Abstract
Objective
To evaluate whether early Psoriasis Area Severity Index (PASI) improvements can predict week 28 tildrakizumab responders and nonresponders.
Methods
Psoriasis patients pooled from two tildrakizumab phase 3 trials randomized to receive tildrakizumab 100 mg at weeks 0, 4, 16, and 28 were included. Patients were grouped by week 28 PASI responses (<50, 50–74, 75–89, and 90–100). PASI improvements from baseline at weeks 4 and 16 were analyzed for each response group.
Results
Of 575 patients included, 8.3%, 14.3%, 23.8%, and 53.6%, respectively, achieved PASI <50, 50–74, 75–89, and 90–100 at week 28. Of patients with PASI <50 at week 16, 85% did not achieve PASI ≥75 at week 28 (nonresponders). Rapid response, defined as PASI ≥50 at week 4 (after a single tildrakizumab dose), was observed in 41% of patients. Of these patients, 87% were week 28 responders (PASI ≥75); 67% were ‘super responders’ (PASI 90–100). Among week 28 responders and super responders, 45% and 50% achieved PASI ≥50 at week 4, respectively.
Conclusions
Tildrakizumab week 28 nonresponders can be identified by week 16 PASI response. PASI improvements as early as week 4 can predict patients’ week 28 PASI improvement status.
Introduction
Treatment for moderate-to-severe psoriasis has improved in the past two decades with the advent of the biologic era and continuing efforts to optimize patient outcomes [Citation1]. Optimization of therapy requires a tailored approach to ensure that patients do not receive prolonged treatment that is unlikely to be adequate and are not exposed to unnecessary risks for adverse effects, as well as improving patient quality of life and reducing overall costs.
Given that there is no established biomarker at this time that can reliably predict response to psoriasis treatment, early indicators of treatment response are valuable and may provide physicians with information to help tailor psoriasis therapy. Several studies have investigated the utility of short-term responses to psoriasis treatments in predicting longer-term outcomes. For example, pooled data from phase 3 studies of the interleukin (IL)-17A inhibitor secukinumab show a correlation between early onset of response, defined as a ≥50% improvement in Psoriasis Area Severity Index score (PASI 50) at week 4 or 8, and sustained efficacy at week 16 [Citation2]. Similarly, for the IL-17 inhibitor ixekizumab, early PASI 40 response at weeks 4 and 6 was predictive of PASI 75 response at week 12 [Citation3]. Studies in patients with moderate-to-severe psoriasis treated with tofacitinib, an oral Janus kinase inhibitor, have reported that PASI improvement at weeks 8 and 12 was highly predictive of PASI 75 or greater response at week 16 [Citation4]. Early response to certolizumab pegol in patients with psoriatic arthritis has also been associated with greater achievement of treatment targets at later time points; however, skin response in the total population was not investigated [Citation5].
Tildrakizumab, a humanized, immunoglobulin G1/κ monoclonal antibody that selectively inhibits the p19 subunit of interleukin (IL)-23, is approved for the treatment of adult patients with plaque psoriasis in the US, Europe, Australia, and Japan. Tildrakizumab 100 and 200 mg were effective in two phase 3 randomized controlled trials (reSURFACE 1 and 2) enrolling patients with moderate-to-severe plaque psoriasis [Citation6]. A pooled post hoc analysis demonstrated separation of the response curves at week 8 for patients achieving week 28 PASI responses of <50% and ≥50%, suggesting that patients unlikely to respond to tildrakizumab could be identified after just two doses [Citation7]. Furthermore, patients likely to be ‘super responders’ (defined as achieving a PASI 90 response or higher at week 28) could be identified as early as week 4 [Citation7].
Based on these findings, the objective of this pooled post hoc analysis was to further evaluate whether PASI improvement at weeks 4 and 16 in patients treated with tildrakizumab could identify PASI responders (PASI ≥75) and super responders (PASI ≥90) at week 28.
