Abstract
Background
Psoriasis, psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA) are chronic inflammatory diseases that often affect women of childbearing age. Detailed information about pregnancy and related outcomes across these indications in patients exposed to ixekizumab is lacking.
Objectives
To evaluate pregnancy outcomes after maternal or paternal exposure to ixekizumab in patients with psoriasis, PsA, or axSpA.
Methods
Pregnancy cases from clinical trials and post-marketing reports, associated with either maternal or paternal exposure to ixekizumab cumulatively through 22 March 2019, were identified in the Eli Lilly Global Safety Database and described separately.
Results
One hundred and ninety-three ixekizumab-exposed pregnancies were identified. Maternal exposure occurred in 51.3% of pregnancies (clinical trials: n = 58; post-marketing: n = 41). The majority of paternal exposure pregnancies occurred in clinical trials (91 of 94). Live births were reported for 53.8 and 61.1% of known outcomes in maternal exposure pregnancies during clinical trials and post-marketing surveillance, respectively. No congenital malformations resulting from maternal exposure were reported in clinical trials: one case, not causally related to ixekizumab therapy, was recorded in the post-marketing setting.
Conclusions
This integrated safety analysis provides relevant information for clinicians treating patients with psoriasis, PsA, or axSpA with ixekizumab. No new safety signals were identified in patients receiving ixekizumab.
Introduction
Psoriasis, psoriatic arthritis (PsA), and axial spondyloarthritis (axSpA) are chronic inflammatory conditions that require long-term treatment, sometimes with systemic therapies. Disease onset commonly occurs in early adulthood, and sometimes even earlier in the case of psoriasis and PsA (Citation1–3). Consequently, women of childbearing age may often be affected by these conditions. However, most non-biologic systemic therapies used to treat these conditions are contraindicated during pregnancy, except for ciclosporin for the treatment of psoriasis, which is not recommended unless the potential benefit to the mother justifies the potential risk to the fetus (Citation4). A growing body of literature suggests that the use of biologics during pregnancy is not detrimental to pregnancy outcomes (Citation5–7); however, clinical studies in this population are missing, and data on outcomes among women exposed to newer classes of biologics, such as interleukin (IL)-17 inhibitors are still limited.
Ixekizumab is a high-affinity monoclonal antibody that selectively targets IL-17A, which plays a key role in the pathogenesis of certain inflammatory conditions (Citation8). Ixekizumab is effective for moderate-to-severe plaque psoriasis in adult and pediatric patients (≥6 years of age) and adult patients with active PsA and axSpA (Citation9–11). It has shown a consistent safety profile across 21 randomized controlled trials totaling 8228 patients (cumulative ixekizumab exposure of 20,895.9 patient-years) (Citation12).
Human immunoglobin G (IgG), the predominant means of fetal immunity, crosses the placental barrier in a linear fashion as the pregnancy progresses, with the greatest transfer of IgG in the third trimester (Citation13). Preferential transport occurs for IgG subclass 1 (IgG1), followed by IgG4 (Citation13); therefore, as an IgG4, ixekizumab may be transmitted from the mother to the developing fetus (Citation9). However, transplacental passage of ixekizumab is estimated to be low during the early phases of pregnancy, when Fc receptors have yet to develop on fetal trophoblasts (Citation14). Hence, only limited amounts of ixekizumab should theoretically reach the fetus during the first trimester of pregnancy. Substantial transfer of ixekizumab across the placenta is not expected until mid-gestation, particularly during the third trimester (Citation13).
In an embryofetal development study conducted in cynomolgus monkeys, ixekizumab was demonstrated to cross the placenta (Citation9). No malformations or embryofetal toxicity were observed in fetuses from pregnant monkeys exposed to ixekizumab, from weekly subcutaneous injections during organogenesis to doses up to 19 times the maximum recommended human dose near parturition. No ixekizumab-related effects on functional or immunological development were observed in the infants from birth through 6 months of age (Citation9). Observational safety studies assessing maternal and fetal outcomes following exposure to ixekizumab are currently ongoing; however, the effects of ixekizumab on pregnancy have not been studied in clinical trials.
