Abstract
Background
Eczema control is a new construct to be measured in atopic dermatitis (AD).
Objectives
Measuring patient-perceived eczema control and treatment satisfaction in AD patients, treated with dupilumab between 16 and 52 weeks.
Methods
Cross-sectional questionnaire study. Patients from the Dutch BioDay registry completed the Atopic Dermatitis Control Test (ADCT), Recap of Atopic Eczema (RECAP) and Treatment Satisfaction Questionnaire for Medication, Version II (TSQM v. II), along with other Patient Reported Outcome Measures (PROMs).
Results
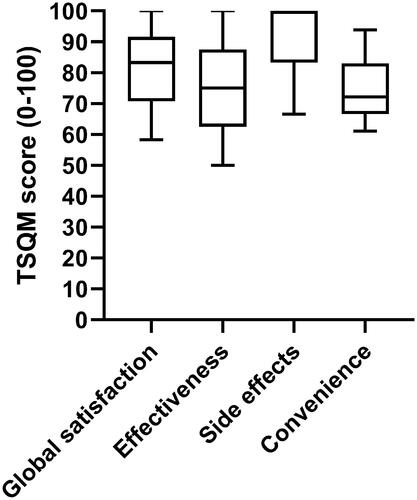
104/157 patients responded (response rate 66.2%). Median ADCT score was 4 (interquartile range [IQR] 5); median RECAP score was 5 (IQR 6); median TSQM v.II global satisfaction score was 83.3 (IQR 25.0). According to the ADCT, 38.5–66.3% perceived their AD was ‘in control’, depending on the interpretability method used. Minimally clinically important difference (MCID) of ≥4 points for the DLQI and POEM was achieved respectively in N = 66 (84.6%) and N = 63 (78.8%) patients.
Conclusion
When considering the favorable scores on other PROMs and the TSQM v. II, and comparing these to the relatively low percentage of patients perceiving control according to the ADCT, interpretability of eczema control still appears difficult. Treatment satisfaction in the studied cohort was high.
Introduction
Atopic dermatitis (AD) is one of the most common chronic and relapsing inflammatory skin diseases worldwide (Citation1). Because of the relapsing nature of AD, single or even repeated measures of disease severity or quality of life assessments may not be representative for ‘control’ of the disease. The Harmonizing Outcome Measures for Eczema (HOME) initiative explored the feasibility and acceptability of different ways to measure eczema control (Citation2). Therefore, two new Patient-reported outcome measures (PROM’s) were developed, the Recap of atopic eczema (RECAP) questionnaire (Citation3) and the Atopic Dermatitis Control Tool (ADCT) (Citation4,Citation5).
Recent results from the BioDay registry on dupilumab treatment for AD patients in daily practice show a clinically relevant improvement of physician-reported outcome measures and patient-reported outcome measures after 3–12 months (Citation6,Citation7). Currently, there are no data on the use of the new tools measuring eczema control, ADCT and RECAP, in daily practice. Therefore, we primarily aimed to assess eczema control in patients participating in the BioDay registry by using the ADCT and RECAP. A secondary aim was to assess treatment satisfaction with dupilumab.
Methods
Study design, population, and recruitment
This was a cross-sectional study carried out in patients who participate in the BioDay registry (Citation6). The BioDay registry is a prospective multicenter registry in which patients treated with new systemic treatments for AD in daily practice are included. For this cross-sectional study, data collection was carried out between April 10 2020 and May 8 2020. Patients (aged ≥ 18 years) who had been treated with dupilumab were eligible for the current study when they had been treated between 16 and 52 weeks at inclusion. This group was included because a steady state concentration of dupilumab from 16 weeks has been reported (Citation7–9). On the 10th of April 2020, a digital questionnaire was sent to eligible patients, using Castor Electronic Data Capture (Citation10). Two weeks later, a reminder was sent. The data were locked another 2 weeks later. The BioDay registry was reviewed and approved by the Medical Ethical Review Board of the University Medical Center Utrecht (METC 18/239 and 19/240).
Measurements
Demographics, severity data, and PROMs collected during regular BioDay visits were used in this study, as recommended by the HOME initiative (Citation11), including Eczema Area and Severity Index (EASI) (Citation12); Investigator Global Assessment (IGA) (range 0–5: clear, almost clear, mild, moderate, severe, very severe); Dermatology Life Quality Index (DLQI) (Citation13); weekly average Numerical Rating Scale (NRS) pruritus/pain (range 0–10); and Patient Oriented Eczema Measure (POEM) (Citation14). For an approximation of disease status at the moment of questionnaire completion, PROM data from the closest regular visit (± max. 8 weeks) in the BioDay registry were used.