Methods
Study population
For this post hoc analysis, pooled data from the reSURFACE 1 and reSURFACE 2 trials of tildrakizumab were examined [Citation6]. Briefly, eligible participants were aged 18 years or older, had moderate-to-severe chronic plaque psoriasis for at least 6 months at baseline, and were candidates for phototherapy or systemic therapy [Citation6]. Both trials had 3 parts. In Part 1 (0–12 weeks), participants were randomized to receive subcutaneous tildrakizumab 100 mg or 200 mg, or placebo at weeks 0, 4, and every 12 weeks thereafter, with appropriate additional placebo injections given to maintain the blind. In Part 2 (12–28 weeks), placebo-treated patients were re-randomized to receive tildrakizumab 100 mg or 200 mg at weeks 12, 16, and every 12 weeks thereafter. The reSURFACE 2 trial also included a subcutaneous etanercept 50 mg arm, with treatment given twice weekly in Part 1 and once weekly in Part 2[Citation6] of the study. In Part 3 (28–64 weeks, reSURFACE 1; 28–52 weeks, reSURFACE 2), patients who were randomized to tildrakizumab in Part 2 and had a PASI 50 response or higher at week 28 were re-randomized to placebo, or continued or increased their tildrakizumab dose, while patients with PASI <50 at week 28 were discontinued [Citation8]. This post hoc analysis only included patients who were randomized to receive tildrakizumab 100 mg at weeks 0, 4, 16, and 28.
Statistical analyses
For this analysis, four mutually exclusive groups were created based on each patient’s PASI improvement at week 28: PASI <50, PASI 50–74, PASI 75–89, and PASI 90–100. Patients with PASI ≥75 improvement at week 28 were considered to be ‘responders,’ those with PASI ≥90 improvement were considered ‘super responders,’ those with PASI 50–74 improvement at week 28 were considered ‘partial responders,’ and those with PASI <50 at week 28 were considered ‘nonresponders.’ In order to examine PASI changes over time by week 28 response group, mean percent PASI improvements from baseline to week 28 were examined for each group.
The association between PASI improvements at weeks 4 and 16 and PASI response status (nonresponder, partial responder, responder, and super responder) at week 28 was analyzed descriptively. Early PASI improvements were evaluated at weeks 4 and 16 and patients were categorized using a cutoff threshold of PASI 50 (i.e. if patients achieved a PASI improvement <50 or ≥50). At both time points, within each PASI 50 response subgroup, the proportions of patients who went on to be week 28 PASI nonresponders, partial responders, responders, and super responders were calculated.
Positive predictive values (PPV) and negative predictive values (NPV) for use of early PASI improvements at weeks 4 and 16 to predict PASI responder and nonresponder status at week 28 were calculated. For all analyses, calculations were based on observed data. Percent of PASI improvements were reported as mean and 95% confidence intervals (CI).
Results
Overall, 575 patients received tildrakizumab 100 mg from baseline to week 28 and were included in this analysis. The mean age of patients was 45.6 years, and 69.5% were male. The majority (77.4%) of patients achieved a PASI 75 response or greater by week 28. The proportions of patients achieving PASI <50, PASI 50–74, PASI 75–89, and PASI 90–100 improvements at week 28 were 8.3%, 14.3%, 23.8%, and 53.6%, respectively (). Compared with patients with PASI 90–100 improvement at week 28, patients with PASI <50 improvement had longer duration of disease and higher body weight, although there was no clear relationship between higher weight and lower PASI response (). Patients with PASI <50 improvement at week 28 were older, and higher proportions were male, previously used biologics before receiving tildrakizumab, and had comorbid cardiovascular disease relative to patients with PASI ≥50 response at week 28. The proportion of patients with psoriatic arthritis was higher among super responders (PASI 90–100; 16.9%) compared with nonresponders (PASI <50; 14.6%).
Figure 1. Percent PASI improvements from baseline to week 28 by week 28 PASI response subgroup. Percent values by the end of each line represent the percentage of patients in each week 28 PASI category. PASI responders were defined as patients with PASI ≥75, super responders as patients with PASI ≥90, partial responders as patients with PASI 50–74, and nonresponders as patients with PASI <50 improvement at week 28. CI: confidence interval; PASI: Psoriasis Area and Severity Index.
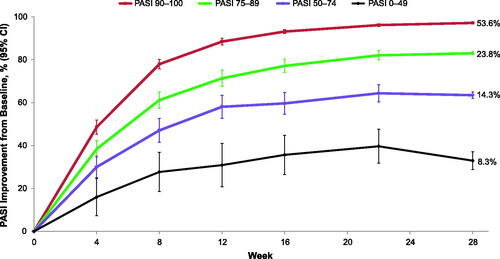
Table 1. Baseline demographic and clinical characteristics.
