Abstract
Background/objectives
Tightly-controlled dose reduction was possible during 1 year in psoriasis patients on adalimumab, etanercept or ustekinumab with low disease activity (CONDOR trial). Extended observation is needed to ensure long-term effectiveness and safety of the strategy. With prolonged follow-up, we investigated the clinical effects and safety of the strategy, the proportion of patients with successful dose reduction, and assessed if patients with a disease flare regained remission.
Methods
Two-year follow up of a subgroup of patients previously included in a randomized pragmatic study comparing usual care (UC) with stepwise dose reduction (DR). Effectiveness (Psoriasis Area and Severity Index, PASI), Dermatology Life Quality Index (DLQI), adverse events, proportion of patients with successful DR and proportion of persistent disease flares were analyzed.
Results
DR leads temporarily to a slightly increased PASI groupwise, but on the long-term patients regained low PASI. DLQI scores remained stable during follow-up. No serious adverse events due to DR were reported. Forty-one percent of patients remained on a low dose up to 2 years. The number of persistent flares was low in DR and UC.
Conclusions
The proposed dose reduction strategy is effective for a significant part of patients and remains safe up to 2 years of follow-up.
Introduction
Treatment of psoriasis has improved dramatically in the last decades due to the introduction of targeted biologic therapies. These drugs are effective, reduce skin symptoms and improve quality of life of patients (Citation1–4). Biologics are administered in fixed dosages during many years. However, this may not be necessary in patients with a very good response. It is important to prevent overtreatment and use healthcare costs appropriately, and this can be achieved by striving for the lowest effective dose in patients with stable low disease activity.
The effectiveness of a tightly controlled dose reduction strategy has been described previously with evaluation of the Psoriasis Area and Severity Index (PASI), the Dermatology Life Quality Index (DLQI) and safety in a randomized pragmatic controlled trial containing 1 year follow up (CONDOR trial) (Citation5). Non-inferiority was not demonstrated regarding PASI at 12 months, whereas DLQI was non-inferior and safety was reassuring (Citation5). After 1 year, successful dose reduction was possible in 53% of psoriasis patients with stable low disease activity.
However, more insight into the longer-term risks and benefits of such a dose reduction strategy is important for its further development and implementation. In this study, we investigated the extension phase of the CONDOR study with a follow-up duration of up to 2 years regarding clinical effects and safety. In addition we addressed if patients who were treated successfully with a low dose after 1 year, could maintain this up to 2 years. Lastly, we investigated whether and how patients with a disease flare after a dose reduction attempt regained their state of low disease activity.
Methods
Study design, participation, randomisation and procedures
This is a 12-month open-label extension of the CONDOR study. The CONDOR study was a 1 year, pragmatic, open label, randomized, controlled, non-inferiority trial in patients with psoriasis (Citation5). In the original study, a tightly controlled dose reduction (DR) strategy of adalimumab, etanercept and ustekinumab was compared with usual care (UC). Patients with plaque psoriasis were eligible for inclusion in the original CONDOR study when they had stable and low disease activity on the authorized full dose of the biologics for at least 6 months. For the design and results of the study, we refer to previous papers (Citation5,Citation6). In short, patients in the DR received identical care as the UC, but the time interval between their injections was prolonged in two steps, resulting in 67% and 50% of their full authorized dose. In case of a short disease flare patients were advised to returned to their previous effective dose or original dose. A disease flare was defined as a PASI score >5 and/or a DLQI score >5 once (short flare), or ≥3 months (persistent flare).
The present paper describes the extension phase of CONDOR and was conducted from March 2017 to June 2019 in the Radboudumc, Nijmegen. In patients in the DR group who were still on a low dose at the end of CONDOR, the low dose was continued in this extension phase unless PASI or DLQI exceeded the threshold of 5 (i.e. disease flare). Other treatment choices, as well as treatment of patients in the UC group, were made by the treating physician based on pertaining guidelines or usual care.
