Abstract
Background
To evaluate the risk of neutropenia during treatment with anti-IL-23 antibodies in patients with psoriasis.
Method
We conducted an observational study with cohort design using MID-NET® in Japan. We identified patients with psoriasis who were newly prescribed anti-IL-23 antibodies, anti-IL-17-antibodies, adalimumab, or apremilast between January 1, 2009, and March 31, 2021. We estimated the adjusted hazard ratio (aHR) of anti-IL-23 antibodies compared to that of anti-IL-17 antibodies, adalimumab, or apremilast, for the risk of grade 2 (neutrophil count < 1,500/μL) or grade 3 (neutrophil count < 1,000/μL) neutropenia.
Results
Overall, 287 patients on anti-IL-23 antibodies, 189 patients on anti-IL-17 antibodies, 293 patients on adalimumab, and 540 patients on apremilast were included. Compared with anti-IL-17 antibodies, the aHR (95% confidence interval (CI)) of anti-IL-23 antibodies was 0.83 (0.27–2.51) for grade 2 and 0.40 (0.02–7.60) for grade 3 neutropenia; that when compared with adalimumab was 0.76 (0.28–2.06) for grade 2 but was not calculated for grade 3 as no cases were found; and that compared with apremilast was 3.88 (0.62–24.48) for grade 2 and 0.43 (0.02–11.63) for grade 3 neutropenia.
Conclusion
No clear increase in the risk of neutropenia with anti-IL-23 antibodies was observed.
Introduction
Interleukin-17 (IL-17) and interleukin-23 (IL-23) are effective therapeutic targets for inflammatory skin diseases (Citation1), and various monoclonal antibody preparations have been developed and widely used for treating psoriasis (Citation2). Neutropenia is one of adverse reactions caused by the administration of anti-interleukin-17 antibody preparations (anti-IL-17 antibodies) (Citation3–7). Although the mechanism underlying the neutropenia is unclear, IL-17 is known to contribute to neutrophil recruitment through neutrophil-specific chemokine release (Citation8), and inhibiting IL-17 is suspected to cause a decrease in neutrophil counts (Citation9–11). On the other hand, neutropenia risks by anti-IL-23 antibody preparations (anti-IL-23 antibodies) are still unclear. Since IL-23 plays an important role in the proliferation and maintenance of IL-17-producing T cells (Citation12), anti-IL-23 antibodies could induce neutropenia via reduction of IL-17 production. Further investigations are necessary about the risk of neutropenia by anti-IL-23 antibodies because limited clinical trial data are only available, indicating no clear conclusions due to inconsistent results among studies (Citation4,Citation13–15).
In Japan, a decreased neutrophil count is described as a ‘Clinically Significant Adverse Reaction’ (CSAR) in the package insert (PI) and as an important identified risk in the Risk Management Plan (RMP) for anti-IL-17 antibodies such as brodalumab, secukinumab and ixekizumab. In contrast, a decreased neutrophil count is not mentioned in the CSAR of the PI for anti-IL-23 antibodies such as risankizumab, ustekinumab, and guselkumab, though it is listed as an important potential risk in the RMP for these drugs. Therefore, further study investigating the neutropenia risk by anti-IL-23 antibodies under real-world setting would be useful for appropriate drug safety assessment at post-marketing stage which may be different situation from clinical trials.
Consequently, the Pharmaceuticals and Medical Devices Agency (PMDA) conducted a pharmacoepidemiological study about the risk of neutropenia during treatment with anti-IL-23 antibodies in patients with psoriasis.
Materials and methods
Database
Real-world data from MID-NET®, a reliable and valuable database in Japan (Citation16,Citation17), were used for analysis in this study because MID-NET® stores electronic medical records, administrative claim data, and diagnosis procedure combination (DPC) data of over 8 million patients (as of December 2023) in cooperation with 10 healthcare organizations, including 33 university hospitals and regional core hospitals. The laboratory test results necessary for evaluating neutropenia, such as white blood cell counts and percentage of neutrophil sequestration, are available for analysis from MID-NET®. The study period spanned from January 1, 2009 to March 31, 2021.
Utilization of MID-NET® for this study was approved on December 25, 2020, through a discussion by the expert committee of MID-NET® (Citation18). The actual data extraction from MID-NET® for analysis was carried out between April 28, 2021 and May 10, 2021. The anonymized dataset was only provided to the authors from the MID-NET® management office.