PASI responses improved continuously from baseline over time for all week 28 PASI response groups, with non-overlapping response curves observed as early as week 8 (). Week 28 responders experienced greater PASI improvements throughout the 52-week study period compared with week 28 nonresponders (data not shown).
Among patients with PASI <50 at week 16, 85% did not achieve PASI responder status (PASI ≥75) at week 28 (). Among patients with PASI <50 improvement at week 28, 58% did not achieve the PASI ≥50 improvement threshold at week 16. The PPV of PASI <50 improvement at week 16 to predict week 28 nonresponder status (PASI <75) was 84.8% (i.e. for a patient with PASI <50 improvement at week 16, the probability of being a week 28 nonresponder was 84.8%) ().
Figure 2. Week 16 PASI responses are predictive of week 28 nonresponders/partial responders (PASI <75). PASI responders were defined as patients with PASI ≥75, super responders as patients with PASI ≥90, partial responders as patients with PASI 50–74, and nonresponders as patients with PASI <50 improvement at week 28. PASI: Psoriasis Area and Severity Index.
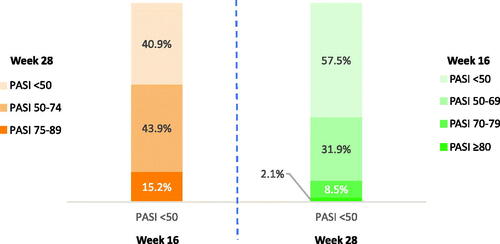
Table 2. Predictive value of week 16 PASI improvement for predicting week 28 partial responders/nonresponders (PASI <75).
Rapid response, defined as PASI ≥50 improvement at week 4, was observed in 41% of tildrakizumab-treated patients (i.e. after receiving a single dose of tildrakizumab at baseline). Among these patients, 87% achieved responder status (PASI ≥75) at week 28, and two-thirds achieved super responder status (PASI ≥90) (). Among week 28 responders and super responders, 45% and 50%, respectively, met the PASI ≥50 improvement threshold at week 4 (). The PPV of PASI ≥50 improvement at week 4 to predict week 28 responder and super responder status was 87.0% and 66.7%, respectively (). Thus, if a patient met the PASI ≥50 improvement threshold at week 4, the probability of becoming a PASI responder or super responder at week 28 was 87.0% and 66.7%, respectively.
Figure 3. Week 4 PASI responses are predictive of week 28 super responders (PASI ≥90) and responders (PASI ≥75). PASI responders were defined as patients with PASI ≥75, super responders as patients with PASI ≥90, partial responders as patients with PASI 50–74, and nonresponders as patients with PASI <50 improvement at week 28. PASI: Psoriasis Area and Severity Index.
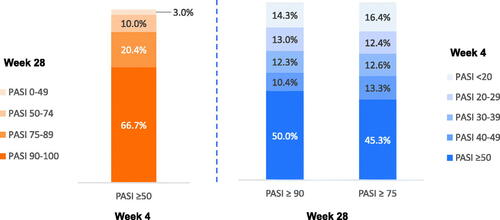
Table 3. Predictive value of week 4 PASI improvement for predicting week 28 responders (PASI ≥75) and super responders (PASI ≥90).
Discussion
Early prediction of treatment response may avoid potential treatment-related side effects and wastage of economic resources in patients unlikely to respond to treatment. The benefits include improved patient quality of life, reduced healthcare expenditures associated with ineffective treatment, and allowing healthcare providers to switch patients to more effective treatment tailored to their needs. This pooled post hoc analysis provides important information on the ability of early response or nonresponse to tildrakizumab 100 mg to predict later outcomes. Among patients with moderate-to-severe plaque psoriasis treated with tildrakizumab 100 mg, approximately three-quarters became PASI responders by week 28. Over 80% of patients who did not meet the PASI ≥50 improvement threshold at week 16 (i.e. after two doses of tildrakizumab) did not become week 28 responders. At week 4, after receiving one dose, over 40% of patients met the PASI ≥50 improvement threshold; two-thirds of these patients became PASI super responders (PASI ≥90 improvement) at week 28, and an additional 20% achieved PASI 75 response or greater. The PPV of PASI <50 improvement at week 16 to predict week 28 nonresponse, and the PPV of PASI ≥50 improvement at week 4 to predict week 28 response, were both above 80%. Response to tildrakizumab as early as week 4 is therefore an informative predictor of later response; nonresponse by week 16 is predictive of later nonresponse.
