Abstract
Background and objectives
Once-daily, fixed-combination calcipotriol 50 μg/g (Cal) plus betamethasone dipropionate 0.5 mg/g (BD) is available in aerosol foam and cream formulations. As no head-to-head data are available, we use a matching-adjusted indirect comparison (MAIC) approach to compare Cal/BD foam and cream.
Methods
Anchored and unanchored MAIC analyses were conducted using individual patient data (IPD) from five Cal/BD foam trials and two trials of Cal/BD cream. Outcomes of interest were the proportion of patients with Physician's Global Assessment (PGA) success and the mean reduction in modified Psoriasis Area and Severity Index (mPASI).
Results
In the anchored MAIC, patients were more likely to achieve PGA success after 4 weeks of Cal/BD foam than after 8 weeks of Cal/BD cream and had larger mean improvements in mPASI (p < .01 in EU mPASI analysis). In unanchored analyses, 4 weeks of Cal/BD foam treatment was statistically significantly more efficacious in inducing PGA success than 8 weeks of Cal/BD cream (p < .01 in five of six comparisons). Mean reductions in mPASI were consistently statistically significantly greater with Cal/BD foam than with Cal/BD cream.
Conclusions
Use of Cal/BD foam consistently shows significantly greater improvements in PGA and mPASI outcomes, compared with Cal/BD cream.
Introduction
Plaque psoriasis is a chronic, inflammatory, immune-mediated skin disorder characterized by well-defined red, scaly plaques. The symptoms of plaque psoriasis impact patients’ health-related quality of life. Itching and scaling are typically the most distressing symptoms for patients, with many patients experiencing skin pain or discomfort, while psychosocial effects including isolation, stigmatization and embarrassment are common (Citation1–3). Topical medications (alone or in combination with systemic drugs) are the most common treatment for patients with plaque psoriasis, with multiple agents available.
The addition of the vitamin D derivative calcipotriol has been shown to increase the efficacy of topical corticosteroids for plaque psoriasis (Citation4), and fixed-combination calcipotriol 50 µg/g (Cal) plus betamethasone dipropionate 0.5 mg/g (BD), used once-daily, is a recommended first-line treatment (Citation5). Cal/BD is available in multiple formulations. Cal/BD aerosol foam (Enstilar®, LEO Pharma), available since 2017, has been shown in randomized controlled trials (RCTs) to be statistically significantly more efficacious than the Cal/BD gel and ointment formulations (Citation6,Citation7). The increased efficacy of Cal/BD aerosol foam over gel and ointment formulations appears to relate to the formation of a supersaturated layer of product which allows greater penetration compared with other formulations (Citation8,Citation9).
Recently, a further Cal/BD formulation, a water-containing cream (Wynzora®, MC2 Therapeutics), has been approved in several regions of the world. Cal/BD cream has been compared with Cal/BD gel in RCTs (Citation10,Citation11), but no head-to-head data comparing the foam and cream formulations are available.
Clinically, it is important to understand if there is an efficacy difference between the diverse formulations of Cal/BD in order to provide individual patients with the most appropriate treatment to improve their symptoms and control their disease (topical therapies including Cal/BD can be used both as monotherapy and, for patients with moderate-to-severe psoriasis, in combination with systemic drugs). In the absence of head-to-head trials, alternative comparison methods can be used to compare therapies (Citation12). Matching-adjusted indirect comparison (MAIC) is an approach to comparing therapies that uses individual patient data (IPD) from clinical trials of one intervention and aggregate data from trials of the second, weighting the IPD to align the mean characteristics of the intervention populations (Citation12,Citation13). MAIC can be used either when no common comparator is available (‘unanchored’) or when the two interventions have been investigated in RCTs with a common comparator arm (‘anchored’; ) – where possible, anchored comparisons are the preferred approach (Citation12). Previously, MAIC analyses have been used to compare the efficacy of Cal/BD foam with that of non-biological systemic treatments and of halobetasol propionate 0.01%/tazarotene 0.045% lotion (Citation14,Citation15).