Cohort and study design
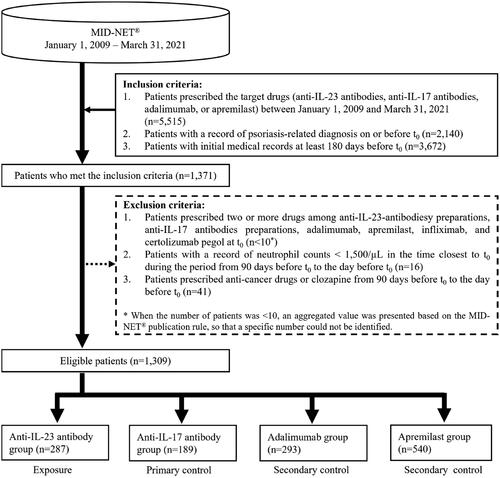
In this study, a new user cohort design was selected to consider the different risks of neutropenia among new and prevalent users. Patients who met all of the following conditions were included in the study (see Supplementary Figure S1 for more details of the study design): (1) patients prescribed the target drugs (anti-IL-23 antibodies, anti-IL-17 antibodies, adalimumab, or apremilast) during the study period (see ‘Definition of exposure and control’ below for the reasons for selecting adalimumab and apremilast, and Supplementary Table S1 for the entire list of the target drugs considered in this study), (2) patients with a record of psoriasis-related diagnosis on or before t0 (the earliest prescription date of any of the target drugs), and (3) patients with initial medical records at least 180 days before t0.
For the analysis, patients who met one or more of the following criteria were excluded: (1) patients prescribed two or more drugs among anti-IL-23 antibodies, anti-IL-17 antibodies, adalimumab, apremilast, infliximab, and certolizumab pegol at t0. Infliximab and certolizumab pegol, which were biologics other than the target drugs available for treating psoriasis in Japan at the time of study planning, were also included in this exclusion criterion because of potential effects on the risk of neutropenia (Citation19–21), (2) patients with a record of neutrophil count < 1,500/µL at the time closest to t0 during the period from 90 days before t0 to the day before t0, and (3) patients prescribed anti-cancer drugs or clozapine from 90 days before t0 to the day before t0.
Definition of follow-up and prescription periods
The follow-up period started at t0 and ended at the earliest date among the following: (1) the onset date of the outcome, (2) the end date of the prescription period, (3) the start date of another different prescription of psoriasis drugs from t0 among anti-IL-23-antibodies, anti-IL-17 antibodies, adalimumab, apremilast, infliximab, and certolizumab pegol, (4) the start date of the prescription of anti-cancer drugs or clozapine, (5) the date of the last medical record for a patient, and (6) the end date of the study period (March 31, 2021).
The prescription period encompassed the start date (t0) and the duration of prescription with a gap and grace periods, considering the prescription interval for each target drug (anti-IL-23 antibodies, anti-IL-17 antibodies, adalimumab, or apremilast) and deviation from the scheduled visit time (See Supplementary Table S2 for more details on the prescriptions, gaps, and grace periods of each drug).
Definition of exposure and control
The categorization of the exposure groups for the analysis was based on the drug at first prescription. The anti-IL-23 antibody group included risankizumab, ustekinumab, and guselkumab, whereas the anti-IL-17 antibody group (primary control group) included brodalumab, secukinumab, and ixekizumab. Adalimumab, a tumor necrosis factor-α (TNF-α) inhibitor, was set as a secondary control group (likely as a positive control), because it is widely used for treating psoriasis in Japan, has hematological side effects including cytopenia (Citation21–23), and has a similar clinical positioning (e.g., a subcutaneously injectable formulation) as that of anti-IL-17 and anti-IL-23 antibodies. Additionally, apremilast, which regulates the expression of inflammatory cytokines, including IL-17, by inhibiting phosphodiesterase-4 (Citation24), was set as another secondary control group for supplementary evaluation because no warning on neutropenia was included in the PI of this drug (Citation25) and no safety concerns regarding neutropenia were observed in the clinical trials of this drug submitted for new drug application (Citation26). It should be noted that risk of neutropenia by apremilast is currently unestablished, and its clinical positioning differs from that of other biological drugs.
Definition of outcome
Neutropenia risk was classified into two different severity grades based on the criteria of neutrophil count decrease in the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 (Citation27). Grade 2 and 3 neutropenia were defined as neutrophil counts < 1,500/μL and < 1,000/μL, respectively. The neutrophil count was calculated using the following formula: neutrophil count = white blood cell count × percentage of neutrophil sequestration. If only the data of the fraction for the neutrophil rod and segmented nuclei were available, the percentage of neutrophil sequestration was calculated as the sum of those fractions.
Neutropenia cases were identified when neutrophil count in a patient firstly met the definition of outcome (as described above) during the follow up period.
Definition of covariates
The covariates used in this study included the following factors: (1) sex (male/female) and age (age < 65 years, 65 years and older) at t0, (2) types of psoriasis based on the diagnosis name before t0, (3) use of TNF-α inhibitor (infliximab or certolizumab pegol): from 180 days to 1 day before t0, (4) concomitant drugs (from 90 days to 1 day before t0 except for antiviral drugs: from 30 days to 1 day before t0), (5) comorbidity (from 180 days to 1 day before t0), (6) baseline neutrophil counts (from 90 days to 1 day before t0) (see Supplementary Table S3 for the list of covariates considered in this study).
