Abstract
Background
Baricitinib previously demonstrated improvements in itch and sleep disturbance versus placebo in adults with moderate-to-severe atopic dermatitis (AD).
Objectives
Examine if itch and sleep improvements are associated with better quality of life (QoL) and productivity in patients with AD.
Methods
Data were drawn from BREEZE-AD5 (NCT03435081). Itch and sleep improvement at Week 16 were defined using ≥4-point improvements in the Itch Numeric Rating Scale and ≥1.5 decreases in the number of nighttime awakenings since baseline, respectively. Patients with and without improvements were compared on Dermatology Life Quality Index (DLQI) and Work Productivity and Activity Impairment-AD scores. Changes from baseline were analyzed using ANCOVA with last observation carried forward. Proportions were analyzed using logistic regression with non-responder imputation.
Results
Greater proportions of patients with versus without itch improvement indicated no impact of AD on QoL (37.7 vs. 1.8%). Patients with itch improvement had greater decreases in work time impaired (−29.3 vs. −5.6%). More patients with versus without sleep improvement reported no effect of AD on QoL (25.5 vs. 1.1%); patients with better sleep experienced larger reductions in work time spent impaired (−33.3 vs. −6.1%).
Conclusions
Patients with AD who experienced itch and sleep improvement had significantly better QoL and productivity.
Introduction
Atopic dermatitis (AD) is a heterogenous, chronic, relapsing, highly symptomatic, inflammatory skin disease often characterized by pruritic, erythematous, and eczematous skin lesions (Citation1). Signs and symptoms of AD can lead to significant disease burden in patients (Citation2). An estimated 85–91% of patients report daily itch (Citation2,Citation3), the primary source of morbidity in AD (Citation4). Patients may experience pruritic symptoms for most of the day (Citation2), resulting in persistent scratching, skin damage, and sleep disturbance.
As two of the most bothersome symptoms of AD, itch and its resultant sleep disturbance can significantly worsen quality of life (QoL), limit patients’ lifestyle, and lead to psychological distress (Citation5–7). Further, these symptoms can adversely affect patients’ daily functioning and productivity at work, as frequent itching causes distractions and poor sleep quality results in daytime fatigue and poor concentration (Citation8). Addressing these burdensome symptoms is thus a primary goal of AD treatment (Citation9).
Janus kinase (JAK) inhibitors are an emerging class of therapeutics for the treatment of AD (Citation10). Evidence suggests JAK inhibition can improve skin barrier function and reduce itch (Citation11). In a Phase 3 study of patients with moderate-to-severe AD, baricitinib, an oral, selective JAK1/JAK2 inhibitor, demonstrated significantly greater improvements in itch and sleep disturbances due to itch compared to placebo (Citation12). Using data from this clinical trial, this analysis sought to examine if improvements in itch and sleep are associated with enhanced QoL and work productivity.
Materials and methods
Study population
This is a post hoc analysis of the first 16 weeks of BREEZE-AD5 (NCT03435081), a Phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study evaluating the efficacy and safety of once-daily baricitinib 1 mg and 2 mg versus placebo in adults with moderate-to-severe AD in the United States and Canada (Citation12). The study was conducted in accordance with ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol and amendments were approved by the appropriate institutional review boards/ethics committees at each study site, and all patients provided written informed consent. No additional ethical approval was required to conduct the present analysis.
Patients were ≥18 years and had a diagnosis of AD, as defined by the American Academy of Dermatology, at least 12 months prior to screening. Patients had moderate-to-severe disease at screening and baseline as defined by an Eczema Area and Severity Index (EASI) score ≥16, a validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD™) score ≥3, and ≥10% body surface area (BSA) involvement. In addition, patients were required to have a documented history of inadequate response to or intolerance of topical medications.
The following analysis was conducted on the intent-to-treat (ITT) population. Also of interest was the ITT subpopulation of patients with baseline BSA 10–50%, who have been identified as having an enhanced response rate with baricitinib 2 mg (Citation13).
Measures
Itch severity
Itch was measured with the Itch Numeric Rating Scale (NRS), a single item designed to capture information on self-reported severity of worst itching each day. Patients were asked to rate itching severity based on the worst level of itching in the past 24 hours using an 11-point scale from 0 (‘no itch’) to 10 (‘worst itch imaginable’). This information was entered into an electronic diary at the end of each patient’s day. Weekly mean scores using the previous 7 days were calculated if at least 4 of the 7 diary values were non-missing. Patients with baseline Itch NRS ≥4 were classified as having improvements in itch (yes or no) if they achieved a ≥ 4-point decrease in the Itch NRS at Week 16; this cutoff was derived from the minimal clinically important difference (MCID) (Citation14).