The primary objective of this research was to provide information about exposure to ixekizumab during pregnancy and related clinical outcomes in patients with psoriasis, PsA, or axSpA using data from clinical trials and post-marketing reporting.
Materials and methods
Data source
This analysis used the Eli Lilly Global Safety Database, which captures adverse events and pregnancies reported in any ixekizumab indication across clinical trials (), post-marketing studies, and spontaneous reports. The database was searched to identify pregnancy cases associated with either maternal or paternal exposure to ixekizumab, cumulatively through 22 March 2019. All cases reporting a pregnancy in the global safety database and/or satisfying the Medical Dictionary for Regulatory Activities (MedDRA) Version 22.1 System Organ Class search query ‘Pregnancy, puerperium and perinatal conditions’ or related Preferred Terms were included in the analysis.
Table 1. Overview of clinical trials in psoriasis, psoriatic arthritis, and axial spondyloarthritis for which safety data was reported in the Eli Lilly Global Safety Database through 22 March 2019.
Analyses
Maternal ixekizumab exposure pregnancies (i.e. females who received ixekizumab when they became pregnant and/or during their pregnancies), and paternal ixekizumab exposure pregnancies (i.e. females who became pregnant while their partners received ixekizumab), from clinical trials and post-marketing reports, were described. Pregnancy outcomes were reported as the number and proportion of live births, non-live births (stillbirth and infant death within 28 days post-partum as a measure of perinatal death), spontaneous abortion or miscarriages, elective terminations, and those with unknown outcomes due to lost to follow up or missing information. Live births were further categorized as full term, pre-term, or term unknown. Dates of conception were estimated based on several pregnancy-related recordings, such as expected delivery dates, maturity estimates, and routine monitoring dates. If no information was available, live births were categorized as ‘term unknown.’ The number of congenital malformations among infants was also reported where information was available.
Results
As of 22 March 2019, the Eli Lilly Global Safety Database included 8421 patients exposed to ixekizumab in clinical trials (6091 psoriasis, 1401 PsA, and 929 axSpA), and 74,200 patients exposed to ixekizumab in the post-marketing setting. A total of 193 ixekizumab-exposed pregnancies were identified from the database. Of these, 99 (51.3%) were maternal exposure pregnancies (58 in clinical trials, 41 during post-marketing surveillance) and 94 were paternal exposure pregnancies (91 in clinical trials, three during post-marketing surveillance).
Maternal ixekizumab exposure pregnancies
Clinical trial data
In total, 58 female clinical trial patients with a mean (±standard deviation [SD]) age of 30.22 (±6.25) years were exposed to ixekizumab while pregnant: all exposure occurred in the first trimester, except for two cases for which the trimester of exposure was not reported ( and ) and outcomes were reported for 89.7% (n = 52) of pregnancies. In accordance with study protocols, all but two women with confirmed pregnancies discontinued ixekizumab treatment (96.6%): one patient underwent elective termination of her pregnancy without discontinuing ixekizumab, while the other patient experienced a spontaneous abortion after 12 weeks of pregnancy and did not interrupt ixekizumab therapy.
Figure 1. Time of maternal exposure to ixekizumab and pregnancy outcomes by age group for patients in clinical trials.
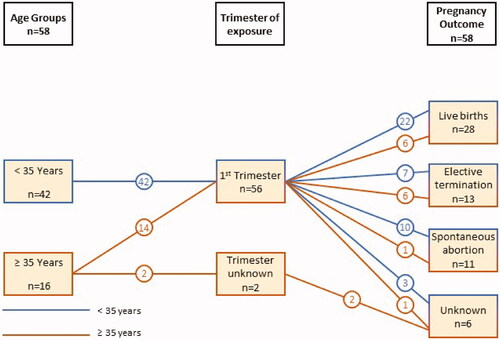
Table 2. Exposure information and outcomes for maternal ixekizumab exposure pregnancies (N = 99)a captured in the Eli Lilly Global Safety Databaseb.