Various thresholds for this analysis were carefully considered. In clinical practice, some physicians may evaluate treatment efficacy before week 16. However, although this may be a relevant time point for older biologics with more frequent dosing schedules, we considered nonresponse at week 16 to allow for the full effect of two doses of tildrakizumab. In this analysis, early PASI improvement was defined as 50% improvement from baseline, which is generally considered the lowest threshold for clinical response to psoriasis treatment. It was therefore thought to be an appropriate early threshold to predict greater PASI improvement at a later time point.
A PASI 75 response has historically been used as the primary endpoint in clinical trials. However, more recently, as therapies have improved and expectations have risen, PASI 90 response has been used to define treatment success. Achieving PASI 90 response is associated with improved quality of life compared to achievement of PASI 75 response [Citation9]. Focusing on this threshold, two-thirds of patients with an early response to tildrakizumab achieved PASI ≥90; the PPV of using week 4 early improvement to assess week 28 PASI ≥90 response was 66.7%.
The predictiveness of early response to psoriasis treatment has not been studied extensively. However, few studies among psoriasis patients treated with other therapies have used early treatment response to predict patients likely to achieve later treatment targets. The results of the current analysis support previous work demonstrating that early response is a good predictor of later response. In particular, the results are in line with a study in patients with moderate-to-severe psoriasis treated with tofacitinib, in which meeting the PASI 50 improvement threshold at week 8 was a good predictor of PASI 75 response at week 16 [Citation4]. The PPV and NPV for use of week 8 response to tofacitinib to predict PASI 75 at week 16 were 80% and 81%, respectively. Similarly, week 4 and 6 improvements of PASI 50 in patients receiving ixekizumab were highly predictive of PASI 75 response at week 12, with NPVs of 71% and 89%, and PPVs of 94% and 89%, respectively [Citation3].
While previous work supports the finding here that early PASI response is a valid predictor of later response, there is a lack of information on outcomes after week 16. As psoriasis is a chronic disease, sustained response to therapy is key. Thus, week 28 responses were examined in the present study. Although this study assessed a longer response period than previous reports, week 28 is still relatively short-term. Long-term efficacy is critical, and the ability to predict durable response would be valuable in future research. In addition, previous work has not consistently analyzed whether early nonresponse can predict later nonresponse. This information is equally valuable for clinicians, who may opt to discontinue a treatment that is not likely to prove efficacious.
Despite careful study design, there are a number of limitations to the current analysis, most notably the post hoc nature of a pooled study and the use of observed data. However, although observed data were used, data for only a few patients were missing through week 28 (1 to 2 missing patients at each time point), and using imputed data would not have changed the results. The study was based on clinical trials with selected patients, and therefore the findings may not be generalizable to all patients with psoriasis. This analysis used percentage PASI improvement from baseline to evaluate clinical response, which may limit clinical utility, and future studies may consider a similar analysis using other measures typically used by clinicians in their real-world practice, such as absolute PASI improvement. Multivariate analysis was not used to assess other predictors of clinical response. However, previous work has found that early response is a stronger predictor of later response relative to baseline characteristics [Citation4].
Given the burden of psoriasis and its treatment, it is important for physicians to select an effective treatment for each patient and to diminish exposure to treatment that could lead to potentially unnecessary risk and cost. Methods to determine early response can be a tool to aid in this decision process. The majority of patients with moderate-to-severe psoriasis treated with tildrakizumab 100 mg achieved PASI 75 or greater responses at week 28. PASI improvements as early as week 4 may be used to predict week 28 PASI responder (PASI ≥75) and super responder (PASI 90–100) status. Patients who did not achieve PASI 50 by week 16 were unlikely to become responders (PASI ≥75) at week 28. By week 16, few patients were nonresponders, and early nonresponders were unlikely to become responders by week 28. In comparison, two-thirds of patients who achieved PASI ≥50 at week 4 achieved PASI ≥90 by week 28, while another 20% achieved PASI ≥75.
Acknowledgments
Editorial support was provided by Clare Byrne, Becky Hanna, and Alan Pedder of Asclepius Analytics, and by Hilary Durbano, PhD, of AlphaBioCom, LLC.