Statistical analysis
Patient background data, including covariates, such as concomitant drugs and comorbidities during the baseline period, were tabulated. The crude hazard ratio (cHR) and adjusted hazard ratio (aHR) (adjusted for the covariates described above) of the anti-IL-23 antibody group to the controls were estimated using the Cox proportional hazards model. The aHR was weighted by the inverse of the propensity score, which was estimated using a logistic regression model that included the covariates described above (see Supplementary Table S3 for the list of covariates considered in this study).
Further, sensitivity analyses were conducted based on the following points: (1) the study population was limited to patients with normal neutrophil counts (2,000/μL to 7,500/μL) during the period of 90 days before t0 to the day before t0 (Citation28), (2) the gap and grace periods were double the original periods in the primary analysis, (3) infliximab, certolizumab pegol, anti-cancer drugs, and clozapine were excluded from the list of drugs considered for terminating the follow-up period.
Results
Cohort and patient background
In all, 5,515 patients were determined to be prescribed anti-IL-23 antibodies, anti-IL-17 antibodies, adalimumab, or apremilast during the study period. Of these, 1,309 were included in the analysis after applying all the inclusion and exclusion criteria (), including 287 patients in the anti-IL-23 antibody group, 189 in the anti-IL-17 antibody group, 293 in the adalimumab group, and 540 in the apremilast group.
shows the patient background of each group before weighting by propensity score. The cohort mainly included patients with mean ages of 51–59 years and slightly more males (58–64%). Neutrophil counts at the baseline in many patients (> 76%) were normal (≥ 2,000/μL); however, the neutrophil counts for more than half of patients in the apremilast group were unknown. Many patients in the cohort were diagnosed with psoriasis vulgaris, followed by psoriasis arthropathica and generalized pustular psoriasis, and were co-prescribed corticosteroids. Patients prescribed high-risk and very-high-risk drugs for developing neutropenia constituted approximately 21–45% and 3–14%, respectively. Although some differences were observed before weighting, the absolute standardized mean differences after weighting were 0.20 or less for all patient background factors between the anti-IL-23 antibody group and the control (anti-IL-17 antibody group or adalimumab group) or between the anti-IL-23 antibody group and the reference (apremilast group) (see Supplementary Tables S4–S6 for more details of patient background between groups before and after propensity score weighting).
Table 1. Summary of patient background at the baseline.
The median (1st and 3rd interquartile ranges) follow-up durations was 496 (196, 1098) days for the anti-IL-23 antibody group, 367 (127, 840) days for the anti-IL-17 antibody group, 280 (99, 836) days for the adalimumab group, and 154.5 (68, 386) days for the apremilast group. Regarding the frequency of testing neutrophil counts during the follow-up period in the cohort, the test rate per 100 patient-days was 1.31 for the anti-IL-23 antibody group, 1.46 for the anti-IL-17 antibody group, 1.73 for the adalimumab group, and 0.96 for the apremilast group.
Comparison of the risk of neutropenia
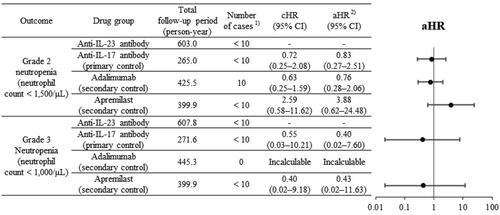
As shown in , in comparing the risk of neutropenia in the anti-IL-23 group with that in the anti-IL-17 group, the aHR (95% confidence interval (CI)) was 0.83 (0.27–2.51) for grade 2 neutropenia and 0.40 (0.02–7.60) for grade 3 neutropenia. Compared with adalimumab, the aHR (95% CI) was 0.76 (0.28–2.06) for grade 2 neutropenia; however, the aHR for grade 3 neutropenia could not be estimated because of no cases of grade 3 neutropenia were found in the adalimumab group. Compared with apremilast, the aHR (95% CI) was 3.88 (0.62–24.48) for grade 2 neutropenia and 0.43 (0.02–11.63) for grade 3 neutropenia, showing different trends.
Figure 2. Comparison of neutropenia in anti-IL-23 antibody group with anti-IL-17 antibody, adalimumab, and apremilast groups.
CI: confidence interval, cHR: crude hazard ratio, aHR: adjusted hazard ratio, –: not applicable.
1: When the number of patients was <10, an aggregated value was presented based on the MID-NET® publication rule, so that a specific number could not be identified.