Figure 1. Schematic of MAIC process. IPD from trials of one intervention is selected and weighted to match the mean population characteristics of a second treatment. After adjustment, trial outcomes can be compared either directly (‘unanchored’) or via a common comparator (‘anchored’). BD, betamethasone dipropionate; Cal, calcipotriol; IPD, individual patient data; MAIC, matching-adjusted indirect comparison; RCT, randomized controlled trial.
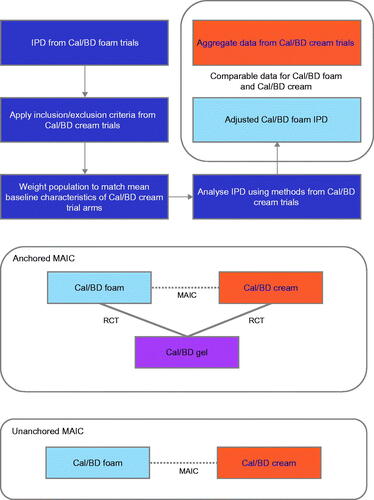
In this study, a MAIC approach was used to compare Cal/BD foam and Cal/BD cream. The objectives of the analysis were to estimate the efficacy of Cal/BD foam in a population matching that of the Cal/BD cream trial using MAIC and to compare the efficacy of Cal/BD foam and Cal/BD cream in inducing Physician's Global Assessment (PGA) success and in reducing modified Psoriasis Area and Severity Index (mPASI).
Materials and methods
Efficacy outcomes
The primary efficacy comparison was between Cal/BD foam results at 4 weeks and Cal/BD cream outcomes at 8 weeks, reflecting the licensed treatment durations and indications, as well as the time points at which the primary endpoints of the relevant trials were assessed. Where possible, results were also compared for both treatments at 4 weeks. Outcomes of interest were the proportion of patients with PGA success, defined as a score of 0 (clear) or 1 (almost clear) with an improvement of ≥ 2 points from baseline, and the mean reduction in mPASI.
MAIC methods and source data
Anchored and unanchored MAIC analyses were conducted as described by Signorovitch et al. (Citation13,Citation16); the overall MAIC process is illustrated in . The clinical trials included in the analysis are summarized in . For the anchored analysis, Cal/BD foam IPD were taken from the PSO-ABLE RCT, which was conducted in the USA and the EU and compared Cal/BD foam with Cal/BD gel (Citation6). PSO-ABLE results were compared with the available data from the US and EU trials of Cal/BD cream, in which Cal/BD gel was a comparator ().
Figure 2. (A) Comparison of included trials. (B) Summary of available data for MAIC analyses. BD, betamethasone dipropionate; Cal, calcipotriol; MAIC, matching-adjusted indirect comparison.
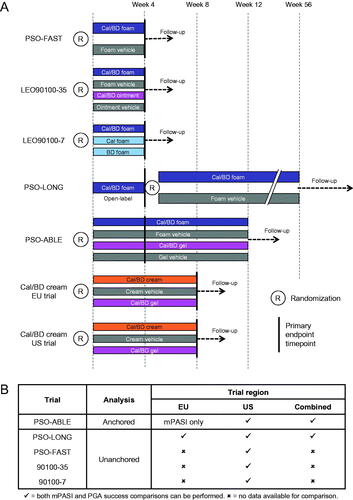
Aggregate data for the Cal/BD cream trials were taken from two published papers and the study clinicaltrials.gov records (Citation10,Citation11,Citation17,Citation18). Available data from the Cal/BD cream EU trial were limited, although pooled US and EU results have been published (Citation10); data availability is summarized in .
For the unanchored analysis, Cal/BD foam IPD were taken from three US RCTs – PSO-FAST (Citation19), LEO90100‒35 (Citation7) and LEO90100‒7 (Citation20), and from the 4-week open-label phase of the PSO-LONG Cal/BD foam trial, which included patients in both the USA and the EU (Citation21) ().