Atopic Dermatitis Control Test (ADCT)
The ADCT is a validated PROM designed to assess patient-perceived control of AD in adults. It is found to have good-to-excellent content validity, construct validity, internal consistency, reliability and discriminating ability in patients with AD; as well as in a group of patients treated with dupilumab for AD (Citation4,Citation5). It is translated to Dutch (Citation15). The ADCT includes six items with a 7-day recall period. Each item is scored from 0 (none) to 4 (extreme), with a total score of 0–24. Lower scores indicate a higher perceived control of disease. There are three methods to identify patients ‘in control’ and ‘not in control’. For the first method, a total score of 7 or more points (derived by adding up item scores) was identified as an optimum threshold to identify patients whose AD is ‘not in control’. The second and third method equally produced the highest sensitivity (0.96) and acceptable level of specificity (0.68). They are based on answering a single item above a certain threshold: one out of all six items (method 2) or one out of the first four items (method 3) (Citation4,Citation5,Citation15).
RECAP of atopic eczema (RECAP)
The RECAP is a validated PROM designed to capture ‘eczema control’ over the past week. It includes seven questions. Each of the questions carry equal weight and is scored from 0 to 4 (total score of 0–28), with lower scores indicating higher control (Citation3). The RECAP has no validated cutoff scores to determine eczema control. The instrument has been translated to Dutch (Citation16).
Additional question
The ADCT and RECAP measure a comparable construct (eczema control). To identify patient preference, patients were asked the (global) question which of both questionnaire they preferred.
Treatment Satisfaction Questionnaire for Medication, Version II (TSQM v. II)
The TSQM was designed as a measure for treatment satisfaction with medication and was later methodologically refined into a shorter, more consistently worded version, the TSQM v. II. It includes 11 questions covering four dimensions: effectiveness, side effects, convenience, global satisfaction. Items have varying amounts of response options. Scores ranging from 0 to 100 are calculated for each dimension, with higher scores indicating more satisfaction (Citation17).
Statistical analysis
The design of the digital questionnaire allowed for missing data. Missing data were handled in agreement with instructions by the questionnaire designers. For the RECAP and TSQM v. II missing values are allowed to a certain extent; for the ADCT missing values are not allowed (Citation3,Citation15,Citation17). Due to the coronavirus disease 2019 (COVID-19) pandemic, several BioDay visits were not conducted, leading to missing values for clinical scores and PROMs.
Results
Sample characteristics
A total of 157 patients were included, with a response rate of 66.2% (N = 104). Non-responder analysis showed that non-responders were significantly younger (median 46 versus 33 years) and had a significantly higher EASI score at baseline (median 12.6 versus 14.0). PROMs did not differ significantly at baseline. Ten potential eligible patients had discontinued dupilumab prior to the current study (reason of discontinuation: side effects (N = 4), ineffectiveness (N = 3), personal reasons (N = 3)). See for basic characteristics.
Table 1. Basic characteristics of total study population (n = 104).
Eczema control
Median reported values for eczema control were 4 for ADCT and 5 for RECAP. All PROMs measured within a maximum time of 8 weeks of these values differed significantly from baseline. See . The minimally clinically important difference (MCID) of ≥ 4 points for the DLQI and POEM was achieved respectively in 66 (84.6% of patients without missing values) and 63 (78.8% of patients without missing values) patients. For ADCT, 66% of patients were ‘in control’ according to the first method. According to the second and third method, this number dropped to around 40% (). When asked which questionnaire was favored by patients, 11% chose ADCT and 9% RECAP; 80% of patients indicated no preference.
Table 2. ADCT, RECAP, and TSQM v.II values, along with closest reported patient reported outcome measures at regular BioDay visits.
Table 3. Control according to the Atopic Dermatitis Control Tool (ADCT).
Discussion
In this study, between 38.5 and 66.3% of patients, using dupilumab between 16 and 52 weeks, perceived their AD as ‘in control’. Treatment satisfaction of this cohort was high.
The current study showed a relatively high percentage of patients ‘not in control’ according to the ADCT. Taking into consideration the significant improvement from baseline for signs, symptoms and various PROMs, seen in previous studies from the BioDay registry (Citation6,Citation7), resulting in low scores on these instruments after 16–52 weeks, the cutoff for ‘not in control’ may be too strict. In a validation paper, the interpretation of the ADCT was assessed using a patient global assessment scale as a reference. Patients indicating their AD was ‘not at all controlled’, ‘a little controlled’ or ‘moderately controlled’, were all categorized as being ‘not in control’ according to the ADCT (Citation4). Using the current binary cutoff values, along with the three different analyzing methods, the use of the ADCT in daily practice may lead to premature discontinuation or change of treatment. Moreover, as patient perceived eczema control is an individual experience, it would be beneficial to investigate the MCID rather than a binary cutoff point for control regarding the ADCT. Future research is definitely needed.
In the current study, values for ADCT and RECAP were similar. However, no interpretability studies have been performed for the RECAP, which impedes the current interpretation of the RECAP in clinical practice and its comparison to the ADCT. It will be interesting to see how interpretability studies on the RECAP will produce values for patients perceiving their AD to be ‘in control’, and how this will relate to ADCT values.