Live births were reported for 53.9% (n = 28) of known outcomes (n = 52) and the majority of those were full term (78.6%, n = 22) ( and ). No stillbirths or infant deaths were reported. In total, 11 spontaneous abortions or miscarriages and 13 elective terminations were recorded. Reasons for terminating the pregnancy were provided by six patients: pregnancy was unwanted by three patients, two patients feared fetal abnormalities, and one patient stated advanced age/contraception failure as a reason for the elective termination. No cases of congenital malformation were reported.
Post-marketing surveillance data
During post-marketing surveillance, a total of 41 maternal exposures to ixekizumab were reported (). All maternal exposures with known exposure timing (n = 21) occurred in the first trimester. Psoriasis was the most frequent treatment indication (75.6%), although the indication was not reported in 22.0% (n = 9) of cases. Where treatment status was known, the majority of pregnant women discontinued treatment with ixekizumab (88.2%, n = 30). In 16 of these patients, ixekizumab was permanently discontinued, in two patients ixekizumab was continued after an elective termination of the pregnancy, and one case mentions first-trimester exposure without providing any further information on action taken with ixekizumab or pregnancy outcome. A longer exposure was reported for two patients with a full-term pregnancy and live birth outcomes. One patient had temporary discontinuation during the second trimester and restarted ixekizumab in the third trimester due to recurrence of psoriasis. This patient had a full-term live birth with no pregnancy complications. The other patient continued ixekizumab throughout her pregnancy and had an emergency cesarean section for hypertension, which was reported as not related to ixekizumab exposure by the physician. This patient gave birth to a live infant, though no information was provided on the infant’s health status.
Overall, pregnancy outcomes were known for 43.9% (n = 18) of maternal exposure pregnancies during post-marketing surveillance (). Eleven of these pregnancies resulted in live births while three spontaneous abortions or miscarriages and four elective terminations accounted for the other pregnancy outcomes. No stillbirths or fetal deaths were reported. Reasons for termination of pregnancy were provided in two cases; one patient experienced an ectopic pregnancy, while the other patient decided on abortion for reasons other than ixekizumab treatment, which were unspecified. One elective termination was performed after 5.5 weeks, the timing of the others was unknown. There was no report of a fetal abnormality in any of the four terminated pregnancies.
One case of a congenital malformation was reported in a male infant with esophageal atresia, tracheoesophageal fistula, and hypospadias following maternal exposure to ixekizumab during the first trimester of pregnancy. The mother, who reported the event, did not designate the event as related to ixekizumab therapy.
Paternal ixekizumab exposure pregnancies
Clinical trial data
Ninety-one pregnancies were reported in 86 partners of male ixekizumab clinical trial patients, including five females who reported becoming pregnant twice while their partners were on ixekizumab. Outcomes were known for 75 partner pregnancies (82.4%). The majority of paternal exposures occurred in clinical trials of psoriasis (89.0%), followed by PsA (7.7%), and axSpA (3.3%). Most patients whose partners were pregnant continued treatment with ixekizumab (91.2%, n = 83); three patients discontinued treatment, and the treatment course was unknown for five patients.
In total, 69.2% (n = 63) of the partner pregnancies resulted in live births: 53 were full-term, seven pre-term, and term status was unknown for three. Of the remaining 12 pregnancies with known outcomes, there were three elective terminations and nine spontaneous abortions or miscarriages. Reasons for termination were provided in two of three elective termination cases: the threat of teratogenicity due to maternal methotrexate exposure and an abnormality in fetus growth that was discovered by ultrasound. In the third case, it was stated that the patient and his partner decided to end the unplanned pregnancy without providing a reason. In all three cases, the male patient continued with ixekizumab therapy.
Congenital malformations were reported in three infants and included heart abnormality, syndactyly, and widened pelvis. In the first case (heart abnormality), the mother experienced a miscarriage during the pregnancy. The defect was identified during an examination of the fetus. Reported risk factors included advanced maternal age (37 years), and maternal history of smoking and alcohol use. In the second case (syndactyly), a live, full-term baby boy was delivered. Upon delivery, it was discovered that his left and middle finger were webbed. Risk factors for syndactyly included the maternal history of webbed fingers and toes and a paternal family history of webbed fingers. In the third and final case (widened pelvis), the mother delivered a full-term baby boy. Ultrasound of the baby’s abdomen revealed congenital widening of the right renal pelvis. Risk factors for this malformation included toxoplasmosis during pregnancy.