Sleep disturbance
The Atopic Dermatitis Sleep Scale (ADSS) captures the self-reported impact of itch on sleep each day, including difficulty falling asleep (Item 1) and number of nighttime awakenings (Item 2) the previous night. Each item is scored individually. For Item 1, patients selected a score ranging from 0 (‘not at all’) to 4 (‘very difficult’). For Item 2, patients selected the number of times they woke up each night, ranging from 0 to 29 times. Weekly mean scores were computed if at least 4 of 7 previous diary values were non-missing. As the MCID has been defined for the ADSS Item 2 (Silverberg et al., unpublished data, 2021), only Item 2 is used in the present analysis. Patients with a baseline ADSS Item 2 score ≥1.5 were categorized as having experienced sleep improvement (yes or no) if they had a ≥ 1.5 decrease in the number of nighttime awakenings from baseline per weekly average at Week 16.
Quality of life
Patients’ QoL was assessed using the Dermatology Life Quality Index (DLQI), a validated, self-administered measure of the impact of AD on QoL (Citation15). The 10-item DLQI asks about experiences in the ‘last week’ and covers 6 domains, including symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment. Responses range from 0 (‘not at all’) to 3 (‘very much'); scores span from 0 to 30, with higher scores representing greater effect on QoL.
In this analysis, the proportion of patients with a DLQI total score of 0 or 1 at Week 16 was determined, representing patients for whom AD has no impact on QoL. Also calculated were the change from baseline in the DLQI score, the proportion of patients at Week 16 with a total score ≤5, indicating a small or no effect of AD on QoL (Citation16), and the proportion of patients who achieved ≥4-point improvement from baseline, which is considered the MCID threshold (Citation17).
Work productivity and daily living
The Work Productivity and Activity Impairment Questionnaire – Atopic Dermatitis (WPAI-AD) was used to evaluate impairment due to AD in the last 7 days. The WPAI-AD includes 6 items grouped into 4 domains: absenteeism (work time missed), presenteeism (work time spent impaired), overall work impairment (total productivity loss associated with absenteeism and presenteeism), and daily activity impairment. Scores are calculated as percentages, with higher scores indicating greater impairment and less productivity (Citation18).
Statistical analysis
The proportion of patients achieving ≥4-point improvements in the Itch NRS and ≥1.5-point decreases in the ADSS Item 2 at Week 16 were compared between treatment groups in the ITT population with baseline Itch NRS ≥4 and baseline ADSS Item 2 ≥ 1.5, respectively. Data collected after rescue or treatment discontinuation were considered missing, and missing data were imputed as non-responders. Logistic regression models with terms for baseline disease severity (vIGA-AD), baseline value, and treatment group were applied. Treatment effects on itch and sleep improvement were also assessed in the subgroup of patients who had baseline BSA 10–50%, as a previous study suggests this population may benefit most from baricitinib 2 mg (Citation13).
At Week 16, QoL and productivity were compared between patients who experienced improvements in itch or sleep versus those who did not. For QoL comparisons, analysis was limited to patients with DLQI scores >5, ≥4, or ≥1, depending on the outcome assessed. Proportions of patients achieving DLQI responses were analyzed using logistic regression models with terms for baseline disease severity (vIGA-AD), baseline value, treatment group, itch/sleep improvement (yes or no), and the interaction of treatment and itch/sleep response, with non-responder imputation applied for missing data. Changes from baseline in DLQI were analyzed using ANCOVA with missing data imputed by last observation carried forward.
For associations of itch and sleep improvements with productivity, the percentage of impairment in daily activity was determined for all patients, and the percentages of presenteeism, absenteeism, and total work impairment were calculated for employed patients. Changes from baseline in WPAI-AD scores were analyzed using ANCOVA with missing data imputed by last observation carried forward.
Results
Patient characteristics
A total of 440 patients were included in the ITT population. Baseline characteristics of the study population, overall and by itch and sleep improvement subgroups, are listed in . Patients with baseline BSA 10–50% made up 69.3% of the ITT population.
Table 1. Baseline demographic and clinical characteristics, overall and by itch and sleep improvement subgroups.
Treatment effects on itch severity
After 16 weeks of treatment, 61/386 (18.8%) patients with Itch NRS score ≥4 at baseline achieved a ≥ 4-point decrease in itch severity score. Compared to 7/123 (5.7%) placebo-treated patients, 33/131 (25.2%) patients treated with baricitinib 2-mg and 21/132 (15.9%) patients receiving baricitinib 1-mg were categorized as having improvements in itch severity (p < .0001 and p = .0114 versus placebo, respectively).