This extension study was approved by the local ethics committee (Commissie Mensgebonden Onderzoek region Arnhem-Nijmegen, NL54557.091.15). Written informed consent was obtained from all patients in the DR group entering the extension study on a low dose. Data from patients from UC (on a normal dose) was extracted from BioCAPTURE registry, for which patients also provided informed consent (Citation7).
Analyses
In general, for continuous variables, means and standard deviations (SD) or medians and interquartile ranges [IQR] were reported, depending on skewness of the data. Patients that were lost to follow-up were left out of the analysis after their lost to follow-up date. If there was no visit at 24 months, available data within a 3 month time window were used. Statistical analyses were performed with SPSS statistical package, version 23.0 (SPSS Inc., Chicago, IL, USA).
Disease activity and QOL during dose tapering and usual care (t = 0 to24 months)
Baseline characteristics and treatment characteristics of patients entering the extension phase were described using descriptive statistics. To gain insight into the long term effects of the introduction of a dose strategy in general, the total study period (CONDOR and extension, t = 0 until t = 24 months) was described using a per protocol approach. PASI and DLQI scores were compared between DR and UC at each time point using the Mann-Whitney U test. Patients in DR that returned to a normal dose before the end of study (either CONDOR or the extension phase) remained in the DR group for this per protocol analysis, due to the fact that ‘returning to a normal dose in case of a (short) flare’ was part of the study protocol.
Safety of dose tapering and usual care (t = 12 to 24 months)
We added the safety of the extension phase (t = 12 to t = 24 months) to the existing knowledge as reported in CONDOR (t = 0 to t = 12) (Citation5). All adverse events and their relation to dose reduction were assessed by two reviewers (SA, JvdR). SAEs and adverse events of special interest (AEoSI) were described in event rates per month. AEoSI included infectious events, cardiovascular events, malignancies and nonmelanoma skin cancer, musculoskeletal events, arthritis, skin events and other clinically relevant events. For this safety analysis specifically, patients who started on a low dose in the CONDOR extension were classified as the ‘low dose-group’; patients that were treated with usual care or those who were on a normal dose when the extension phase started (i.e. patients that failed dose reduction already in CONDOR) were analyzed as the ‘normal-dose group’. Observation-time ended when the dose was changed in an individual patient and the patient did not belong to the original dose group anymore. Formal statistical comparisons were not made because the study was not powered for this purpose.
Maintenance of dose tapering (t = 12 to 24 months)
Patients who were on a low dose at the start of the extension phase were selected. The proportion of these patients that continued dose tapering during this observational follow up period was calculated for month 15, 18, 21 and 24. It was also assessed which proportion of patients on a low dose was successful (definition of successful: PASI and DLQI ≤5). Furthermore, the course of disease activity (PASI) and quality of life (DLQI) of this selection of patients was described for the CONDOR phase and extension phase (t = 0 until t = 24 months).
Analysis of course of disease flares in dose tapering and usual care (t = 0 to 24 months)
The number of persistent disease flares in patients that were initially randomized to UC and DR in CONDOR was reported. This analysis comprised data of the CONDOR phase and the extension phase (t = 0 until t = 24 months). Statistical comparisons were not made due to differences in follow-up duration between groups. The number of patients that were still in a state of persistent disease flare at 24 months was reported, as well as the number of patients that needed a higher than normal dose or a switch to another biologic due to disease flare.
Results
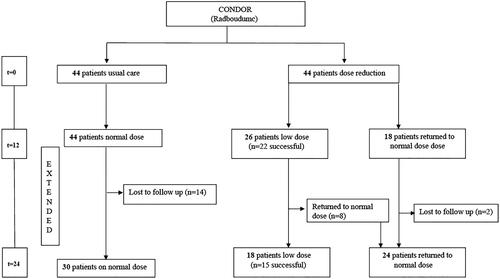
The cohort of this CONDOR extension study consisted of 88 patients and comprised only patients from Radboudumc that were included in the original CONDOR study. Of this cohort, 44 were randomized to UC and 44 to DR in the initial CONDOR study. Twenty-six out of 44 patients (59%, 95%CI 43–73%) that were randomized to DR were still on a low dose at the end of the CONDOR study. Twenty-two out of these 26 patients were classified as having ‘successful’ dose reduction (i.e. PASI and DLQI ≤ 5 on a low dose) and four out of 26 were classified as ‘unsuccessful’ (low dose but high PASI/DLQI > 5). The other 18 DR patients returned to their normal dose due to failure of dose reduction before starting this extension phase. All 44 UC patients that finished the CONDOR study were included for this extension study as a comparison cohort, and they continued usual care. At start of the extension phase, all 44 were on a normal dose but in time, a subgroup of patients (n = 14) were actively switched to lower dosages and were excluded from analyses from that moment on. shows the flow of patients throughout the CONDOR trial and this extension phase.