Data from the Cal/BD cream trials were rounded to the nearest integer number of patients. The change in mPASI at week 4 in the Cal/BD cream US trial was read from a graph (Citation11). The standard deviation (SD) for mPASI at week 4 was not available for this trial. Instead, the mPASI SD at week 8 was assumed to apply to the week 4 data and was used for both time points. A sensitivity analysis was conducted by using a week 4 mPASI SD calculated from a weighted average of week 4 variances across all five US Cal/BD foam arms as an alternative estimate of the SD in the Cal/BD cream US RCT.
Matching trial populations
IPD from the Cal/BD foam trials were selected by applying the inclusion criteria from the Cal/BD cream trials. Cal/BD foam trial IPD was then adjusted by weighting to match the mean baseline characteristics of patients treated with Cal/BD cream. Matched EU and US IPD were subsequently pooled for the comparison with the pooled EU and US data for Cal/BD cream. The baseline characteristics matched were age, sex, Fitzpatrick skin type, percentage of body surface area affected, mPASI and PGA; the prognostic relevance of these characteristics was confirmed by discussion among the authors.
Results
Baseline characteristics
For each of the MAICs informed by the different Cal/BD foam trials, the matched baseline characteristics of the Cal/BD foam arms were well balanced with the Cal/BD cream treatment arms after matching (; Supplementary Tables 1 and 2). In the anchored analysis, the effective sample sizes after matching were 88.5% and 82.0% of the original population in the US Cal/BD foam and Cal/BD gel arms, respectively. By contrast, in the EU analysis, the corresponding effective sample sizes were 38.3% and 43.4%, suggesting greater differences between trial populations in the EU comparison. In the unanchored analyses, the effective sample sizes after matching were 74.8–96.5% of the original patient numbers, suggesting substantial overlap between the Cal/BD foam and Cal/BD cream treatment arm populations.
Table 1. Baseline characteristics before and after matching – PSO-ABLE vs Cal/BD cream trials (anchored MAIC).
Anchored MAIC – PSO-ABLE RCT arms
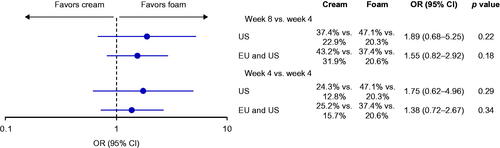
In the anchored MAIC, US patients were more likely to achieve PGA success after 4 weeks of Cal/BD foam than after 8 weeks of Cal/BD cream, although the confidence intervals were wide and the difference was not statistically significant (). Similar results were seen in the pooled US plus EU analysis at 8 weeks, and in the corresponding comparisons with 4 weeks of Cal/BD cream.
Figure 3. Odds ratios for achieving PGA success is anchored MAIC of PSO-ABLE versus US and EU RCTs of Cal/BD cream. PGA response rates are for the comparators vs Cal/BD gel. BD, betamethasone dipropionate; Cal, calcipotriol; CI, confidence interval; MAIC, matching-adjusted indirect comparison; OR, odds ratio; PGA, Physician's Global Assessment; RCT, randomized controlled trial.
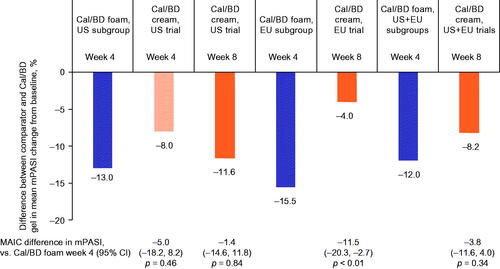
In the US, EU and pooled EU plus US analyses, patients receiving Cal/BD foam for 4 weeks had larger mean improvements in mPASI than those treated with Cal/BD cream for 8 weeks or 4 weeks (only US data could be compared at 4 weeks); the difference between treatments was statistically significant in the EU analysis only (p < .01; ).