The reported values for treatment satisfaction are on the higher end of the spectrum. In a large real-world study in patients on systemic therapy for AD, TSQM v.II values tended to be lower in all domains (Citation18). Especially the side effects satisfaction score stands out with a median of 100. A possible explanation for this is probably that many patients have had multiple treatment failures before starting dupilumab. Therefore, when treated with dupilumab, patients may trivialize their side effects if they perceive a high satisfaction regarding effectiveness of the treatment. However, a proper interpretation of the values reported here is not entirely possible due to the lack of interpretability studies for the TSQM v.II.
A limitation to our study is that the COVID pandemic resulted in various missing values. Furthermore, its cross-sectional design makes it impossible to compare the ADCT, RECAP, and TSQM v.II with values at baseline or other time points. Longitudinal studies are needed.
In conclusion, our study shows that the interpretability of the ADCT and RECAP regarding eczema control and its applicability in clinical decision making may benefit from further investigation. Treatment satisfaction during dupilumab treatment in daily practice is high.
Disclosure statement
Marjolein S. de Bruin-Weller has been a consultant, advisory board member, and/or speaker for AbbVie, Almirall, Eli Lilly, Galderma, Janssen, Leo Pharma, Pfizer, Regeneron, Sanofi-Genzyme, and UCB. Marie L. A. Schuttelaar: Sanofi Genzyme, Pfizer, LEO Pharma, Eli Lilly – advisory board member; AbbVie, Novartis, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme – investigator; Regeneron Pharmaceuticals, Inc. – consultant; Sanofi Genzyme, Novartis – research grant. Marlies de Graaf has been an advisory board member and/or speaker for Sanofi-Genzyme, Eli Lilly and Leo Pharma. The other authors report no conflict of interest.
References
- Silverberg JI. Atopic dermatitis in adults. Med Clin North Am. 2020;104(1):157–176.
- Howells L, Thomas KS, Sears AV, Long-Term Control of Eczema Working Group for the HOME Initiative, et al. Defining and measuring 'eczema control': an international qualitative study to explore the views of those living with and treating atopic eczema. J Eur Acad Dermatol Venereol. 2019;33(6):1124–1132.
- Howells L, Chalmers JR, Gran S, et al. Development and initial testing of a new instrument to measure the experience of eczema control in adults and children: recap of atopic eczema (RECAP). Br J Dermatol. 2019;183(3):524–536.
- Pariser DM, Simpson EL, Gadkari A, et al. Evaluating patient-perceived control of atopic dermatitis: design, validation, and scoring of the Atopic Dermatitis Control Tool (ADCT). Curr Med Res Opin. 2019;36(3):367–376.
- Simpson E, Eckert L, Gadkari A, et al. Validation of the Atopic Dermatitis Control Tool (ADCT©) using a longitudinal survey of biologic-treated patients with atopic dermatitis. BMC Dermatol. 2019;19(1):15.
- Ariëns LFM, van der Schaft J, Bakker DS, et al. Dupilumab is very effective in a large cohort of difficult-to-treat adult atopic dermatitis patients: first clinical and biomarker results from the BioDay registry. Allergy. 2020;75(1):116–126.
- Ariëns LF, van der Schaft J, Spekhorst LS, et al. Dupilumab shows long-term effectiveness in a large cohort of treatment-refractory atopic dermatitis patients in daily practice: 52-weeks results from the Dutch BioDay registry. J Am Acad Dermatol. 2020;84(4):1000–1009.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303.
- Dupixent – summary of product characteristics. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent.
- Castor EDC. Available from: https://www.castoredc.com.
- Harmonizing Outcome Measures for Eczema (HOME). Available at: http://www.homeforeczema.org/index.aspx.
- Tofte S. Eczema area and severity index (EASI): a new tool to evaluate atopic dermatitis. J Eur Acad Dermatol Venereol. 1998;11:S197.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use . Clin Exp Dermatol. 1994;19(3):210–216.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513–1519.
- Atopic dermatitis control tool. Available from: https://www.adcontroltool.com/.
- RECAP – The University of Nottingham. Available from: https://www.nottingham.ac.uk/research/groups/cebd/resources/recap.aspx.
- Atkinson MJ, Kumar R, Cappelleri JC, et al. Hierarchical construct validity of the Treatment Satisfaction Questionnaire for Medication (TSQM Version II) among outpatient pharmacy consumers. Value Heal. 2005;8(SUPPL. 1):S9–S24.
- Wei W, Ghorayeb E, Andria M, et al. A real-world study evaluating adeQUacy of Existing Systemic Treatments for patients with moderate-to-severe Atopic Dermatitis (QUEST-AD): baseline treatment patterns and unmet needs assessment. Ann Allergy, Asthma Immunol. 2019;123(4):381–388.e2.