Abstract
Purpose: The incidence of cutaneous paradoxical reactions associated with IL-17 inhibitors has gained attention in recent literature. Our report aims to investigate the characteristics of one rare paradoxical reaction, presenting as Behcet’s disease.
Methods: We reported one case of Behcet’s-like disease induced by secukinumab in a patient with psoriasis. This patient, a young woman with a long history of psoriasis, showed significant improvement in her psoriatic condition after receiving four doses of secukinumab. Unexpectedly, she developed symptoms such as high fever, painful oral and genital ulcers, facial maculopapules, and erythema nodosum-like lesions on her lower limbs. Despite neutrophilia, there was no evidence of infection found in her laboratory tests. Histological analysis of a skin biopsy highlighted subcutaneous panniculitis and a mixed inflammatory cell infiltrate in the dermis. The patient was consequently diagnosed with secukinumab-induced Behcet’s-like disease. Additionally, we have reviewed nine other documented cases of Behcet’s-like disease triggered by IL-17 inhibitors.
Results: This group showed no significant gender preference, suffering from conditions such as psoriasis, ankylosing spondylitis, and hidradenitis suppurativa. Oral and genital ulcers were prevalent among the paradoxical reactions noted. Marked improvement was observed in all patients upon discontinuation of the IL-17 inhibitors.
Conclusions: Our report serves to alert physicians to this uncommon but significant paradoxical effect that may arise with anti-IL-17 treatment.
Introduction
Biologics targeting interleukin-17 (IL-17) are widely used for moderate to severe psoriasis with great efficiency. Nonetheless, their usage has sporadically resulted in paradoxical reactions, defined as the emergence or intensification of immune-mediated pathologies while these agents are being employed to treat a different condition. Several cutaneous paradoxical reactions have been reported in association with IL-17 inhibitors, such as eczema, sarcoidosis-like eruptions, alopecia areata, pyoderma gangrenosum, and so on. However, mucocutaneous ulcers, a characteristic manifestation of Behcet’s disease (BD), are rarely observed as paradoxical reactions associated with IL-17 inhibitors [Citation1]. Here, we report a case of Behcet’s-like disease potentially induced by an IL-17 inhibitor used for treating psoriasis.
Case report
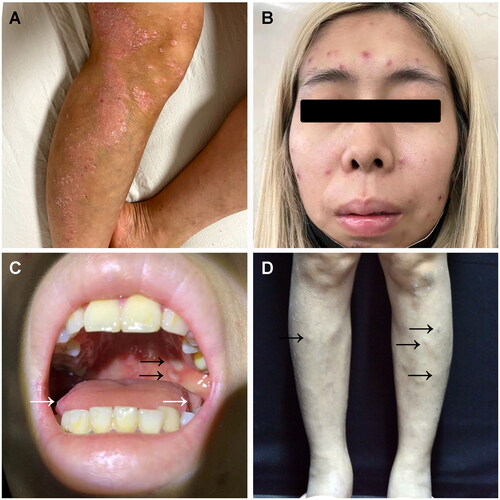
A 28-year-old female patient with a 20-year history of psoriasis presented to our clinic for secukinumab therapy (). Although her psoriasis improved markedly within four weeks (300 mg per week), she developed high fevers, painful oral and genital ulcers, and asymptomatic facial eruptions. Subsequently, painful skin eruptions and tender lower limb swelling appeared. She reported anorexia, weight loss, and fatigue but no ocular or gastrointestinal symptoms (abdominal pain, black stool, and diarrhea). Physical examination revealed scattered papulopustules or maculopapules on the face (). Besides, ulcers and erosions were found in her mouth, especially involving her tongue and palate (). One erosion was also noticed on her labia majora. Several subcutaneous nodules were observed on her lower limbs ().
Figure 1. Clinical findings of the patient. (A) Psoriasis plaques involving her lower leg before secukinumab therapy; (B) Multiple eruptive papulopustules on the face; (C) Several oral ulcers involving her palate and tongue (as arrows); (D) Scattered subcutaneous nodules on her legs (as arrows).
