) (). In BREEZE-AD2, significant improvement in HADS was observed at week 16 for baricitinib 4-mg with an LSM CFB of −3.7 for baricitinib 4-mg and −1.3 for placebo (p=.004) (). At week 16, significant improvement relative to placebo was observed for the proportion of patients with a HADS Depression score <8 at baseline and ≥8 at follow up (BREEZE-AD1: 35.7% (4-mg) vs. 13.0%, p=.021 and BREEZE-AD2: 19.4% (2-mg) vs. 5.5%, p=.024) and the proportion of patients with a HADS Anxiety score <8 at baseline and ≥8 at follow-up (BREEZE-AD1: 41.0% (4-mg) vs.12.0%, p<.001 and BREEZE-AD2: 25.6% (4-mg) vs. 11.4%, p=.036).
Health-related quality of life and work productivity
Significant improvement in EQ-5D was observed at week 16 with baricitinib 4-mg treatment (LSM CFB of 9.1 for baricitinib 4-mg and 2.0 for placebo (p=.017) in BREEZE-AD1 and 11.2 and 10.5 for baricitinib 4-mg and 2-mg, vs. 2.3 for placebo in BREEZE-AD2 ()). Significant improvement in the WPAI-AD was observed with baricitinib 4-mg at week 16 in BREEZE-AD1 with an LSM CFB of −13.9 for baricitinib 4-mg and −2.6 for placebo (p=.010) ().
Correlation between improvements in itch, skin pain, and sleep disturbance and patient global index of severity – atopic dermatitis and Dermatology Life Quality Index
In BREEZE-AD1, PGI-S-AD had substantial-to-excellent correlations (0.757–0.808) with Itch NRS, substantial correlations with Skin Pain NRS (0.736–0.793), and fair-to-moderate correlations with ADSS Item 2 (0.352–0.474), but only fair correlations with EASI (0.322–0.393) (). Similarly, DLQI had moderate-to-substantial correlations (0.552–0.601) with Itch NRS, moderate-to-substantial correlations (0.495–0.628) with Skin Pain NRS, and fair-to-moderate correlations (0.342–0.481) with ADSS Item 2, but fair correlations (0.302–0.376) with EASI. In BREEZE-AD2, most correlations were consistent with BREEZE-AD1 except for those with EASI, which showed fair-to-moderate correlations (0.396–0.526) with PGI-S-AD and fair-to-moderate correlations with DLQI (0.356–0.559).
Table 2. Correlation between changes from baseline at week 4 and week 16 in Itch NRS, Skin Pain NRS, ADSS Item 2, and EASI (LOCF) and changes from baseline at week 4 and week 16 in PGI-S-AD and DLQI (LOCF) and by baricitinib treatmenta.
Discussion
Effective long-term treatment options for AD that address patients’ concerns remain a relatively unmet need (Citation38–40). At the Patient-Focused Drug Development meeting for eczema held in 2019, 1508 survey respondents indicated that the most challenging signs and symptoms of AD were itch (in 79% of patients), red, inflamed skin (in 47% of patients), and sleep disturbance (in 29% of patients) (Citation41). In addition, patient-reported symptoms and self-assessed disease severity may be discordant with clinician disease severity assessment or response to therapy. In a US cross-sectional survey with 678 patients, approximately one-third of patients rated severity of AD differently from their physicians (Citation42). Torrelo et al. also found poor agreement in perceptions of AD severity by patients and physicians (Citation43). Emphasizing treatment targets that incorporate patient input about their experience with AD and treatment response, including symptoms, QoL, and long-term control of flares might complement clinician-reported measures (Citation26).
Rapid symptom control and speed of onset of treatment are important elements in the treatment of AD. Itch can be significantly disruptive to sleep and QoL for patients with AD and caregivers and can impact a patient’s HRQoL and sleep (Citation44,Citation45). Repeated scratching that breaks the skin can cause open wounds and cracks, which increases the susceptibility of patients to various infections. Skin pain in AD that is more frequent and/or severe, has also been associated with decreased QoL (Citation8). Here, a higher correlation of AD symptoms with QoL and patient assessment of improvement in disease severity was observed earlier in the study (week 4) compared to later (week 16).