Figure 1. Flow chart CONDOR Extension study, Controlled Dose Reduction of Biologics with 24 months follow up.
Disease activity and QOL during dose tapering and usual care (t = 0 to 24 months)
shows patient and treatment characteristics of DR and UC. Course of PASI an DLQI were compared between DR and UC (of note, this concerns patients that were randomized to DR and UC, and remained in this group if the protocol was followed, including protocolized return to a normal dose). For these per protocol analyses, all 44 DR and 44 UC patients were described and compared for the extension phase until lost to follow up.
Table 1. Patient characteristics condor extension.
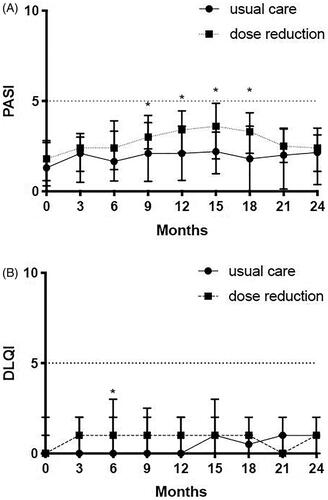
shows the course of PASI. The PASI was significantly higher in DR compared to UC at month 9–18, but this difference was not present after 18 months. shows the course of DLQI. There were no significant differences between both groups during the extension phase for DLQI.
Figure 2. PASI and DLQI. (A) Psoriasis Area and Severity Index (PASI) comparison between usual care and dose reduction. PASI scores (median and IQR) are depicted for the original CONDOR study (month 0, 3, 6, 9 and 12) and for the CONDOR extension phase (month 15, 18, 21, 24). Significant differences were seen at 9 months (p = .005), at 12 months (p = .001), at 15 months (p = .002) and at 18 months (p = .003). (B) Dermatology Quality and Life Index (DLQI) depicted for the original CONDOR study (month 0, 3, 6, 9 and 12) and for the CONDOR extension phase (month 15, 18, 21, 24). Significant differences between dose reduction and usual care were only observed at 6 months (p = .005). No significant differences were observed in the extension period. *Significant differences that were observed between dose reduction and usual care.
Safety of dose tapering and usual care (t = 12 to 24 months)
Sixty-two patients added observation time to the ‘normal dose group’ (i.e. n = 44 UC and n = 18 DR patients that were on a low dose before entering the extension phase), and 26 patients added observation time to the ‘low dose group’ (i.e. all DR patients that entered the extension phase on a low dose). One patient (4%, 95%CI 0.2–22%) reported one severe adverse event (SAE) in the low dose group and five patients (8%, 95%CI 3–19%) reported 12 SAEs in the normal dose group during the extension phase. No SAE was assumed to be causally related to dose reduction in the low dose group. No patients had been admitted to the hospital due to a psoriasis exacerbation. In , AEoSI and SAEs and corresponding rates (event rates per month) were specified. As stated, statistical comparisons were not performed as the study was underpowered for this purpose.
Table 2. Safety during condor extension (t = 12 to 24 months).
One patient reported musculoskeletal complaints when using a low dose. Five patients in the normal dose group reported musculoskeletal complaints. No exacerbation or newly developed psoriatic arthritis (PsA) was reported in the normal dose group. In the low dose group, one patient was formally diagnosed with PsA in the extension phase. This diagnosis was based on a monoarthritis which occurred during the extension phase, combined with the fact that he had one episode of arthritis in the past. All AEs per patient in the extension phase are reported in Supplemental Table 2.