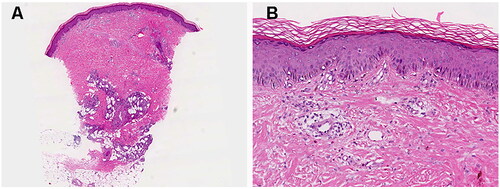
One skin biopsy taken from her subcutaneous nodule demonstrated a predominant lymphocytic infiltrate with neutrophils and eosinophils around vessels in the superficial and deep dermis (). Mixed lobular and septal panniculitis were also showed (). Repeated bacterial, viral, and fungal cultures of the lesions and her blood were all sterile. Laboratory examination indicated neutrophilia of 8770/mm3 without any evidence of infection. Autoantibody screens were negative. She was also found to have elevated erythrocyte sedimentation rate (87 mm/h, normal range: 0–20 mm/h) and C-reactive protein (159.3 mg/L, normal range: 0–8 mg/L). Additionally, the occult blood test on her stool yielded negative results. Despite negative results of pathergy and human leukocyte antigen (HLA)-B51 tests, a diagnosis of Behcet’s-like disease was considered, probably prompted by secukinumab administration. Upon cessation of secukinumab and initiation of oral corticosteroids, the patient’s condition improved within three weeks.
Figure 2. Histological findings of the patient. (A) A pattern of inflammatory cell infiltration in both the superficial and deep dermal layers, accompanied by mixed lobular and septal panniculitis; (B) A predominance of lymphocytes, neutrophils, and eosinophils within the inflammatory infiltrate. Hematoxylin and eosin, original magnification: (a) ×20; (b) ×100.
Discussion
Secukinumab is a selective inhibitor of IL-17A. By impeding the interaction between IL-17A and its receptor, it hinders the release of proinflammatory cytokines including tumor necrosis factor-alpha, interferon-gamma, IL-6, and other chemokines, which are pivotal for the pathophysiology of psoriasis. It has demonstrated its efficacy and safety in treating patients with moderate to severe psoriasis for years. Recently, there have been increasing reports of Behcet’s-like disease following the use of IL-17 blocking agents. Consequently, we have summarized the characteristics of these cases [Citation2–8].
As shown in , no apparent gender predilection was seen in the documented patients with Behcet’s-like disease occurring under IL-17 inhibitor treatment. The age of onset ranged widely, from 29 to 56 years. These cases included individuals with various underlying conditions, such as psoriasis [Citation2,Citation4–6], ankylosing spondylitis [Citation3,Citation8], and hidradenitis suppurativa [Citation7]. It is noteworthy that while most patients received treatment with secukinumab, one patient was administered Ixekizumab [Citation6], suggesting that this paradoxical reaction is not solely triggered by one type of IL-17 inhibitor. The onset of paradoxical reactions ranged from 2 to 40 weeks after the administration of IL-17 inhibitors, implying that the development of this condition may be either immediate or significantly delayed.
Table 1. Reported patients’ characteristics of IL-17 inhibitor associated behcet’s-like disease.
The most common symptoms among these patients included high fever, and oral and genital ulcers. Notably, two patients simultaneously presented with esophageal, gastric, and duodenal ulcers [Citation2,Citation9]. Papulopustules, another characteristic lesion, were observed on the trunk and lower limbs of three documented patients [Citation4,Citation5,Citation7], while they were present on our patient’s face. Our patient and two others also exhibited erythema nodosum-like painful lesions on the lower extremities [Citation4]. Uveitis was reported in only two previous cases [Citation3,Citation4].
A subset of the patients tested positive for HLA-B51 or HLA-B13/B50, suggesting a potential genetic predisposition for the development of Behcet’s-like disease in response to IL-17 inhibitors. As proven by prior research, BD exhibits various genetic markers [Citation10], of which HLA-B51 is most prevalently reported [Citation11]. HLA-B51 is associated with neutrophil hyperfunction [Citation12]. This implies caution should be exercised when administering IL-17 inhibitors to patients with HLA-B51.
While IL-17 is pivotal in BD pathophysiology and despite its reported success in treating refractory BD [Citation13], the underlying cause of Behcet’s-like disease onset following anti-IL-17 therapy remains unclear. One study showed IL-17A could exert immune-regulatory effects through negative feedback on Th17 cells and subsequent inhibition of IL-17F and granulocyte-macrophage colony-stimulating factor secretion [Citation6]. Therefore, the inhibition of IL-17A alone may lead to an imbalance of other cytokine levels, potentially triggering BD-like symptoms. Moreover, recent studies unveiled that dysbiosis in the gut or oral microbiota could disturb the balance between regulatory T cells (Tregs) and Th1/Th17 cells through metabolic processes, consequently provoking pathological reactions in BD [Citation14]. It’s been reported that IL-17 inhibitors treatment led to noticeable changes in the composition of gut microbiota in psoriasis patients [Citation15]. Therefore, one hypothesis is that IL-17 inhibitors might cause an imbalance of immune homeostasis by altering gut microbiota, then triggering the onset of BD-like symptoms. However, this assumption still needs to be verified.
