Abstract
Background
The recent introduction of biological drugs specifically targeting the interleukins involved in psoriasis pathogenesis revolutionized the therapeutic scenario of moderate to severe forms of psoriasis. Among these, risankizumab, an anti-IL-23, was shown to be effective both in clinical trials and real-life experiences. However, data on its use on very severe forms of psoriasis, defined by a Psoriasis Area Severity Index (PASI) of at least 30, are scant. In this context, our study aimed to investigate the outcomes of patients with very severe psoriasis, and the involvement of difficult-to-treat areas treated with risankizumab for up to 2 years.
Methods
A retrospective, observational study enrolled patients with very severe plaque psoriasis and the involvement of difficult-to-treat areas undergoing treatment with risankizumab. Clinical and demographic data were collected at baseline. Moreover, at baseline and each dermatological examination (16, 28, 40 and 104 weeks), clinical improvement was measured using the percentage of patients achieving PASI 75/90/100 response, site-specific Psoriasis Global Assessment and Dermatology Life Quality Index.
Results
At baseline, the mean PASI was 35.1 ± 5.1. A significant reduction was observed since week 16 and maintained up to week 104. Moreover, the Psoriasis Global Assessment and Dermatology Life Quality Index improved as well.
Conclusions
Risankizumab showed to be effective and safe in patients affected by very severe forms of psoriasis with the involvement of difficult-to-treat areas.
Introduction
Psoriasis is a chronic relapsing cutaneous disease with an estimated prevalence of 2–4% of the worldwide population [Citation1]. Plaque psoriasis is the most common clinical presentation (up to 90% of cases) [Citation1].
Moreover, several comorbidities can be associated with psoriasis, such as psoriatic arthritis, cardiovascular diseases, diabetes, inflammatory bowel disease, etc.
The knowledge of psoriasis pathophysiology, particularly the role of the interleukin (IL)-23/17 axis, has greatly advanced in the last decades, leading to the development of new target treatments and allowing improved outcomes [Citation2].
The treatment of plaque psoriasis changes with the disease severity. Indeed, mild cases are usually managed with topical prescription therapy, while mild-to-severe cases need systemic interventions. The choice of systemic treatments depends on the level of severity, previous treatments, comorbidities, and the patient’s history, which may show personal issues in responsiveness and tolerability.
Beyond classic systemic drugs, such as methotrexate, acitretin, dymethil fumatare and ciclosporin, several biologics are available for the systemic treatment of psoriasis, targeting cytokines, including IL-17, IL-23, IL-12/23 and TNF-α [Citation3, Citation4]. As a result, goals, such as 90% improvement (Psoriasis Area and Severity Index score [PASI] 90) of severe psoriasis and successful management of very serious cases, have been achievable [Citation5, Citation6].
Risankizumab, an IgG1-humanized monoclonal antibody, is a recently approved IL-23 inhibitor that has been licensed for the treatment of moderate to severe plaque psoriasis, psoriatic arthritis, and Chron’s disease [Citation7]. Its efficacy and safety have been demonstrated by several clinical trials [Citation8–11] and was confirmed by studies in the real-life setting [Citation12–15]. Further investigation of data retrieved from the clinical practice may improve knowledge about the durability of responses, interactions with comorbidities, and possible patients’ profiles, suggesting populations with higher or reduced probability of benefit. Specifically, clinical trial data are not easily generalizable to patients with very severe disease, as they are usually excluded by specific trials’ criteria, which tend to enroll only subjects affected by moderate to severe plaque psoriasis [Citation8–10]. Indeed, guidelines for severe forms of psoriasis are lacking, as well as this category may shed light on specific unmet medical needs in this segment of the population with psoriasis [Citation11].
This study aimed to investigate the outcomes of patients with very severe psoriasis and the involvement of difficult-to-treat areas treated with risankizumab in two dermatological centers in Italy and followed up for up to 2 years.
Patients and methods
A retrospective observational study was performed between January 2021 and January 2024 in San Gallicano Dermatological Institute and the Dermatology Department of the University of Naples Federico II, enrolling patients with very severe plaque psoriasis, defined as PASI ≥ 30, treated with risankizumab.
Risankizumab was administered following the Italian Guidelines for the management of plaque psoriasis [Citation16, Citation17]. The study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data collection
Clinical and demographic data collected before the initiation of treatment with risankizumab included age, gender, body mass index (BMI), comorbidities, previous exposure to biological drugs, and the involvement of difficult-to-treat areas (scalp, palms/soles, genitalia, and nails). At baseline and each dermatological examination (16, 28, 40 and 104 weeks), clinical outcomes were measured by the following parameters: 1) PASI – the improvements of 75%, 90% and 100% in PASI score compared with baseline (PASI 75, 90 and 100); 2) site-specific (scalp, palmoplantar, genital, nail) Psoriasis Global Assessment (PGA) of clear, almost clear, moderate, severe and very severe level.
