Abstract
Background
Chronic pruritus of unknown origin (CPUO) is a highly debilitating disease that lacks effective treatments. This study explores a new therapeutic strategy with dupilumab.
Objectives
To examine whether patients with CPUO demonstrate clinical response to dupilumab.
Patients and methods
This is a retrospective case series examining all patients with CPUO who were treated with dupilumab from March 2017 to December 2019 at a tertiary referral clinic at Washington University School of Medicine in St. Louis, MO. Numerical rating scale (NRS) itch score changes over time were recorded and analyzed.
Results
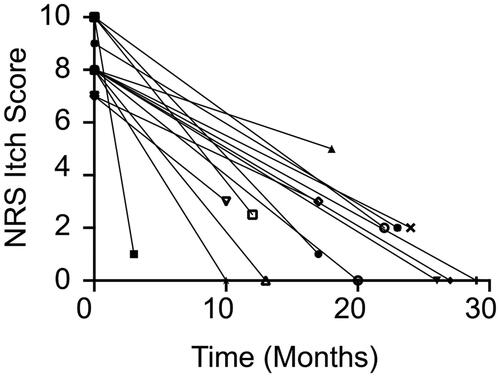
Fifteen patients (67% women; mean [SD] age, 68.7 [12.6] years [range, 42–88 years]) were included in the analysis. All patients had a diagnosis of CPUO for a mean [SD] 2.6 [2.8] years. The median [IQR] pruritus NRS itch score before dupilumab injection was 8 [8–10] and the final median [IQR] NRS itch score was 1 [0–2.5]. The mean [SD] reduction in the NRS itch score was 7.0 [1.9]. Dupilumab was well tolerated with one report of mild injection site reaction that was self-resolving.
Conclusion
This study suggests that dupilumab may be an effective treatment for patients with CPUO and supports the design of future randomized placebo-controlled trials to prove its efficacy.
Introduction
Classically, chronic pruritus has been viewed as a symptom of a primary dermatologic or systemic medical disorder. Thus, treatments have focused predominantly on the underlying disease rather than the symptom itself. However, it is increasingly recognized that many forms of itch can arise in the absence of a well-defined rash or medical condition. In this context, chronic pruritus of unknown origin (CPUO) is one such disorder for which there is no single FDA-approved treatment (Citation1,Citation2).
CPUO is a relatively recently recognized entity that presents with chronic pruritus for greater than 6 weeks without a defined causative underlying medical condition. Although patients with CPUO can present with excoriations and eczematous lesions in the skin, these findings are considered secondary changes. Further, recent studies suggest that both local and systemic type 2 inflammation underlie CPUO (Citation3) and that blocking the activity of the type 2 cytokines interleukin (IL)-4 and IL-13 on sensory neurons may act to alleviate itch by broadly suppressing neural hypersensitivity (Citation4). Based on these findings, we report our experience with dupilumab (anti-IL-4Rα monoclonal antibody [mAb]) in patients with CPUO in a retrospective observational fashion.
Methods
The patients were diagnosed with CPUO according to a recently published set of consensus recommendations (Citation1). The numerical rating scale (NRS) itch score is a single-question assessment tool measured over the prior 24 h with a scale of 0 (no itch) to 10 (worst imaginable itch) where itch is typically stratified between 0–3 (mild), 4–6 (moderate), and ≥7 (severe).
This retrospective case series had the following inclusion criteria: (1) chronic pruritus >6 weeks duration, (2) baseline NRS itch score of ≥7 (severe), (3) consecutive recruitment between March 2017 to December 2019, and (4) dupilumab (600 mg subcutaneous [SC] injection followed by 300 mg SC biweekly) treatment. Exclusion criteria included: (1) any primary dermatologic disorder that could account for the pruritus, and (2) any lab or radiologic abnormality based on recent consensus recommendations that could account of their itch (Citation1). Further, if patients met diagnostic criteria for atopic dermatitis or had evidence of prurigo nodularis or any other defined dermatitis, they were excluded. Recruitment was made by non-selectively enrolling all patients meeting the above-mentioned criteria regardless of the treatment results and concomitant diseases.