Figure 4. Mean change in mPASI in anchored MAIC of PSO-ABLE versus US and EU RCTs of Cal/BD cream. Data shown are the difference in mean mPASI change between the comparators and Cal/BD gel. BD, betamethasone dipropionate; Cal, calcipotriol; CI, confidence interval; MAIC, matching-adjusted indirect comparison; mPASI, modified Psoriasis Area and Severity Index; RCT, randomized controlled trial.
Unanchored MAIC – Cal/BD foam RCT arms
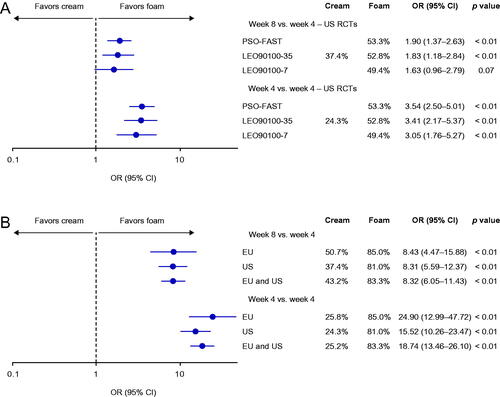
In the unanchored MAIC of Cal/BD foam RCTs, 4 weeks of Cal/BD foam treatment was statistically significantly more efficacious in inducing PGA success than 8 weeks of Cal/BD cream in two of the three comparisons (PSO-FAST and LEO90100-35, both p < .01), and numerically more efficacious in the third (LEO90100-7; ). When 4 weeks of Cal/BD foam was compared with 4 weeks of Cal/BD cream, Cal/BD foam was statistically significantly more efficacious in all three analyses (all p < .01).
Figure 5. Odds ratios for achieving PGA success in unanchored MAIC of (A) PSO-FAST, LEO90100-35 and LEO90100-7 versus US RCTs of Cal/BD cream, and (B) PSO-LONG versus US and pooled US and EU RCTs of Cal/BD cream. BD, betamethasone dipropionate; Cal, calcipotriol; CI, confidence interval; MAIC, matching-adjusted indirect comparison; OR, odds ratio; PGA, Physician's Global Assessment; RCT, randomized controlled trial.
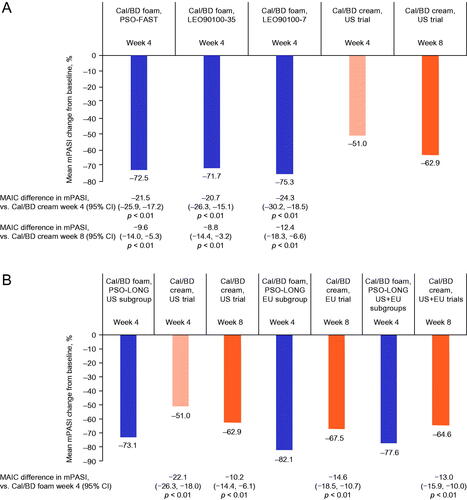
The mean reduction in mPASI with 4 weeks of Cal/BD foam treatment was statistically significantly greater than with 8 weeks of Cal/BD cream use in all three analyses (all p < .01; ). Similar results were found in the comparison with 4 weeks of Cal/BD cream treatment (all p < .01).
Figure 6. Mean change in mPASI in unanchored MAIC of (A) PSO-FAST, LEO90100-35 and LEO90100-7 versus US RCTs of Cal/BD cream, and (B) PSO-LONG versus US and pooled US and EU RCTs of Cal/BD cream. BD, betamethasone dipropionate; Cal, calcipotriol; CI, confidence interval; MAIC, matching-adjusted indirect comparison; mPASI, modified Psoriasis Area and Severity Index; RCT, randomized controlled trial.