Among patients with baseline BSA 10–50%, 5/81 (6.2%) of placebo-treated patients had ≥4-point improvements in the Itch NRS, compared to 24/90 (26.7%) of baricitinib-2-mg-treated patients and 16/90 (17.8%) of baricitinib 1-mg-treated patients (p = .0004 and p = .0338 versus placebo, respectively).
Improvements in itch severity
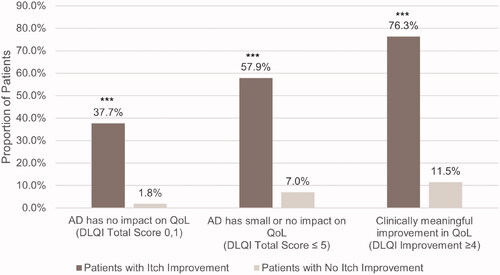
There was no significant interaction between itch improvement groups and treatment arms at the α level of 0.1. Across treatment arms, a higher proportion of patients who experienced itch improvement versus no improvement had a DLQI total score of 0 or 1 at Week 16 (37.7 vs. 1.8%, p < .0001), indicating no impact of AD on QoL (). Similarly, compared to those without itch improvement, greater proportions of patients with relief in itch severity obtained a DLQI total score ≤5 (57.9 vs. 7.0%, p < .0001) and a clinically meaningful ≥4-point change in DLQI score (76.3 vs. 11.5%, p < .0001). Patients with itch improvement versus no improvement also had significantly greater decreases in DLQI score from baseline (−11.7 vs. −3.4, p < .0001).
Figure 1. Proportion of patients with and without itch improvement achieving DLQI endpoints at Week 16. AD: atopic dermatitis; DLQI: Dermatology Life Quality Index. ***p < .0001. Itch improvement is defined as a ≥ 4-point decrease in the Itch Numeric Rating Scale at Week 16.
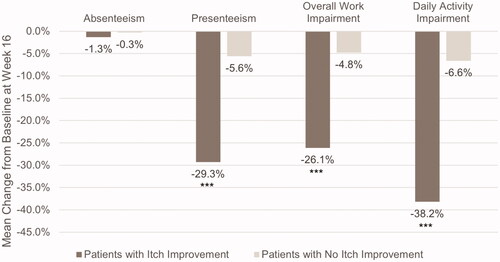
Among employed patients (n = 253), patients with better itch response saw larger decreases in presenteeism (−29.3 vs. −5.6%, p < .0001) and in total work impairment (−26.1 vs. −4.8%, p = .0001) than those without itch improvement (). The difference in absenteeism between the two subgroups was not significant (−1.3 vs. 0.3%, p = .7263). Among all patients, those with decreases in itch severity experienced significantly less daily activity impairment than those without itch improvement (−38.2 vs. −6.6%, p < .0001).
Figure 2. Mean change from baseline in work productivity and daily activity impairment in patients with and without itch improvement. Scores are from the Work Productivity and Activity Impairment Questionnaire – Atopic Dermatitis. ***p ≤ .0001. Itch improvement is defined as a ≥ 4-point decrease in the Itch Numeric Rating Scale at Week 16. Absenteeism, presenteeism, and overall work impairment were measured in employed patients only (n = 253).
Treatment effects on sleep disturbance
At Week 16, 51/231 (22.1%) patients with baseline ADSS Item 2 score ≥1.5 had at least a 1.5-point decrease in the number of nighttime awakenings due to itch since baseline. Compared to 6/70 (8.6%) placebo-treated patients, 29/88 (33.0%) patients treated with baricitinib 2 mg and 16/73 (21.9%) patients treated with baricitinib 1 mg experienced better sleep (p < .001 and p = .036 versus placebo, respectively).
Similar results were observed for patients with baseline BSA 10–50%. Compared to 4/41 (9.8%) of patients treated with placebo, 23/58 (39.7%) and 10/42 (23.8%) of patients treated with baricitinib 2 mg and 1 mg, respectively, experienced ≥1.5-point reductions in the number of nighttime awakenings due to itch (p = .0011 and p = .1415 versus placebo, respectively).