Two other noteworthy paternal exposure cases are presented here. In one case, a pulmonary complication was reported for a baby born to a mother whose male partner was an ixekizumab patient. In this case, the infant was part of a set of triplets born at 31 weeks and 6 days gestation via c-section. The baby’s length and weight at birth were 36 cm and 980 g, respectively. The mother had no known complications. Risk factors were not reported. In another case, the mother decided to terminate the pregnancy after abnormal growth was detected via ultrasound. No additional information was available.
Post-marketing surveillance data
In the post-marketing setting, three partner pregnancies of male patients exposed to ixekizumab were recorded; very limited additional data and no information on the pregnancy or infant outcomes were available.
Discussion
Women of childbearing age with chronic inflammatory conditions, such as psoriasis, PsA, and axSpA may require systemic biologic therapies. Ixekizumab, a high-affinity monoclonal antibody that selectively targets interleukin-17A, has a positive benefit/risk profile in several placebo-controlled and head-to-head studies compared with different biologic disease-modifying anti-rheumatic drugs (bDMARDs) (Citation15–19). The purpose of this study was to provide information on the clinical outcomes of pregnancies in patients with psoriasis, PsA, and axSpA after exposure to ixekizumab. One hundred and ninety-three ixekizumab-exposed pregnancies were recorded in the ixekizumab integrated safety database, of which 99 were maternal exposure pregnancies (58 in clinical trials, 41 during post-marketing surveillance). The majority of maternal exposures occurred in the first trimester of pregnancy, a time period before active transfer of IgG across the placenta occurs (Citation13). A review of this data did not identify any congenital malformations resulting from maternal exposure in clinical trials, while one event of congenital malformation was identified during post-marketing surveillance. The proportion of congenital malformations among infants with maternal exposure to ixekizumab in the overall dataset (1.4% of known maternal exposure pregnancy outcomes; 2.6% of live birth outcomes) was similar to that reported in the general US and European populations (Citation20,Citation21).
Over half of the maternal ixekizumab exposures reported during post-marketing surveillance had an unknown outcome (56.1%), compared with only 10.3% in clinical trials. The high proportion of missing pregnancy outcomes from post-marketing surveillance in the ixekizumab global safety database is consistent with reports for other products used in the treatment of psoriasis (Citation22,Citation23); however, it could result in underestimation of the incidence of live births.
More information on pregnancy outcomes was provided in the clinical trial setting compared with the post-marketing setting. This is not unexpected, as patients participating in a clinical trial do so with an expectation of continued follow-up.
The overall proportion of spontaneous abortions among maternal exposure pregnancies with known outcomes (21.2% of clinical trial cases, 16.7% of post-marketing cases) was similar to that reported for another IL-17 inhibitor in a similarly aged population (Citation22). The risk of spontaneous abortion varies depending on maternal age and these findings are consistent with that observed in the general population for this age demographic (15–20%) (Citation24).
Approximately a quarter and a fifth of maternal exposure pregnancies with known outcomes observed in the clinical trial and post-marketing settings, respectively, ended in elective termination. This is higher than that observed among female psoriasis patients treated with a biologic in a post-marketing safety registry (Citation25) but similar to reports from safety databases for other biologic medications (Citation22,Citation26). Possible reasons could include that data from clinical trials overrepresent this outcome due to the unknown effects of an investigational drug. Furthermore, clinical trial inclusion criteria require birth control, which may result in a potential bias toward the inclusion of patients who do not wish to become pregnant and would elect for a termination of pregnancy independent of study protocol-related factors. This hypothesis is consistent with the reasons given by mothers in clinical trials for terminating their pregnancy; four mothers cited unwanted pregnancy or failure of birth control. However, there is no feasible method to prevent this potential bias toward elective pregnancy termination in clinical study settings.