Unanchored MAIC – PSO-LONG open-label phase
In the PSO-LONG MAIC, US patients treated with Cal/BD foam were statistically significantly more likely to have PGA success at week 4, compared with 8 weeks of Cal/BD cream treatment (p < .01; ). Similarly, in the EU analysis and in the pooled analysis of EU and US trial data, 4 weeks of Cal/BD foam use was associated with a statistically significantly greater likelihood of achieving PGA success, compared with 8 weeks of treatment with Cal/BD cream (p < .01). Both analyses found statistically significantly higher response rates when 4 weeks of Cal/BD foam was compared with 4 weeks of Cal/BD cream (both p < .01).
Patients receiving open-label Cal/BD foam for 4 weeks in the US cohort in PSO-LONG had statistically significantly greater improvements in mPASI than those using Cal/BD cream for 8 weeks (p < .01; ). Similar results were seen in the comparison with 4 weeks of Cal/BD cream (p < .01). In the EU analysis and in the pooled analysis of EU and US trial data, improvements in mPASI were statistically significantly greater with 4 weeks of open-label Cal/BD foam than with 8 weeks of Cal/BD cream (p < .01; ).
Discussion
This study used a MAIC approach to compare Cal/BD foam and Cal/BD cream, using IPD from five clinical trials of Cal/BD foam and publicly available, aggregate data from US and EU trials of Cal/BD cream. Overall, all comparisons found Cal/BD foam to have greater efficacy than Cal/BD cream in inducing PGA success and improving mPASI; the difference between the treatments was statistically significant in the majority of comparisons ().
The comparisons between 4 weeks of Cal/BD foam and 8 weeks of Cal/BD cream are of particular clinical relevance, as these are the licensed treatment periods for daily use of the two therapies (in the EU and the UK, patients with a response to 4 weeks of treatment with Cal/BD foam can continue with proactive twice-weekly maintenance treatment (Citation22)). The MAIC results suggest that 4 weeks of Cal/BD foam treatment is more efficacious in inducing PGA success than 8 weeks of Cal/BD cream, with an even greater difference seen between the two formulations after 4 weeks of each. These findings indicate that, in addition to greater efficacy over the treatment period, Cal/BD foam works more quickly than the cream. Fast response to treatment is important to patients, with rapid improvements in skin symptoms and quality of life potentially improving treatment adherence, which plays a key role in the effectiveness of topical therapies; patients are less likely to remain adherent during an 8-week course of treatment, compared with a 4-week course.
The rapid response seen with Cal/BD foam is likely to be a reflection of the aerosol foam formulation, which does not crystallize, allowing the rapid evaporation of the propellant and leaving a supersaturated layer of Cal/BD on the skin; this supersaturated layer has also been associated with greater penetration through thick lesions, compared with gel or ointment formulations (Citation8,Citation23–25).
After the matching process, the proportion of patients with PGA success in the included Cal/BD foam trials was similar to the results of the original trial populations (Citation6,Citation7,Citation19–21); this is consistent with the high effective sample size in most of the MAICs and suggests that the Cal/BD foam study populations were not substantially different from those of the Cal/BD cream trials. A numerical difference was observed between the PGA success rate (but no change in mPASI) at week 4 in the Cal/BD gel arms of the PSO-ABLE RCT (Citation6) and the US trial of Cal/BD cream (Citation11). The reason for this is unclear, and it may reflect unadjusted differences between the trials. In general, there are some differences between PSO-ABLE and the other Cal/BD foam RCTs (Citation6,Citation7,Citation19–21). In particular, PSO-ABLE had a higher proportion of patients with mild psoriasis than the other RCTs, which may have made PGA success (which for patients with the mild disease requires complete clearance of psoriasis) difficult to achieve (Citation6) – variation between PASI and PGA assessment methods has previously been described for therapies providing near-complete clearance of psoriasis (Citation26).