Improvements in sleep disturbance
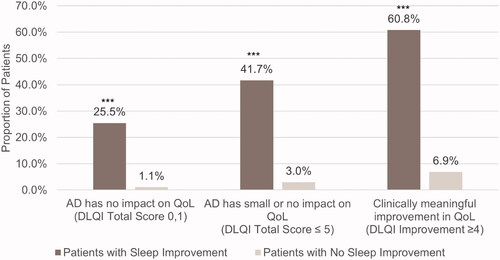
There was no significant interaction between sleep improvement groups and treatment arms at the α level of 0.1. Across treatments, compared to patients without sleep improvement, a greater proportion of patients who slept better had a DLQI score of 0 or 1 at Week 16 (25.5 vs. 1.1%, p < .0001) (). Similarly, more patients who slept better reported small or no impact of AD on QoL (DLQI ≤ 5, 41.7 vs. 3.0%, p < .0001) and a ≥ 4-point decrease on the DLQI (60.8 vs. 6.9%, p < .0001). Patients with fewer nighttime awakenings also had significantly greater improvements in DLQI score at Week 16 (−12.8 vs. −3.4, p < .0001).
Figure 3. Proportion of patients with and without sleep improvement achieving DLQI endpoints at Week 16. AD, atopic dermatitis; DLQI, Dermatology Life Quality Index. ***p < .0001. Sleep improvement is defined as a ≥ 1.5-point decrease in Atopic Dermatitis Sleep Scale Item 2 score at Week 16.
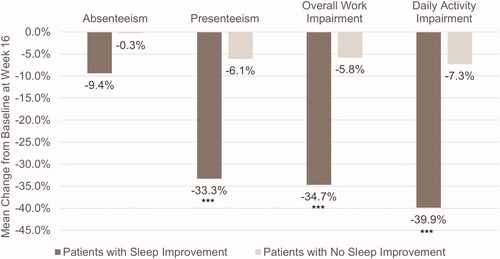
Among employed patients (n = 148), patients with fewer nighttime awakenings had greater reductions in presenteeism than those without sleep improvement (−33.3 vs. −6.1%, p < .0001) (). Patients with fewer nighttime awakenings also had significant decreases in total work impairment (−34.7 vs. −5.8%, p < .0001), though there were no significant differences in absenteeism between the two groups (−9.4 vs. −0.3%, p = .1104). Among all patients, those with sleep improvement also experienced significantly less daily activity impairment than those without fewer nighttime awakenings (−39.9 vs. −7.3%, p < .0001).
Figure 4. Mean change from baseline in work productivity and daily activity impairment in patients with and without sleep improvement. Scores are from the Work Productivity and Activity Impairment Questionnaire – Atopic Dermatitis. ***p < .0001. Sleep improvement is defined as a ≥ 1.5-point decrease in Atopic Dermatitis Sleep Scale Item 2 score at Week 16. Absenteeism, presenteeism, and overall work impairment were measured in employed patients only (n = 148).
Discussion
In this analysis of Phase 3 data of adults with moderate-to-severe AD, patients treated with baricitinib versus placebo were more likely to achieve clinically meaningful 4-point improvements in itch severity and a 1.5-point reduction in the number of nighttime awakenings due to itch. These findings were also found in the subpopulation of patients with baseline BSA involvement of 10–50%, who have been identified as having the highest response rates with baricitinib 2 mg (Citation13). Further, patients who experienced clinically relevant decreases in itch severity or sleep disturbance were significantly more likely to achieve a state in which AD had a small or no impact on their QoL compared to patients without itch or sleep improvements. Similarly, patients with reduced itch severity or fewer nighttime awakenings reported less impairment at work and in their daily lives. Though it is already understood that itch, sleep disturbance due to itch, and skin lesions are correlated with each other and QoL, this study highlights how relief in key symptoms related to itch are needed to ultimately improve the lives of patients with AD.
While the positive impacts of baricitinib treatment on QoL and productivity measures have been previously reported (Citation12), this study further explored whether improvements in key symptoms of itch and sleep disturbance translate into clinically important aspects of patients’ lives. Itch and nighttime awakening due to itch have been identified by patients as being among the most bothersome symptoms associated with their AD (Citation7), and the findings of this study suggest these symptoms are also core to the impact of the disease on QoL and daily functioning. Thus, assessment of these symptoms throughout the course of therapy may inform whether patients will ultimately achieve meaningful improvements in their daily lives and work productivity. The results of this study also reinforce the clinical relevance of the MCIDs of the Itch NRS (4 points) and ADSS Item 2 (1.5 points) and support the use of these MCIDs in defining patient subgroups for whom external criteria, such as QoL or work productivity, can be reliably distinguished.