These reports, along with our case, suggest that the onset of Behcet’s-like disease following the use of an IL-17 blocking agent could be considered a novel paradoxical reaction. Discontinuation of the causative drug is typically recommended for paradoxical reactions [Citation1]. Following the cessation of the IL-17 inhibitor and the commencement of steroid therapy, Behcet’s-like disease symptoms improved in all cases, signaling a positive response to this treatment approach. Furthermore, switching to an alternative medication was also recommended [Citation1]. As for our patient, our next step will involve treating her psoriasis with apremilast.
Our case serves to raise awareness among physicians regarding this paradoxical effect that can occur during the treatment of anti-IL-17 drugs. Early detection of the symptoms and timely discontinuation of the biologics may foster better patient outcomes. Additionally, more cases are required to delineate this immune-mediated adverse reaction accurately, and long-term monitoring of these individuals were also mandatory. Further investigation into the precise underlying mechanism is warranted.
Informed consent
The patient has given informed consent to publication of her case details and images.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Murphy MJ, Cohen JM, Vesely MD, et al. Paradoxical eruptions to targeted therapies in dermatology: a systematic review and analysis. J Am Acad Dermatol. 2022;86(5):1–6. doi: 10.1016/j.jaad.2020.12.010.
- Shiga H, Fukuda S, Iijima K. Interleukin-17A inhibitor-induced Crohn’s disease/Behcet’s disease-like lesions. Inflamm Bowel Dis. 2017;23(6):E38–E39. doi: 10.1097/MIB.0000000000001142.
- Dincses E, Yurttas B, Esatoglu SN, et al. Secukinumab induced Behcet’s syndrome: a report of two cases. Oxf Med Case Reports. 2019;2019(5):omz041. doi: 10.1093/omcr/omz041.
- Barrado-Solís N, Rodrigo-Nicolás B, De la Morena-Barrio I, et al. Report of two cases of Behcet’s disease developed during treatment with secukinumab. J Eur Acad Dermatol Venereol. 2020;34(10):e587–e589. doi: 10.1111/jdv.16454.
- Calleja Algarra A, Aragón Miguel R, Andrés Lencina JJ, et al. Behcet’s-like disease during secukinumab treatment: new paradoxical reaction? J Dtsch Dermatol Ges. 2021;19(1):116–118. doi: 10.1111/ddg.14196.
- Chen D, Zhou M, Xu A, et al. Behcet’s-like disease in a patient treated with ixekizumab for chronic plaque psoriasis. Scand J Rheumatol. 2022;51(4):336–337. doi: 10.1080/03009742.2021.2014105.
- Avcı C, Akın G, Akarsu S, et al. Pyoderma gangrenosum and Behcet’s-like disease induced by secukinumab: a paradoxical drug reaction. J Dermatolog Treat. 2023;34(1):2235040. doi: 10.1080/09546634.2023.2235040.
- Ogasawara M, Taniguchi Y. IL-17 inhibitor-induced Behcet-Like disease. J Clin Rheumatol. 2023;30(2):e65. doi: 10.1097/RHU.0000000000002025.
- Sun FSK, Chiu NSY, Chung HY. Potential gastrointestinal Behcet’s disease flare after treatment with anti-interleukin 17a therapy. BMC Rheumatol. 2023;7(1):25. doi: 10.1186/s41927-023-00344-9.
- van der Houwen TB, van Hagen PM, van Laar JAM. Immunopathogenesis of Behçet’s disease and treatment modalities. Semin Arthritis Rheum. 2022;52:151956. doi: 10.1016/j.semarthrit.2022.151956.
- Takeno M. The association of Behçet’s syndrome with HLA-B51 as understood in 2021. Curr Opin Rheumatol. 2022;34(1):4–9. doi: 10.1097/bor.0000000000000846.
- Bodis G, Toth V, Schwarting A. Role of human leukocyte antigens (HLA) in autoimmune diseases. Rheumatol Ther. 2018;5(1):5–20. doi: 10.1007/s40744-018-0100-z.
- Di Scala G, Bettiol A, Cojan RD, et al. Efficacy of the anti-IL 17 secukinumab in refractory Behcet’s syndrome: a preliminary study. J Autoimmun. 2019;97:108–113. doi: 10.1016/j.jaut.2018.09.002.
- Joubert M, André M, Barnich N, et al. Microbiome and Behçet’s disease: a systematic review. Clin Exp Rheumatol. 2023;41(10):2093–2104. doi: 10.55563/clinexprheumatol/zbt4gx.
- Huang YH, Chang LC, Chang YC, et al. Compositional alteration of gut microbiota in psoriasis treated with IL-23 and IL-17 inhibitors. Int J Mol Sci. 2023;24(5):4568. doi: 10.3390/ijms24054568.