While patient adherence is dependent on many factors, rapid improvement in disease characteristics is desired by patients with various chronic dermatological diseases in which the intensity of symptoms typically fluctuates over time (Citation46). Adherence to treatment of chronic diseases with topical medications is typically poor (Citation47) while overall dermatological adherence status is significantly better for oral medications than for topical medications (Citation48). In this first assessment of PRO and clinician-reported outcome correlation to improvements in QoL and patient assessment of disease severity in clinical trials, several important points regarding the analysis should be noted. Although the DLQI questionnaire includes a question that focuses on itch and skin pain and questions that allude to the appearance of signs of skin inflammation, there are no questions included in the DLQI related to the impact of AD on sleep. Sleep disturbance had a moderate correlation with DLQI, found to be similar, or at times higher than improvements in skin inflammation (EASI) despite inclusion of questions 2 and 4 in the DLQI. The results of this correlation analysis further emphasize the importance of sleep disturbance in AD, as captured by SCORAD or PO-SCORAD (Citation49).
Despite not having a specific mention of disease domains in the question, the correlations found between PGI-S-AD and DLQI follow a similar trend to those correlations observed with the DLQI; itch and skin pain had the highest correlations while skin inflammation had the lowest correlation.
Some limitations to this manuscript must be considered. The studies included a large, diverse global population, though patients were predominantly Caucasian. In addition, the choice of weeks 4 and 16 as early and late time points in the correlation analysis presented here may not be representative of the moderate-to-severe disease time course.
Baricitinib rapidly improved skin inflammation, itch, skin pain, sleep disturbance, QoL, and patient assessment of disease severity. Although primary endpoints in AD studies often focus on clinician-reported outcomes, improvement in PROs for itch, skin pain, and sleep disturbance showed a higher correlation with improvements in QoL and patient assessment of disease severity in this study. This emphasizes the importance of including the PRO assessment to complement standard clinician-reported outcomes in both clinical practice and clinical trials to identify treatments that can manage the full patient experience of AD.
Acknowledgements
The authors would like to thank the patients and families involved in these trials. We thank Shannon E. Gardell, PhD, of Evidera for assistance with preparation of this manuscript.
Disclosure statement
K Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Biogen-Idec, Boehringer Ingelheim, Celgene, Covagen, Forward Pharma, Fresenius Medical Care, Galapagos, GlaxoSmithKline, Janssen-Cilag, Kyowa Kirin, LEO Pharma, Eli Lilly and Company, Medac, Merck Sharp & Dohme, Miltenyi, Novartis, Ocean Pharma, Pfizer, Samsung Bioepis, Sanofi, Takeda, UCB Pharma, Valeant, XBiotech, and XenoPort. AM DeLozier, FP Nunes, JA Ross Terres, SD Watts, and Y-F Chen are current employees and shareholders of Eli Lilly and Company. JP Thyssen has been an advisory board member, and/or received speaker honoraria, and/or has participated in clinical studies for: Eli Lilly and Company, LEO Pharma, AbbVie, Regeneron, Pfizer, and Sanofi-Genzyme. LF Eichenfield has been an advisory board member, and/or speaker, and/or consultant, and/or has participated in clinical studies for Amgen, AbbVie, Almirall, Arcutis, Asana, Celgene, Dermira, Dermavant, Eli Lilly, Forte, Galderma, Incyte, Leo Pharma, Novartis, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, and Valeant/Ortho Derm. A Wollenberg has received grants as an investigator and/or honoraria, and/or consulting fees from: AbbVie, Almirall, Beiersdorf, Eli Lilly and Company, Galderma, Leo Pharma, MedImmune, Novartis, Pfizer, Pierre Fabre, Regeneron, and Sanofi Genzyme. EL Simpson has been an investigator for: Eli Lilly and Company, Galderma, Leo Pharma, Merck, Pfizer, Regeneron; and a consultant with honorarium for: AbbVie, Boehringer Ingelheim, Dermavant, Eli Lilly and Company, Incyte, Leo Pharma, Pfizer, Pierre Fabre Dermo Cosmetique, Regeneron, and Sanofi Genzyme. JI Silverberg has received grants and/or personal fees from: AbbVie, Dermavant, Dermira, Eli Lilly and Company, Galderma, GSK, Kiniksa Pharmaceuticals, Leo Pharma, Menlo Therapeutics, Pfizer, and Regeneron-Sanofi.
Data availability statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Additional information
Funding
References
- Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125–137.
- Silverberg JI. Atopic dermatitis in adults. Med Clin North Am. 2020;104(1):157–176.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527–1534.
- Gowda S, Goldblum OM, McCall WV, et al. Factors affecting sleep quality in patients with psoriasis. J Am Acad Dermatol. 2010;63(1):114–123.
- Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7(3):349–364.