The effects of paternal exposure to ixekizumab on fetal development are uncertain, and more research is needed. In preclinical studies, no effects on fertility parameters, such as reproductive organs or sperm analysis were observed in sexually mature cynomolgus monkeys dosed with ixekizumab, however, the monkeys were not mated to evaluate fertility (Citation9). Ninety-one pregnancies were reported in 86 partners of male ixekizumab clinical trial patients. The majority of the patients were treated for psoriasis and continued ixekizumab therapy after the pregnancy of their partner was diagnosed. The proportions of elective terminations (4.0%) and spontaneous abortions (12.0%) among paternal-exposed pregnancies with known outcomes were similar to those reported from the safety database of another IL-17 inhibitor in a similarly aged population (Citation22). Three congenital abnormalities were recorded; all cases had risk factors for congenital abnormality, such as advanced age, family history of congenital abnormalities, smoking, alcohol use, or toxoplasmosis infection.
The following limitations should be considered with respect to this analysis. Data from clinical studies are limited due to the low frequency of events and short exposure and observation periods, whereas post-marketing data are limited by the high frequency of unknown outcomes. Cases from post-marketing sources are also less robust compared to those reported during clinical trials. Data pertaining to maternal medical history, obstetric history, high-risk behavior, or concomitant medications are often missing, precluding an assessment of causality for a reported outcome. The high proportion of missing outcomes complicates comparisons between clinical trials and post-marketing sources. We have therefore provided data separately by setting to allow readers to assess the potential impact of missing data.
Conclusions
In line with previous studies of ixekizumab in psoriasis, PsA, and axSpA, which have confirmed an overall favorable safety profile (Citation12), this integrated safety analysis of treatment-emergent maternal and paternal pregnancies did not identify new safety signals in patients receiving ixekizumab or partners of patients receiving ixekixumab. The risk of spontaneous abortion following ixekizumab exposure is consistent with that observed in the general population for this age demographic. This data supports the growing evidence of a well-tolerated and balanced safety profile for bDMARDs in these indications during pregnancy.
Acknowledgments
The authors would like to acknowledge Sue Williamson and Dr. Sue Chambers (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.
Disclosure statement
A. Egeberg has received research funding from Pfizer, Eli Lilly, Novartis, Bristol-Myers Squibb, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as a consultant and/or speaker from AbbVie, Almirall, Leo Pharma, Galápagos NV, Sun Pharmaceuticals, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly and Company, Novartis, Galderma, Dermavant, UCB, Mylan, Bristol-Myers Squibb, and Janssen Pharmaceuticals. L. Iversen has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by: AbbVie, Almirall, Amgen, Astra Zeneca, BMS, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Company, Janssen Cilag, Kyowa, Leo Pharma, MSD, Novartis, Pfizer, Regranion, Samsung, and UCB. A. B. Kimball has received honoraria as a consultant and grants as an investigator from BMS, Janssen, AbbVie, Lilly, Novartis, Pfizer, Meiji, and UCB, and fellowship funding from Janssen and AbbVie. She is on the Board of Directors of Almirall and Advisor to the Organization of Teratology Information Services (OTIS). S. R. Feldman received research, speaking, and/or consulting support from Arcutis, Dermavant, Galderma, GSK/Stiefel, Almirall, Alvotech, Leo Pharma, BMS, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Ortho Dermatology, Abbvie, Samsung, Janssen, Eli Lilly and Company, Menlo, Helsinn, Arena, Forte, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. He consults for others through Guidepoint Global, Gerson Lehrman, and other consulting organizations. He is the founder and majority owner of www.DrScore.com. He is also a founder and part-owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. S. Kelly, E. Grace, H. Patel, W. Xu, G. Gallo, and E. Riedl are employees and minor stakeholders of Eli Lilly and Company.
Additional information
Funding
References
- National Psoriasis Foundation. Psoriasis.org. About psoriasis [online] [updated 2020 May 24; cited 2021 Jul]. Available from: https://www.psoriasis.org/about-psoriasis
- National Psoriasis Foundation. Psoriasis.org. About psoriatic arthritis [online] [updated 2020 Jan 15; cited 2021 Jul]. Available from: https://www.psoriasis.org/about-psoriatic-arthritis
- Ursin K, Lydersen S, Skomsvoll JF, et al. Disease activity during and after pregnancy in women with axial spondyloarthritis: a prospective multicenter study. Rheumatology. 2018;57(6):1064–1071.