Maintenance of dose tapering (t = 12 to 24 months)
Baseline characteristics of the 26 patients that started on a low dose in this extension phase are presented in Supplemental Table 1. shows the proportion of patients that remain on a low dose during the extension phase, for each time point (month 12, 15, 18, 21, 24). Of these patients, 18/26 patients (69%, 95%CI 48–85%) remained on a low dose until the end of the extension phase (at 24 months). Eight out of 26 patients (31%, 95%CI 15–52%) returned to their original authorized full dose at the end of the extension phase. One patient (4%, 95%CI 0.2–22%) had a low dose but a PASI/DLQI > 5 at 24 months, but did not follow the advice to return to his/her previous effective dose earlier, therefore 17/26 patients (65%, 95%CI 44–82%) could be considered as having successful dose reduction (low dose and low PASI/DLQI). Of the patients with a low dose until end of study (n = 18), 11/18 (61%, 95%CI 36–82%) used 50% of their original dose, and 7/18 (39%, 95%CI 18–64%) used 67% of their original dose at 24 months.
Figure 3. Dose, PASI and DLQI of 26 patients that started the CONDOR extension phase on a low dose (1-year extension phase). (A) Proportions of patients with successful dose reduction or failure, and their specific dosages. (B) Psoriasis Area and Severity Index (PASI) course (medians with interquartile ranges (IQR)). Missing M12 = 0, M15 = 2, M18 = 2, M21 = 2 and M24 = 1. 3. (C) Dermatology Quality and Life Index (DLQI) course (medians with IQR). Missing M12 = 0, M15 = 3, M18 = 2, M21 = 2 and M24 = 3.
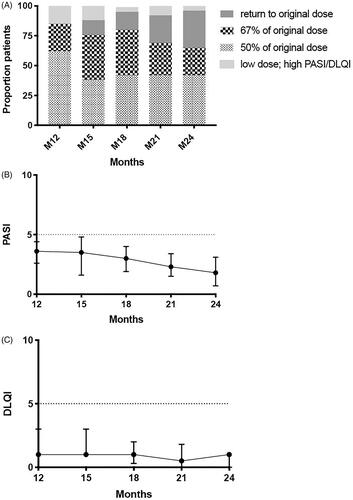
The median PASI scores of the 26 patients who entered the extension phase on a lower dose was 3.6 [interquartile range (IQR) 2.6–4.4] at 12 months, 3.5 [IQR 1.6–4.8] at 15 months, 3.0 [IQR 1.9–4.0] at 18 months, 2.3 [IQR 1.5–3.4] at 21 months, 1.8 [IQR 0.7–3.1] at 24 months, see . The median DLQI scores of 26 patients who entered the extension phase on a lower dose was 1.0 [IQR 0.0–3.0] at 12 months, 1.0 [IQR 1.0–3.0] at 15 months, 1.0 [IQR 0.3–2.0] at 18 months, 0.5 [IQR 0.0–1.8] at 21 months, 1.0 [IQR 0.0–1.0] at 24 months, see .
Analysis of course of disease flares in dose tapering and usual care (t = 0 to 24 months)
In the extension phase, three new persistent flares (7% 95%CI 2–20%) occurred in the DR group. Over the entire study period (CONDOR and extension, t = 0–24), 11 persistent flares (n = 10 patients, 23% 95%CI 12–38%)) were seen in the DR group. In UC, also three new persistent flares (7% 95%CI 2–20%) were observed in the extension period and seven (n = 6 patients, 14% 95%CI 6–28%) over the entire study period (CONDOR and extension) were seen, but note that there were more patients lost to follow up in UC than DR and direct comparisons cannot be made, as this leads to an underestimation of the flare rate in UC. After 24 months, two patients in the DR group and two patients in the UC group were still in a state of persistent flare, but follow up stopped due to end of study.