- Vakharia PP, Chopra R, Sacotte R, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548–552.e3.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Pain is a common and burdensome symptom of atopic dermatitis in United States adults. J Allergy Clin Immunol Pract. 2019;7(8):2699–2706.e7.
- Ronnstad ATM, Halling-Overgaard AS, Hamann CR, et al. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79(3):448–456.e30.
- Thyssen JP, Halling-Sonderby AS, Wu JJ, et al. Pain severity and use of analgesic medication in adults with atopic dermatitis: a cross-sectional study. Br J Dermatol. 2020;182(6):1430–1436.
- Thyssen JP, Hamann CR, Linneberg A, et al. Atopic dermatitis is associated with anxiety, depression, and suicidal ideation, but not with psychiatric hospitalization or suicide. Allergy. 2018;73(1):214–220.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24(5):476–486.
- Alexander H, Paller AS, Traidl-Hoffmann C, et al. The role of bacterial skin infections in atopic dermatitis: expert statement and review from the International Eczema Council Skin Infection Group. Br J Dermatol. 2020;182(6):1331–1342.
- Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(4):360–366.
- Yu SH, Silverberg JI. Association between atopic dermatitis and depression in US adults. J Invest Dermatol. 2015;135(12):3183–3186.
- Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39(6):406–410.
- Shrestha S, Miao R, Wang L, et al. Burden of atopic dermatitis in the United States: analysis of healthcare claims data in the commercial, medicare, and Medi-Cal databases. Adv Ther. 2017;34(8):1989–2006.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Atopic dermatitis in US adults: from population to health care utilization. J Allergy Clin Immunol Pract. 2019;7(5):1524–1532.e2.
- Adamson AS. The economics burden of atopic dermatitis. Adv Exp Med Biol. 2017;1027:79–92.
- Eckert L, Gupta S, Amand C, et al. The burden of atopic dermatitis in US adults: health care resource utilization data from the 2013 National Health and Wellness Survey. J Am Acad Dermatol. 2018;78(1):54–61.e1.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878.
- Dupixent (dupilumab) injection [prescribing information]. Tarrytown (NY): Regeneron Pharmaceuticals; Bridgewater (NJ): Sanofi-Aventis US; 2017.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–255.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290–298.
- Schmitt J, Spuls PI, Thomas KS, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134(4):800–807.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients' perspective. Arch Dermatol. 2004;140(12):1513–1519.
- Newton L, DeLozier AM, Griffiths PC, et al. Exploring content and psychometric validity of newly developed assessment tools for itch and skin pain in atopic dermatitis. J Patient Rep Outcomes. 2019;3(1):42.
- Kunz B, Oranje AP, Labreze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195(1):10–19.
- Stalder JF, Taïeb A. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23–31.
- Lee JY, Kim M, Yang HK, et al. Reliability and validity of the Atopic Dermatitis Symptom Score (ADSS). Pediatr Allergy Immunol. 2018;29(3):290–295.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230(1):27–33.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736.
- Snaith RP. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes. 2003;1:29.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370.
- Wollenberg A, Oranje A, Deleuran M, et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol. 2016;30(5):729–747.
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143(1):1–11.
- Hajar T, Gontijo JRV, Hanifin JM. New and developing therapies for atopic dermatitis. An Bras Dermatol. 2018;93(1):104–107.
- McCleary KK. Understanding the lived experience of eczema. The “Voice of the Patient” report on the eczema patient-focused drug development meeting. Available from: http://www.morethanskindeep-eczema.org/uploads/1/2/5/3/125377765/mtsd_report_-_digital_file.pdf
- Wei W, Anderson P, Gadkari A, et al. Discordance between physician- and patient-reported disease severity in adults with atopic dermatitis: a US Cross-Sectional Survey. Am J Clin Dermatol. 2017;18(6):825–835.
- Torrelo A, Ortiz J, Alomar A, et al. Atopic dermatitis: impact on quality of life and patients' attitudes toward its management. Eur J Dermatol. 2012;22(1):97–105.
- Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–498.
- Chrostowska-Plak D, Reich A, Szepietowski JC. Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2013;27(2):e239–e242.
- Stein Gold L, Kircik LH, Pariser D, et al. Rapid onset of action in patients with moderate-to-severe plaque psoriasis with halobetasol 0.01%/tazarotene 0.045% fixed combination. J Drugs Dermatol. 2018;17(8):863–868.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease – strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33(12):2253–2263.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral and topical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172(1):272–275.
- Stalder JF, Barbarot S, Wollenberg A, et al. Patient-oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe. Allergy. 2011;66(8):1114–1121.