- Novartis Pharmaceuticals UK Ltd. SANDIMMUN® soft gelatin capsules 25 mg, 50 mg, 100 mg (ciclosporin) summary of product characteristics; 2020 [cited 2021 Jul]. Available from: https://www.medicines.org.uk/emc/product/11090/smpc
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3(1):21–25.
- Mariette X, Flynn A, Förger F, et al. OP0017 lack of placental transfer of certolizumab pegol during pregnancy; results from crib, a prospective, postmarketing, multicenter, pharmacokinetic study. Ann Rheum Dis. 2017;76:57–58.
- Johansen CB, Jimenez-Solem E, Haerskjold A, et al. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: a review. Int J Mol Sci. 2018;19(5):E1349.
- Ruiz de Morales JMG, Puig L, Daudén E, et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: an updated review of the evidence focusing on controversies. Autoimmun Rev. 2020;19(1):102429.
- Taltz (ixekizumab) injection, for subcutaneous use. Indianapolis (IN): Eli Lilly and Company; 2020 [cited 2021 Jul]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125521s020lbl.pdf
- Blegvad C, Skov L, Zachariae C. Ixekizumab for the treatment of psoriasis: an update on new data since first approval. Expert Rev Clin Immunol. 2019;15(2):111–121.
- Deodhar A, Poddubnyy D, Pacheco-Tena C, et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol. 2019;71(4):599–611.
- Genovese MC, Mysler E, Tomita T, et al. Safety of ixekizumab in adult patients with plaque psoriasis, psoriatic arthritis and axial spondyloarthritis: data from 21 clinical trials. Rheumatology. 2020;59:3834–3844.
- Yeung J, Gooderham MJ, Grewal P, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg. 2020;24(1_suppl):3S–14S.
- Soh MC, Moretto M. The use of biologics for autoimmune rheumatic diseases in fertility and pregnancy. Obstet Med. 2020;13(1):5–13.
- Griffiths CEM, Reich K, Lebwohl M, et al. UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551.
- Leonardi CL, Blauvelt A, Sofen HL, et al. Rapid improvements in health-related quality of life and itch with ixekizumab treatment in randomized phase 3 trials: results from UNCOVER-2 and UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31(9):1483–1490.
- Gordon KB, Blauvelt A, Papp KA, et al. UNCOVER-1 study group; UNCOVER-2 study group; UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356.
- Reich K, Pinter A, Lacour JP, et al. IXORA-S investigators. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORS-S, a phase III study. Br J Dermatol. 2017;177(4):1014–1023.
- Blauvelt A, Papp K, Gottlieb A, et al. IXORA-R study group. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blind trial. Br J Dermatol. 2020;182(6):1348–1358.
- Centers for Disease Control (U.S.), & Centers for Disease Control and Prevention (U.S.). Morbidity and mortality weekly report: MMWR. Atlanta (GA): U.S. Dept. of Health, Education, and Welfare, Public Health Service, Center for Disease Control. Update on Overall Prevalence of Major Birth Defects – Atlanta, Georgia, 1978–2005; 2008 [cited 2021 Jul]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5701a2.htm
- Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol. 2010;686:349–364.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179(5):1205–1207.
- Geldhof A, Slater J, Clark M, et al. Exposure to infliximab during pregnancy: post-marketing experience. Drug Saf. 2020;43(2):147–161.
- Nybo Andersen AM, Wohlfahrt J, Christens P, et al. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320(7251):1708–1712.
- Kimball AB, Crow JA, Ridley K, et al. Pregnancy outcomes in women with moderate to severe psoriasis: the PSOLAR experience. J Am Acad Dermatol. 2014;70:AB179.
- Yurkon K, Guo CY, Harrison DD, et al. Pregnancy outcomes in women with dermatologic conditions exposed to infliximab. J Am Acad Dermatol. 2014;70:AB179.