For patients who did not attend the scheduled dermatological visits and performed the injection of risankizumab at home or skipped the dose, data from the last available visit were used using the last-observation carried-forward analysis (a missing follow-up visit value is replaced by that subject’s previously observed value).
At each visit, the occurrence of any adverse events (AEs) was recorded, including serious AEs and AEs leading to risankizumab discontinuation.
Statistical analysis
Data were summarized by descriptive analysis. Means, median and standard deviations (SDs) were calculated for continuous variables, while absolute values and frequency (%) were calculated for categorical variables. A t-test performed a comparison of mean values. The Wilcoxon-signed rank test was used to compare repeated measurements in each group, and the Mann–Whitney test compared mean data between groups. Binary logistic regression was used to assess the association of variables. All analyses were performed with IBM SPSS Statistics for Windows, Version 26.0. p < 0.05 was considered significant.
Results
Patients’ characteristics
Demographic and clinical characteristics of patients at baseline are reported in . Overall, 27 patients were included (17 (63%) male, mean age 46.2 ± 14.5). Mean PASI at baseline was 35.1 ± 5.1. Eight (29.6%) subjects had not received systemic therapy before the study. The other 19 (70.3%) patients had received one or more systemic treatments. Classic systemic drugs were: ciclosporin (n = 10, 52.6%), methotrexate (n = 6, 31.6%) or acitretin (n = 3, 15.8%). Eight (42.1%) patients had been treated with UVB phototherapy. Moreover, 1 (9.1%) patient previously failed apremilast.
Table 1. Demographic and clinical characteristics of patients at baseline.
Sixteen (59.3%) patients were naïve to biologics. Among patients who previously failed at least one biologic drug (n = 11, 40.7%), 6 (45.5%) had received adalimumab, 2 (18.2%) etanercept, 2 (18.2%) secukinumab, certolizumab, infliximab, ustekinumab were used by 1 (9.1%) patient each. Three (27.3%) patients had received more than one previous biologic. Of note, previous biologics were discontinued due to loss of efficacy in all cases.
Outcomes of treatment with risankizumab
A total of 26 (96.3%), 25 (92.6%), 23 (85.2%), and 21 (77.8%) reached 16, 28, 40 and 104 years of treatment, respectively.
The mean PASI score was significantly reduced since week 16 (PASI baseline: 35.1 ± 5.1; PASI week 16: 3.0 ± 4.3, p < 0.001) and the mean improvement was maintained at all study time points ().
Table 2. Mean PASI score at study visits.
An improvement of psoriasis up to PASI 75 was reached in 100% of patients by week 40 and maintained at week 104. Moreover, 21/21 (100%) reached PASI 90 by week 104. After 2 years of treatment, 17/21 (81%) patients reached PASI 100. 16/26 (61.5%) patients reached PASI ≤2 by week 16, and the proportion increased at each visit up to 20/21 (95.2%) patients at week 52. The proportions of patients achieving PASI 75, 90, 100 and ≤2 are shown in .
Table 3. Patients achieving PASI 75, 90 and 100 or ≤2 at each study visit.
At least one difficult-to-treat area was involved in each patient. The efficacy of risankizumab in these areas was evaluated by site-specific PGA (). The proportion of patients with clear scalp progressively increased from baseline to week 104. The scalp was clear from psoriasis at week 104 in 20/21 (95.2%) patients and in a similar percentage (22/23, 95.7%) by week 40. Only one patient had moderate palmoplantar psoriasis at baseline and was clear at week 16; two patients had mild palmoplantar lesions at baseline and were both clear by week 28. The genital area was severely involved in 4 (15.4%) patients, who were all clear by week 16 and thereafter. Mild psoriasis of nails was recorded in 3 (11.5%) patients at baseline, persisted at week 28, and was absent by week 40. Moderate nail involvement was observed.
Table 4. Efficacy of risankizumab in difficult-to-treat areas (scalp, palms and soles, genital area, and nails), assessed by site-specific physician global assessment score.
Impact of previous biologic treatment on the efficacy of risankizumab
We evaluated the association of some characteristics at baseline with complete clearance of psoriasis (PASI 100) at week 16. The binary logistic regression analysis showed a significant positive association of PASI 100 at week 16 with being naïve to biologics at baseline (OR = 43.7; 95% CI: 2.1–930.7; p = 0.015). Conversely, no significant association was found with obesity, age, gender, PASI at baseline, and concomitant psoriatic arthritis.