MAIC is a useful method for assessing the comparative efficacy of two treatments using evidence generated in different clinical trials in which baseline characteristics and treatment effect modifiers may differ (Citation12,Citation13,Citation16). The MAIC methodology, originally described by Signorovitch et al. (Citation13,Citation16), is now used widely to assess comparative efficacy. MAIC approaches have been used several times to compare therapies for plaque psoriasis, including comparisons of topical treatments, systemic and biological therapies (Citation14,Citation15,Citation27–29). A challenge in conducting MAICs is that published aggregate data on relevant baseline characteristics can sometimes be limited. In general, it would be beneficial if the provision of the detailed baseline characteristics needed for these types of comparisons became standard practice when publishing trial results, consistent with the current push toward greater public availability of clinical data.
This study has several strengths. First, this is the first analysis to compare the efficacy of Cal/BD aerosol foam and cream. Second, this study includes data from five trials of Cal/BD foam and two trials of Cal/BD cream. Data from individual Cal/BD foam trials were used, rather than pooled data, in order to give an overview of inter-trial variability. Third, in the unanchored analyses, the reduction in effective sample size when data for the Cal/BD foam treatment arms were adjusted to match the Cal/BD cream arms was small, suggesting that the populations of the phase 3 trials were generally similar.
The results of these analyses have some limitations. First, as with all indirect comparisons, there may be some bias due to unobserved differences across the trials, for which it was not possible to adjust. Second, only one trial – PSO-ABLE – could be used for anchored analysis. Third, the effective sample size in the PSO-ABLE trial arms after adjustment to match the Cal/BD cream population was low, limiting the statistical power of the anchored MAIC; however, the results of the anchored analysis were generally consistent with the unanchored comparisons. Fourth, the PGA success analysis for 4 weeks of Cal/BD cream in the US trial relies on data taken from a graph, while for the corresponding mPASI analysis it is necessary to use the week 8 SD. The results of the SD sensitivity analysis (data not shown) are essentially identical to the base-case analysis; however, these results should be treated with some caution. Because the week 4 data were not used in the primary efficacy comparison, the potential sensitivity of these results to changes in SD was not explored further. Fifth, the LEO90100-07 trial MAIC is limited by the small sample size and resultant lack of statistical power. Sixth, the high proportion of patients with PGA success in PSO-LONG may reflect the open-label nature of this trial, thereby limiting the comparability of these results with the RCT data. Accordingly, the latter comparison was presented separately. Finally, this analysis included objective efficacy parameters, with patient-reported outcomes and symptoms data not yet compared between the two therapies.
This study has compared the efficacy of treatment with Cal/BD foam or Cal/BD cream over 4 or 8 weeks, but the longer-term comparative efficacy of, for example, twice-weekly maintenance therapy remains unclear. In the absence of head-to-head studies, a further MAIC of maintenance treatment outcomes would be beneficial. In addition, comparative analyses of safety and of patient-reported outcomes – particularly patient preferences and skin symptoms such as itch and pain – would be clinically useful.
Conclusion
In conclusion, the MAIC results demonstrate that the use of Cal/BD foam as a once-daily treatment for 4 weeks shows significantly greater improvements in PGA and mPASI outcomes, compared with either 4 weeks or 8 weeks of Cal/BD cream treatment. These improvements in disease severity were demonstrated in the MAIC analyses of five different trials of Cal/BD foam, with strong consistency between the results from the different comparisons. Overall, Cal/BD foam has been shown to perform highly consistently.
Supplemental Material
Download PDF (80.9 KB)Acknowledgments
Medical writing support was provided by Symmetron Ltd (London, UK) and Beacon Medical Communications Ltd (Brighton, UK) in accordance with Good Publication Practice (GPP3) guidelines, and was funded by LEO Pharma (Ballerup, Denmark).