Of all patients with a persistent flare, two patients in the DR group and one patient in the UC group received a higher dose than the normal dose in order to regain clinical effectiveness. In both DR and UC, no patient switched to another biologic due to clinical ineffectiveness during the 24 month follow up period. Throughout the total follow up period, four DR patients preferred to stay on their low dose despite PASI/DLQI >5 and our advice to return to a normal dose. Two of those patients had a low PASI and DLQI score next follow-up visit and the other two patients had a persistent state of disease flare.
Discussion
The present study provides an additional year of follow-up for patients randomized in the CONDOR trial (Citation5). In this trial, a dose reduction strategy of adalimumab, etanercept and ustekinumab in psoriasis patients was tested. For further development and implementation of a dose reduction strategy, insight is needed into the effectiveness and safety of patients undergoing the strategy on the long run, and to assess how tenable dose reduction is with this strategy. We saw that in general, dose reduction leads temporarily to a slight increase of PASI scores groupwise, but on the long-term (after 18 months) the PASI decreased again. DLQI scores remained stably low during the whole period indicating that the temporary PASI increases had a limited effect on quality of life. Furthermore we demonstrate that 18 out of 44 (41%) patients from the initial CONDOR study were still on a low dose after 2 years. Of all patients with a low dose at the end of the CONDOR study (Citation5), 70% could maintain their low dose up to 2 years of follow up. Furthermore, we investigated if, and how, patients with a disease flare after a dose reduction attempt regained their state of low disease activity again. In dose reduction (DR) as well as usual care (UC), three persistent flares were observed in each group in the extension phase. In total, two patients in de DR group and one patient in the UC group needed a higher dosage than the authorized dose to treat a disease flare. During the 24 months of follow-up (CONDOR and extension), no patient needed to switch to another biologic to regain low disease activity. This implies that concerns regarding the possibility to regain effectiveness after failure of dose reduction may be redundant.
There were no safety signals that dose reduction led to any related problems like hospital admission due to psoriasis exacerbation or serum sickness due to antibody formation. In the dose reduction group, there was one patient with newly diagnosed psoriatic arthritis but, in hindsight, this patient had suspected complaints already before the CONDOR study. Musculoskeletal complaints were higher in DR than UC in CONDOR, but low in both groups during this extension period (Citation5).
Few studies have been published on long term results of dose tapering of biologics for psoriasis. However, all showed that lower dosages were feasible in a substantial part of patients with low disease activity (Citation8–10). The methodology of the present study (RCT vs. retrospective observational studies) and outcomes (definition of persistent flares) differ substantially from previous reports. In the study of Esposito et al. no clear dosing regimen was used and this contrasts with our tight controlled dose reduction strategy (Citation9). We found a lower proportion of patients with successful dose reduction compared to that study, which might be explained by the fact that we were relatively strict when returning to a higher dose. Furthermore, in a study of Hansel et al., 30 patients who achieved complete clearance (PASI100) under a normal dose underwent dose reduction; 18 of them (60%) maintained complete clearance during the observation period with a median of 60 months (Citation8). They also found that higher BMI was associated with a higher chance of relapse due to dose tapering. Of note, we found that even patients without a PASI100 at baseline could lower their dose successfully and are worthwhile to consider for our strategy, but it is important to note that the definition of success also differs between studies. In addition, in another study of Bezooijen et al. with a follow-up duration of 1.5 years, reassuringly the outcomes of disease activity, quality of life (at baseline) and proportion of patients on dose reduction were very similar to our results (Citation10). Nevertheless, this study was not randomized, small numbers of patients per biologic and different dosing schedules were used
A limitation of the present study was the fact that it was not powered to make strict statistical comparisons, e.g. on safety, as this extension phase was limited to subpopulation included in the CONDOR study. Reassuringly, baseline characteristics, like PASI, age, BMI, were comparable for these UC and DR subpopulations. Another factor that limited these comparisons was the fact that a part of the UC group did not complete the total extension phase, due to a switch to a lower dose.