The demographic and clinical characteristics of naïve and non-naïve patients were similar, although four naïve patients were obese and 0 non-naïve subject, one naïve patient had psoriatic arthritis vs four non-naïve ones. The baseline mean PASI was slightly higher in naïve than in non-naïve patients (37.0 ± 5.0 vs 32.3 ± 4.0; p = 0.021). The improvement of mean PASI versus baseline was significant (p < 0.001) at all study visits, both in naïve and non-naïve patients. Starting from the visit at week 16 and thereafter at all study visits, the patients who had experienced previous biologics had higher mean PASI than naïve patients (). Improvement of PASI was higher in naïve than in non-naïve subjects: PASI 75 was reached more frequently by naïve subjects only at week 16 and was similarly frequent at later visits; PASI 90 was more frequent in naïve subjects at weeks 16 and 28; finally, PASI 100 was obtained more frequently by naïve subjects than by non-naïve ones at all visits ().
Figure 1. PASI comparison between patients who had (non-naïve) or had not (naïve) experienced previous biologics. (A) Mean Pasi at baseline and at each study visit. (B) Proportion of patients with PASI improvement (PASI 75, 90 and 100) at each study visit.
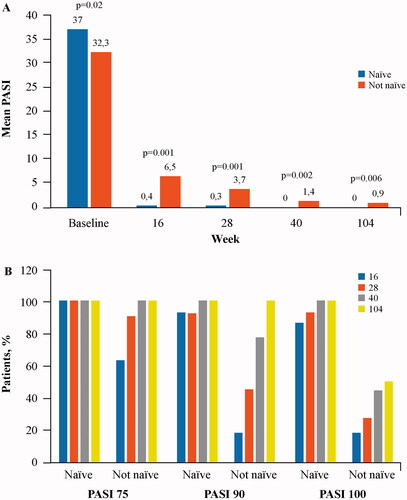
PASI ≤2 was obtained by a higher proportion of naïve than non-naïve patients at weeks 16, 28 and 40; only at week 104, the difference between the groups was not significant, although PASI ≤2 was slightly more frequent among naïve subjects (100% vs 87.5%).
No patient discontinued risankizumab due to adverse events.
Discussion
This retrospective study confirmed the efficacy of risankizumab on patients affected by very severe psoriasis in a real-life setting, also with the involvement of difficult-to-treat areas [Citation8–10].
Currently, data on the use of risankizumab in these patients are scant. Indeed, there is only one clinical trial on Japanese patients affected by erythrodermic psoriasis [Citation18] and few case reports on patients with an erythrodermic form of the disease [Citation19]. However, these populations were not comparable to ours due to the different psoriasis severity at baseline.
Similarly, our population is not directly comparable to the patients of a previous multicenter real-life study in Italy [Citation13]. Indeed, our subjects had a higher mean PASI score at baseline compared to patients of the previous study, as we selected very difficult cases. The efficacy of risankizumab in difficult-to-treat areas has been reported in the real-life setting by Brunasso et al. in eight patients with moderate to severe psoriasis [Citation15]. Also the observational study from registry data published by Strober et al. confirming the efficacy of risankizumab in the real-life setting, does not provide information for the management of very severe cases [Citation12].
Our data showed that within a population with very severe disease, the response to risankizumab is higher, more frequent and is obtained earlier if no other biologic has been used previously. We were not able to analyze whether different biologics had different impacts on the response to risankizumab due to the limited number of patients.
Overall, as in less severe patients [Citation13], a progressive improvement was observed throughout the 2-year study in terms of mean PASI and frequency of PASI 75, 90 and 100. The evaluation of difficult-to-treat areas showed that very severe patients had a relevant benefit from risankizumab also in these sites and not only in classical localizations.
This study has some limitations, including the observational retrospective design, the limited sample number of patients due to the relatively low incidence of very severe cases, as well as the heterogeneity of clinical evaluations from different clinicians.
Conclusion
Our data suggest that risankizumab may be effectively used in patients with very severe plaque psoriasis and that it may obtain better results if used before other biologics.
Authors’ contributions
Study conception and design: Orsini D, Megna M; collection and interpretation of data: Assorgi C, Potestio L; statistical analysis: Assorgi C; manuscript drafting: Cameli N; manuscript editing: Bonifati C; approval to submit: Orsini D, Megna M.