Disclosure statement
KP has received honoraria for advisory board, speaker, consultant services, scientific officer and/or steering committees from AbbVie, Akros, Amgen, Bausch Health/Valeant, Boehringer Ingelheim, Celgene, Celltrion, Coherus, Eli Lilly, Forbion, Galderma, Janssen, Kyowa Hakko Kirin, LEO Pharma, Meiji Seika Pharma, Merck (MSD), Merck-Serono, Mitsubishi Pharma, Novartis, Pfizer, Reistone, Sanofi-Aventis/Genzyme, Sandoz, Takeda, UCB, vTv Therapeutics and Xencor; and has received research grants from AbbVie, Akros, Amgen, Anacor, Arcutis, Astellas, Avillion, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, Bristol-Myers Squibb, Can-Fite Biopharma, Celgene, Coherus, Dermavant, Dermira, Dow Pharma, Eli Lilly, Evelo, Galapagos, Galderma, Genentech, Gilead, GSK, Incyte, Janssen, Kyowa Hakko Kirin, LEO Pharma, Medimmune, Merck (MSD), Merck- Serono, Moberg Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Sun Pharma, Takeda and UCB. HT, HB and MYJB are employees of LEO Pharma. SG has been an adviser and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of AbbVie, Affibody AB, Akari Therapeutics Plc, Almirall-Hermal, Amgen, Anaptys Bio, Argenx BV, AstraZeneca AB, Biogen Idec, Bioskin, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Dermira, Eli Lilly, Foamix, Forward Pharma, Galderma, Hexal AG, Incyte Inc., Janssen-Cilag, Johnson & Johnson, Kymab, LEO Pharma, Medac, MSD, Neubourg Skin Care GmbH, Novartis, Pfizer, Principia Biopharma, Regeneron Pharmaceutical, Sandoz Biopharmaceuticals, Sanofi-Aventis, Trevi Therapeutics and UCB Pharma. MM has been an adviser and/or received speakers’ honoraria for AbbVie, Eli Lilly, Novartis, LEO Pharma, UCB, Janssen, Novartis and Amgen. OY has received speaker honoraria from LEO Pharma, Merck (MSD) and Bristol-Myers Squibb; and acted as a consultant for Almirall, Isispharma and LEO Pharma.
Additional information
Funding
References
- Ljosaa TM, Rustoen T, Mörk C, et al. Skin pain and discomfort in psoriasis: an exploratory study of symptom prevalence and characteristics. Acta Derm Venerol. 2010;90(1):39–45.
- Hrehorów E, Salomon J, Matusiak L, et al. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92(1):67–72.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the national psoriasis foundation survey data 2003-2011. PLOS One. 2012;7(12):e52935.
- Mason AR, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2013;3:Cd005028.
- Körber A, Wilsmann-Theis D, Augustin M, et al. Topische therapie bei psoriasis vulgaris – ein behandlungspfad. JDDG: Journal Der Deutschen Dermatologischen Gesellschaft. 2019;17(S4):3–14.
- Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017;31(1):119–126.
- Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris–a randomized phase II study. J Dermatolog Treat. 2016;27(2):120–127.
- Yélamos O, Alejo B, Ertekin SS, et al. Non-invasive clinical and microscopic evaluation of the response to treatment with clobetasol cream vs. calcipotriol/betamethasone dipropionate foam in mild to moderate plaque psoriasis: an investigator-initiated, phase IV, unicentric, open, randomized clinical trial. J Eur Acad Dermatol Venereol. 2021;35(1):143–149.
- Basse LH, Olesen M, Lacour JP, et al. Enhanced in vitro skin penetration and antipsoriatic effect of fixed combination calcipotriol plus betamethasone dipropionate in an innovative foam vehicle. J Invest Dermatol. 2014;134.S33:abst 192.
- Pinter A, Green LJ, Selmer J, et al. A pooled analysis of randomized, controlled, phase 3 trials investigating the efficacy and safety of a novel, fixed dose calcipotriene and betamethasone dipropionate cream for the topical treatment of plaque psoriasis. Acad Dermatol Venereol. 2022;36(2):228–236.