Disclosure statement
SRF received research, speaking and/or consulting support from Abbvie; Advance Medical; Almirall; Alvotech; Arena; BMS; Boehringer Ingelheim; Caremark; Celgene; Forte; Galderma, GSK/Stiefel, Helsinn; Informa; Janssen; LEO Pharma; Lilly; Menlo; Merck; Mylan; National Biological Corporation; National Psoriasis Foundation; Novan; Novartis; Ortho Dermatology; Pfizer; Qurient; Regeneron; Samsung; Sanofi; Sun Pharmaceutical Industries, Inc.; Suncare Research; and UpToDate. He consults for others through Guidepoint Global, Gerson Lehrman and other consulting organizations. He is founder and majority owner of www.DrScore.com<http://www.DrScore.com>. He is also a founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. JFM is a consultant and/or investigator for AbbVie, Arena, Avotres, Biogen, Bristol-Myers Squibb, Celgene, Dermavant, Eli Lilly, EMD Sorono, Janssen, LEO Pharma, Merck, Novartis, Pfizer; Regeneron; Sanofi; Sun Pharmaceutical Industries, Inc.; and UCB. DMP has served as a consultant for Brickel Biotechnology, Biofrontera AG, Celgene Corporation, Dermira, DUSA Pharmaceuticals Inc., LEO Pharma US, Novartis, Promius Pharmaceuticals, Regeneron, Sanofi, TheraVida, Inc., and Bausch Health Companies, Inc.; has received grant/research support as principal investigator for Abbott Laboratories, Amgen, Asana Biosciences, Brickel Biotechnology, Celgene Corporation, Dermavant Sciences, Eli Lilly, LEO Pharma US, Merck & Co., Inc., Novartis, Novo Nordisk, Ortho Dermatologics, Peplin Inc., Pfizer Inc., Photocure ASA, Promius Pharmaceuticals, Regeneron, Stiefel, a GSK company, and Bausch Health Companies, Inc.; and has been on an advisory board for Pfizer. YZ and JZ were employed by Sun Pharmaceutical Industries, Inc. at the time the study was conducted. AMM is a former employee of Sun Pharmaceutical Industries, Inc., and has individual shares in Johnson and Johnson, and as part of retirement account/mutual funds. ABG received honoraria as an advisory board member and consultant for AnaptypsBio; Avotres Therapeutics; Beiersdorf; Boehringer Ingelheim; Bristol-Myers Squibb Co.; Eli Lilly; Janssen; LEO Pharma; Novartis; Sun Pharmaceutical Industries, Inc.; UCB; and Xbiotech (stock options); and research/educational grants from Boehringer Ingelheim; Janssen; Novartis; Sun Pharmaceutical Industries, Inc.; UCB; and Xbiotech (all paid to Mount Sinai School of Medicine).
Additional information
Funding
References
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82(1):161–201.
- Pinter A, Gerdes S, Papavassilis C, et al. Characterization of responder groups to secukinumab treatment in moderate to severe plaque psoriasis. J Dermatolog Treat. 2020;31(8):769–775.
- Zhu B, Edson-Heredia E, Cameron GS, et al. Early clinical response as a predictor of subsequent response to ixekizumab treatment: results from a phase II study of patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2013;169(6):1337–1341.
- Tan H, Valdez H, Griffins CE, et al. Early clinical response to tofacitinib treatment as a predictor of subsequent efficacy: results from two phase 3 studies of patients with moderate-to-severe plaque psoriasis. J Dermatolog Treat. 2017;28(1):3–7.
- van der Heijde D, Deodhar A, Fleischmann R, et al. Early disease activity or clinical response as predictors of long-term outcomes with certolizumab pegol in axial spondyloarthritis or psoriatic arthritis. Arthritis Care Res (Hoboken). 2017;69(7):1030–1039.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288.
- Blauvelt A, Sofen H, Papp K, et al. Tildrakizumab efficacy and impact on quality of life up to 52 weeks in patients with moderate-to-severe psoriasis: a pooled analysis of two randomized controlled trials. J Eur Acad Dermatol Venereol. 2019;33(12):2305–2312.
- Reich K, Warren RB, Iversen L, et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol. 2020;182(3):605–617.
- Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645–648.