Given that this is a case series, no concomitant medications were stopped, and the use of emollients and topical steroids was allowed. Treatment response was assessed using change in NRS itch score from prior to treatment with dupilumab to the most current NRS itch score at time of data analysis.
Differences were considered statistically significant for a two-tailed p < .05. This study was approved by the Institutional Review Board of Washington University in St. Louis (Missouri, USA) and followed the tenets of the Declaration of Helsinki (IRB ID No. 201412117 and 201605144).
Results
A total of 15 patients were evaluated based on our inclusion/exclusion criteria. The mean [SD] age was 68.7 [12.6] years and 9/15 were female. The mean [SD] duration of itch prior to presentation was 2.6 [2.8] years. The median [interquartile range, IQR] time from baseline NRS itch score to the primary endpoint NRS itch analysis was 19 [10–26] months. All baseline characteristics are presented in .
Table 1. Patient demographics, NRS scores, and follow-up time of improvement of patients.
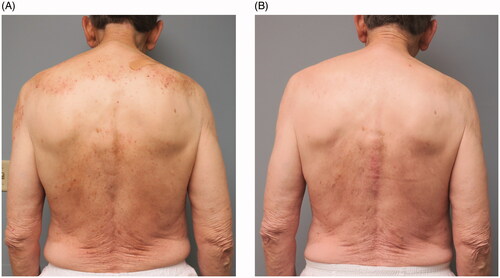
All of the 15 subjects experienced marked reduction in pruritus symptoms as measured by the NRS itch score from baseline (). The median [IQR] baseline 24-h NRS itch score was 8 [8–10] with a final NRS itch score of 1 [0–2.5]. The mean [SD] reduction in the NRS itch score was 7.0 [1.9] (). Given that these patients were evaluated during the course of routine care, systematic daily assessment of itch improvement over time was not acquired from all patients. Notwithstanding this, two of the subjects (Patient 2 and Patient 15) reported notable improvement of itch within 72 h of their first injection of dupilumab. Interestingly, one subject (Patient 9) reported delayed improvement of pruritus between 6 and 10 months after initiating treatment. Four patients (Patients 3, 4, 5, and 13) were followed over 24 months and the efficacy of dupilumab was observed consistently throughout this time period (). All patients did not have rash at baseline, however, some patients did exhibit mild or moderate excoriations on the back () that resolved with treatment (). One patient experienced a mild injection site reaction that then improved and no other adverse events (AEs) were observed such as conjunctivitis or facial dermatitis. Collectively, these findings suggest that dupilumab may be an effective and safe therapeutic for patients with CPUO. However, prospective randomized placebo-controlled clinical trials are needed to prove both efficacy and safety for this indication.
Discussion
In the absence of US Food and Drug Administration-approved therapies for CPUO, a wide range of traditional therapies have been employed including oral and topical corticosteroids, phototherapy, neuromodulators like gabapentin, and steroid-sparing immunosuppressants like azathioprine (Citation5). However, there are currently no universally effective treatments and data on newer agents are limited.
New advances in neuroimmunology have revealed that targeting cytokines at the immune-sensory neuron interface represents a novel therapeutic strategy. The first pruritogenic cytokine, IL-31, is now a biologic target for nemolizumab (anti-IL-31RA mAb) in atopic dermatitis and prurigo nodularis (Citation6,Citation7), and has recently been shown to be elevated in the sera of patients with CPUO (Citation8). In addition to IL-31, recent advances in our understanding of IL-4 and IL-13 and downstream Janus kinase (JAK) on sensory neurons in itch (Citation4) have advanced a paradigm in which JAK inhibitors, in addition to their anti-inflammatory properties, may have neuromodulatory mechanisms of action in itch. Indeed, recent proof-of-concept studies have suggested anti-itch efficacy of both the JAK inhibitor tofacitinib and dupilumab in CPUO (Citation9,Citation10). Thus, the current study lends further support for pharmacologic disruption of cytokine-neural interactions as a potential treatment strategy for CPUO.