- Stein Gold L, Green L, Dhawan S, et al. A phase 3, randomized trial demonstrating the improved efficacy and patient acceptability of fixed dose calcipotriene and betamethasone dipropionate cream. J Drugs Dermatol. 2021;20(4):420–425.
- Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–211.
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947.
- Bewley AP, Shear NH, Calzavara-Pinton PG, et al. Calcipotriol plus betamethasone dipropionate aerosol foam vs. apremilast, methotrexate, acitretin or fumaric acid esters for the treatment of plaque psoriasis: a matching-adjusted indirect comparison. J Eur Acad Dermatol Venereol. 2019;33(6):1107–1115.
- Wu JJ, Hansen JB, Patel DS, et al. Effectiveness comparison and incremental cost-per-responder analysis of calcipotriene 0.005%/betamethasone dipropionate 0.064% foam vs. halobetasol 0.01%/tazarotene 0.045% lotion for plaque psoriasis: a matching-adjusted indirect comparative analysis. J Med Econ. 2020;23(6):641–649.
- Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28(10):935–945.
- clinicaltrials.gov. NCT03308799 2019 [cited 2021 September]. Available from: https://clinicaltrials.gov/ct2/show/NCT03308799.
- clinicaltrials.gov. NCT03802344 2020 [cited 2021 September]. Available from: https://clinicaltrials.gov/ct2/show/NCT03802344.
- Leonardi C, Bagel J, Yamauchi P, et al. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris–a randomized phase III study (PSO-FAST. J Drugs Dermatol. 2015;14:1468–1477.
- Lebwohl M, Tyring S, Bukhalo M, et al. Fixed combination aerosol foam calcipotriene 0.005% (cal) Plus betamethasone dipropionate 0.064% (BD) is more efficacious than cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, phase 2 study. J Clin Aesthet Dermatol. 2016;9:34–41.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84(5):1269–1277.
- LEO Pharma. Enstilar Cutaneous Foam, Summary of Product Characteristics 2021. [cited 2021 April 14]. Available from: https://www.medicines.org.uk/emc/product/2139/smpc.
- Puig L, Carretero G. Update on topical treatments for psoriasis: the role of calcipotriol plus betamethasone dipropionate aerosol foam. Actas Dermosifiliogr. 2019;110(2):115–123.
- Rudnicka L, Olszewska M, Goldust M, et al. Efficacy and safety of different formulations of calcipotriol/betamethasone dipropionate in psoriasis: gel, foam, and ointment. J Clin Med. 2021;10:5589.
- Lind M, Nielsen KT, Schefe LH, et al. Supersaturation of calcipotriene and betamethasone dipropionate in a novel aerosol foam formulation for topical treatment of psoriasis provides enhanced bioavailability of the active ingredients. Dermatol Ther. 2016;6(3):413–425.
- Wu AG, Conway J, Barazani L, et al. Is clear always clear? Comparison of psoriasis area and severity index (PASI) and the physician's global assessment (PGA) in psoriasis clearance. Dermatol Ther. 2020;10(5):1155–1163.
- Hampton P, Borg E, Hansen JB, et al. Efficacy of brodalumab and guselkumab in patients with moderate-to-severe plaque psoriasis who are inadequate responders to ustekinumab: a matching adjusted indirect comparison. PTT. 2021;ume 11:123–131.
- Papp KA, Yang M, Sundaram M, et al. Comparison of adalimumab and etanercept for the treatment of moderate to severe psoriasis: an indirect comparison using individual patient data from randomized trials. Value Health. 2018;21(1):1–8.
- Warren RB, Brnabic A, Saure D, et al. Matching-adjusted indirect comparison of efficacy in patients with moderate-to-severe plaque psoriasis treated with ixekizumab vs. secukinumab. Br J Dermatol. 2018;178(5):1064–1071.