Ethics approval
Institutional review board approval was exempted for this study as its procedure did not deviate from good routine clinical practice. The study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Consent to participate
All patients gave written informed consent for the retrospective retrieval of anonymized data.
Consent for publication
All patients gave written informed consent for the publication of anonymized data.
Acknowledgements
Editorial assistance was provided by Aashni Shah and Laura Brogelli (Polistudium, Milan, Italy).
Disclosure statement
D. Orsini has been a speaker and/or consultant for Abbvie, LeoPharma, UCB, Bristol-Meyer-Squibb and Boehringer- Ingelheim. M. Megna acted as a speaker or consultant for Abbvie, Eli Lilly, Janssen, Leo-Pharma, UCB and Novartis. No other disclosures were reported.
Data availability statement
Additional data supporting the findings of this study are available from the Corresponding Author on reasonable request.
Additional information
Funding
References
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1–6. doi: 10.1001/jama.2020.4006.
- Martin G, Young M, Aldredge L. Recommendations for initiating systemic therapy in patients with psoriasis. J Clin Aesthet Dermatol. 2019;12:13–26.
- Darwin E, Lebwohl M, Han G. Biologic vs conventional therapies: comparing risk of psoriasis-associated comorbidities. J Drugs Dermatol. 2023;22(6):621–622. doi: 10.36849/JDD.7119.
- Zhu B, Jing M, Yu Q, et al. Treatments in psoriasis: from standard pharmacotherapy to nanotechnology therapy. Postepy Dermatol Alergol. 2022;39(3):460–471. doi: 10.5114/ada.2021.108445.
- Mastorino L, Dapavo P, Susca S, et al. Drug survival and clinical effectiveness of secukinumab, ixekizumab, brodalumab, guselkumab, risankizumab, tildrakizumab for psoriasis treatment. J Dtsch Dermatol Ges. 2024;22:34–42.
- Pavia G, Gargiulo L, Spinelli F, et al. Generalized pustular psoriasis flare in a patient affected by plaque psoriasis after BNT162b2 mRNA COVID-19 vaccine, successfully treated with risankizumab. J Eur Acad Dermatol Venereol. 2022;36(7):e502–e505. doi: 10.1111/jdv.18032.
- Elgaard CDB, Iversen L, Hjuler KF. Guselkumab, tildrakizumab, and risankizumab in a real-world setting: drug survival and effectiveness in the treatment of psoriasis and psoriatic arthritis. J Dermatolog Treat. 2023;34:2133531.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650–661.
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184(1):50–59. doi: 10.1111/bjd.19341.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(6):649–658. doi: 10.1001/jamadermatol.2020.0723.
- Fiorillo G, Ibba L, Gargiulo L, et al. Effectiveness and safety of biological therapies in very severe plaque psoriasis: a real-life retrospective study. J Pers Med. 2024;14(2):186. doi: 10.3390/jpm14020186.
- Strober B, Ferris L, Callis Duffin K, et al. Real-world effectiveness of risankizumab in patients with moderate-to-severe psoriasis using the CorEvitas psoriasis registry. J Am Acad Dermatol. 2024;90(1):82–90.
- Gargiulo L, Ibba L, Malagoli P, et al. A risankizumab super responder profile identified by long-term real-life observation-IL PSO (ITALIAN LANDSCAPE PSORIASIS). J Eur Acad Dermatol Venereol. 2024;38(1):e113–e116. doi: 10.1111/jdv.19464.
- Orsini D, Gargiulo L, Ibba L, et al. Effectiveness of risankizumab in plaque psoriasis with involvement of difficult-to-treat areas: a real-world experience from two referral centers. J Dermatolog Treat. 2023;34:2220849.
- Brunasso AMG, Burlando M, Amoruso F, et al. Risankizumab: daily practice experience of high need patients. Biomedicines. 2023;11(6):1769. doi: 10.3390/biomedicines11061769.
- Gisondi P, Fargnoli MC, Amerio P, et al. Italian adaptation of EuroGuiDerm guideline on the systemic treatment of chronic plaque psoriasis. Ital J Dermatol Venerol. 2022;157:1–78.
- European Medicines Agency. Skyrizi (Risankizumab): Summary of product characteristics. 2019. [cited 2024 Apr 5]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/skyrizi.
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study. J Dermatol. 2023;50(2):195–202. doi: 10.1111/1346-8138.16667.
- Megna M, Ruggiero A, Salsano A, et al. A case of erythrodermic psoriasis successfully treated with risankizumab. Clin Cosmet Investig Dermatol. 2023;16:3503–3507.