This study has several limitations. Data are limited to the inclusion and exclusion criteria of the clinical trial and include only adult patients with moderate-to-severe AD. In addition, data are limited to the first 16 weeks of treatment. As AD is a chronic disease, longer-term assessments are needed to better understand the lasting effect of treatment on symptoms and QoL. This analysis did not assess the type of employment or other factors that may be associated with daily activity and work productivity; the impact of AD may vary across these factors. The present analysis does not evaluate the specific factors that directly or indirectly affect QoL, and the magnitude of these impacts. Future mediator analyses may be conducted to better address these questions.
Conclusion
Baricitinib therapy led to clinically meaningful improvements in itch severity and sleep disturbance in adult patients with moderate-to-severe AD. Patients who experienced relief in itch symptoms and reduced sleep disturbance had significantly better QoL and productivity. This study highlights how relief in key symptoms is needed to improve the lives of patients with AD.
Acknowledgments
The authors thank Amy Ellinwood, MPH, PhD, of Eli Lilly and Company for medical writing and editorial support.
Disclosure statement
Dr. Lio reports research grants/funding from Regeneron/Sanofi Genzyme, and AbbVie; is on the speaker’s bureau for Regeneron/Sanofi Genzyme, Pfizer, and Galderma; reports consulting/advisory boards for UCB, Dermavant, Regeneron/Sanofi Genzyme, Dermira, Pfizer, LEO Pharmaceuticals, Abbvie, Kiniksa, Eli Lilly, Menlo Therapeutics, Galderma, IntraDerm, Exeltis, Realm Therapeutics; Dr. Simpson reports grants and fees for participation as a consultant and principal investigator from Eli Lilly and Company, LEO Pharma, Pfizer, and Regeneron; grants for participation as a principal investigator from Galderma and Merck & Co.; and fees for consultant services from AbbVie, Boehringer Ingelheim, Dermavant Incyte, Forte Bio, Pierre Fabre Dermo, and Sanofi Genzyme; Dr. Han is or has been an investigator for Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Eli Lilly, Novartis, Janssen, MC2, PellePharm, Pfizer, and UCB; and a consultant, advisor, or speaker for Abbvie, Eli Lilly, Janssen, LEO Pharma, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, SUN Pharmaceutical, and UCB; Dr. Soung has received honoraria and/or grants as a speaker, advisory board member and/or investigator for: AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Eli Lilly and Company, Galderma, GlaxoSmithKline, Janssen, Kyowa Kirin, Leo Pharma, MedImmune, Menlo, Merck, Novan, Novartis, Pfizer, Roche, Regeneron, Sun Pharma, Sanofi Genzyme, Cassiopeia, UCB, and Valeant; Dr. Eichenfield has served as an investigator, consultant, speaker, or data safety monitoring board member for AbbVie, Almirall, Arcutis, Dermira, Eli Lilly, Forte Biosciences, Galderma, Ichnos/Glenmark, Incyte Corporation, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Otsuka, Pfizer, Realm, Regeneron, and Sanofi Genzyme; Drs. Ball, Sun, Casillas, Ding, and Ms. DeLozier are employees and shareholders of Eli Lilly and Company.
Additional information
Funding
References
- Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125–137.
- Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–498.
- Dawn A, Papoiu AD, Chan YH, et al. Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol. 2009;160(3):642–644.
- Simpson EL. Comorbidity in atopic dermatitis. Curr Dermatol Rep. 2012;1(1):29–38.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60(8):984–992.
- Lifschitz C. The impact of atopic dermatitis on quality of life. Ann Nutr Metab. 2015;66(1):34–40.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347.
- Andersen L, Nyeland ME, Nyberg F. Increasing severity of atopic dermatitis is associated with a negative impact on work productivity among adults with atopic dermatitis in France, Germany, the U.K. and the U.S.A. Br J Dermatol. 2020;182(4):1007–1016.
- Augustin M, Langenbruch A, Blome C, et al. Characterizing treatment-related patient needs in atopic eczema: insights for personalized goal orientation. J Eur Acad Dermatol Venereol. 2020;34(1):142–152.
- Cotter DG, Schairer D, Eichenfield L. Emerging therapies for atopic dermatitis: JAK inhibitors. J Am Acad Dermatol. 2018;78(3):S53–S62.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217–228.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy Phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Academy Dermatol. 2021;85(1):62–70.
- Silverberg JI, Boguniewicz M, Waibel J, et al. Clinical tailoring of baricitinib 2-mg in atopic dermatitis: baseline body surface area and rapid onset of action identifies response at Week 16. Revolutionizing Atopic Dermatitis; 2020. December 13-14, 2020.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–769.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- Hongbo Y, Thomas CL, Harrison MA, et al. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–664.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230(1):27–33.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365.