Despite the small size of the population, the results of this extended observation of a dose reduction study on etanercept, adalimumab, and ustekinumab for psoriasis, provide important insights into the durability of this dose reduction strategy. We showed that 41% of patients could maintain their low dose up to 2 years, and the rise in PASI due to dose reduction was reversible in the vast majority of patients when following the reported dose reduction strategy. Moreover, DLQI scores remained low continuously and no SAEs related to dose reduction were reported. These reassuring results provide further support to implement the dose reduction strategy in psoriasis care for etanercept, adalimumab and ustekinumab, thereby improving personalized care for patients with psoriasis.
Supplemental Material
Download PDF (345.3 KB)Acknowledgements
We thank Mascha Eilander for her important contribution to this study.
Disclosure statement
S Atalay reported grants from Janssen Pharmaceuticals, Abbvie, Celgene and Novartis and congress fee from Celgene. All funding is not personal but goes to the independent Research Fund of the Department of Dermatology of the Radboud University Medical Center Nijmegen, The Netherlands. J M.P.A. van den Reek carried out clinical trials for AbbVie, Celgene and Janssen and has received speaking fees/attended advisory boards from AbbVie, Janssen, BMS, Almirall, LEO Pharma and Eli Lilly and reimbursement for attending a symposium from Janssen, Pfizer, Celgene and AbbVie. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud university medical center Nijmegen, the Netherlands. PCM van de Kerkhof received fees for consultancy service or lecturerships from Celgene, Almirall, Abbvie, Eli Lilly, Novartis, Jansen Pharmaceutica, Leo Pharma, Bristol Mayer Squib and Dermavant. EMGJ de Jong has received research grants for the independent research fund of the department of dermatology of the Radboud university medical center Nijmegen, the Netherlands from AbbVie, Pfizer, Novartis, Janssen Pharmaceuticals and Leo Pharma and has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Janssen Pharmaceutica, Novartis, Lily, Celgene, Leo Pharma, UCB and Almirall. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud university medical center Nijmegen (Radboudumc), the Netherlands. The other authors declare no conflict of interests.
Data availability statement
All data will be shared with restrictions upon reasonable request when legally and ethically permitted by Dutch law.
Trial registration: The CONDOR study was registered at ClinicalTrials.gov, NCT 02602925.
References
- Conrad C, Gilliet M. Psoriasis: from pathogenesis to targeted therapies. Clin Rev Allergy Immunol. 2018;54(1):102–113.
- Ronholt K, Iversen L. Old and new biological therapies for psoriasis. IJMS. 2017;18(11):2297.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet (London, England). 2007;370(9583):263–271.
- Griffiths CEM, Augustin M, Naldi L, et al. Patient-dermatologist agreement in psoriasis severity, symptoms and satisfaction: results from a real-world multinational survey. J Eur Acad Dermatol Venereol. 2018;32(9):1523–1529.
- Atalay S, van den Reek J, den Broeder AA, et al. Comparison of tightly controlled dose reduction of biologics with usual care for patients with psoriasis: a randomized clinical trial. JAMA Dermatol. 2020;156(4):393.
- Atalay S, van den Reek J, van Vugt LJ, et al. Tight controlled dose reduction of biologics in psoriasis patients with low disease activity: a randomized pragmatic non-inferiority trial. BMC Dermatol. 2017;17(1):6.
- Zweegers J, Groenewoud JMM, van den Reek J, et al. Comparison of the 1- and 5-year effectiveness of adalimumab, etanercept and ustekinumab in patients with psoriasis in daily clinical practice: results from the prospective BioCAPTURE registry. Br J Dermatol. 2017;176(4):1001–1009.
- Hansel K, Bianchi L, Lanza F, et al. Adalimumab dose tapering in psoriasis: predictive factors for maintenance of complete clearance. Acta Derm Venerol. 2017;97(3):346–350.
- Esposito M, Gisondi P, Conti A, et al. Dose adjustment of biologic therapies for psoriasis in dermatological practice: a retrospective study. J Eur Acad Dermatol Venereol. 2017;31(5):863–869.
- van Bezooijen JS, van Doorn MBA, Schreurs MWJ, et al. Prolongation of biologic dosing intervals in patients with stable psoriasis: a feasibility study. Ther Drug Monit. 2017;39(4):379–386.