However, not all agents investigated in clinical studies for CPUO have demonstrated success. A recent proof-of-concept phase 2a study of apremilast, a phosphodiesterase 4 inhibitor, demonstrated poor tolerability and could not be evaluated for efficacy in CPUO (Citation11). Additionally, the first randomized placebo-controlled trial in CPUO with the neurokinin 1 receptor antagonist serlopitant did not demonstrate efficacy over placebo in phase 2 studies (Citation12) and also failed to show efficacy in phase 3 studies in prurigo nodularis (Citation13). Collectively, these findings suggest that certain pathways may be more suitable as a therapeutic target in CPUO.
Given that our study is a consecutive case series and these patients were undergoing routine care, there are limitations. The study was not randomized, unblinded, and some variables could not be controlled like concomitant medications and assessment timepoints. Notwithstanding these limitations, our findings coupled with other recent studies (Citation14,Citation15), provide a scientific premise to support a proper evaluation of the efficacy of dupilumab for CPUO.
Conclusions
Dupilumab may have anti-itch efficacy in CPUO and was well tolerated in 15 patients with CPUO. Future randomized double-blind placebo-controlled trials are warranted to determine the efficacy of dupilumab in this highly debilitating medical condition which currently lacks effective therapies.
Disclosure statement
Dr. Kim has served as a consultant for AbbVie, Almirall, Cara Therapeutics, LEO Pharma, Menlo Therapeutics, Pfizier, and Sanofi Genzyme. He has also participated on the advisory board for Almirall, Boehringer Ingelheim, Cara Therapeutics, Kiniksa Pharmaceuticals, Menlo Therapeutics, Trevi Therapeutics, Regeneron Pharmaceuticals, and Sanofi Genzyme. He is also Founder, Chief Scientific Officer, and stockholder of Nuogen Pharma, Inc. He is stockholder of Locus Biosciences. Dr. Kim has a patent pending for the use of JAK inhibitors for chronic itch. All other authors have no conflict of interest to declare.
Additional information
Funding
References
- Kim BS, Berger TG, Yosipovitch G. Chronic pruritus of unknown origin (CPUO): Uniform nomenclature and diagnosis as a pathway to standardized understanding and treatment. J Am Acad Dermatol. 2019;81(5):1223–1224.
- Millington GWM, Collins A, Lovell CR, et al. British Association of Dermatologists' guidelines for the investigation and management of generalized pruritus in adults without an underlying dermatosis, 2018. Br J Dermatol. 2018;178(1):34–60.
- Berger TG, Steinhoff M. Pruritus in elderly patients-eruptions of senescence. Semin Cutan Med Surg. 2011;30(2):113–117.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217–228.e13.
- Maley A, Swerlick RA. Azathioprine treatment of intractable pruritus: A retrospective review. J Am Acad Dermatol. 2015;73(3):439–443.
- Silverberg JI, Pinter A, Pulka G, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2020;145(1):173–182.
- Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382(8):706–716.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351.
- Wang F, Morris C, Bodet ND, et al. Treatment of refractory chronic pruritus of unknown origin with tofacitinib in patients with rheumatoid arthritis. JAMA Dermatol. 2019;155(12):1426.
- Zhai LL, Savage KT, Qiu CC, et al. Chronic pruritus responding to dupilumab—A case series. Medicines. 2019;6(3):72.
- Clark M, Wang F, Bodet ND, et al. Evaluation of apremilast in chronic pruritus of unknown origin: A proof-of-concept, phase 2a, open-label, single-arm clinical trial. Health Sci Rep. 2020;3(2):e154.
- A randomized, double-blind, placebo-controlled study of the efficacy, safety, and tolerability of serlopitant for the treatment of chronic pruritus of unknown origin. ClinicalTrials.gov identifier: NCT03841331. Updated February 10, 2020. Accessed May 8, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT03841331.
- A randomized, double-blind, placebo-controlled study of the efficacy, safety, and tolerability of serlopitant for the treatment of pruritus in adults with prurigo nodularis. ClinicalTrials.gov identifier: NCT03546816. Updated March 5, 2020. Accessed May 14, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT03546816.
- Hendricks AJ, Yosipovitch G, Shi VY. Dupilumab use in dermatologic conditions beyond atopic dermatitis - a systematic review. J Dermatolog Treat. 2021;32(1):19–28.
- Yang EJ, Murase JE. Recalcitrant anal and genital pruritus treated with dupilumab. Int J Womens Dermatol. 2018;4(4):223–226.