2: aHR, weighted by the inverse of the propensity score, which was estimated using a logistic regression model including covariates (see Supplementary Table S3 for the list of covariates considered in this study).
presents the results of sensitivity analyses. When comparing the risk of neutropenia between the anti-IL-23 antibody group and the anti-IL-17 antibody or adalimumab groups, the aHR was consistently lower than 1.0. For example, compared with the anti-IL-17 antibody group, the aHR (95% CI) for grade 2 neutropenia was 0.41 (0.09–1.84) for (1) limiting the study population to patients with normal neutrophil counts, 0.86 (0.31–2.39) for (2) doubling the periods of the gap and grace, and 0.83 (0.27–2.52) for (3) excluding infliximab, certolizumab pegol, anti-cancer drugs, and clozapine from the list of drugs considered for terminating the follow-up period. Notably, the aHR for grade 3 neutropenia compared with adalimumab could not be estimated because there were no such cases in the adalimumab group. In the comparison between anti-IL-23 antibody and apremilast, results consistent with the primary analysis were observed in sensitivity analyses (1)–(3) for grade 3 neutropenia (lower aHR (<1.0)) as well as in sensitivity analyses (2) and (3) for grade 2 neutropenia (higher aHR (>1.0)). However, in sensitivity analysis (1) for grade 2 neutropenia, a result inconsistent with the primary analysis, showing lower aHR (0.48, 95% CI: 0.03–6.67) was observed.
Table 2. Summary of results from the sensitivity analysis (anti-IL-23 antibody vs. anti-IL-17 antibody, adalimumab, or apremilast).
Discussion
The present study evaluated the risk of neutropenia from anti-IL-23 antibodies compared with that from anti-IL-17 antibodies, adalimumab, or apremilast in patients with psoriasis. In the previous studies on the neutropenia risk of anti-IL-23 antibodies, no clear conclusions have been reported owing to inconsistent results in clinical trials but not observational studies (Citation4,Citation13–15). Therefore, this study provides useful real-world evidence of the risk of neutropenia with anti-IL-23 antibodies. The median follow-up period of all groups in this study was more than 150 days, which would be sufficient to detect neutropenia because an increased risk of neutropenia by anti-IL-17 antibodies has been reported in a clinical trial spanning 12 weeks (Citation4,Citation29). The neutropenia cases observed in this study were mainly of grade 2 neutropenia, similar to the results of clinical trials.
The aHRs of anti-IL-23 antibodies to anti-IL-17 antibodies, adalimumab, and apremilast were consistently less than 1.0 for grade 2 and 3 neutropenia, except in the comparison with adalimumab for grade 3 neutropenia, which could not be calculated as no cases were found in the adalimumab group, and in the comparison with apremilast for grade 2 neutropenia, showing aHR higher than 1.0 but inconsistent with the results of the sensitivity analysis for (1) limiting the study population to patients with normal neutrophil counts. These results indicate that there is no clear increased risk of neutropenia from anti-IL-23 antibodies and support the current precaution of describing no neutropenia risk in the CSAR of the PI for anti-IL-23 antibodies. However, the risk of neutropenia from anti-IL-23 antibodies remains controversial because the 95% CIs in this study were relatively large and the inconsistent results were obtained in randomized clinical trials, suggesting a higher neutropenia risk with ustekinumab and guselkumab than that with placebo (Citation4,Citation14) as well as those of no differences compared to the placebo (Citation13,Citation15). The higher aHR in comparison with apremilast for grade 2 neutropenia also suggests the possibility of neutropenia caused by anti-IL-23 antibodies, although it would be difficult to conclude this hypothesis based only on this result because of the inconsistent results observed between primary and sensitivity analyses, as well as the different clinical positioning of anti-IL-23 antibodies from that of apremilast.
Notably, in this analysis, the anti-IL-23 group comprised risankizumab, ustekinumab, and guselkumab while the anti-IL-17 group comprised brodalumab, secukinumab, and ixekizumab, assuming no major differences in the degree of neutropenia risk among the antibodies in a group theoretically based on their pharmacological action. This was because individually examining the risk of each antibody in this study was difficult owing to the limited sample size and lower incidence of neutropenia. Thus, potential differences in neutropenia risk for each anti-IL-23 antibody should be considered when interpreting the study results. As discussed above, further studies with larger sample sizes will be beneficial to clarify the risk of neutropenia posed by each anti-IL-23 antibody in real-world clinical practice.
The strength of this study was the utilization of the laboratory test result of neutrophil counts as an objective parameter to determine the occurrence of neutropenia based on the MID-NET® (Citation16). However, as a limitation, the results may have been affected by other potential confounders, such as the patient’s general condition, prior treatments for psoriasis, other concomitant drugs, and complications that were not considered in this study. In addition, our study had a limited sample size, which led to relatively large confidence intervals and may have resulted in insufficient power to identify group differences. Therefore, these results should be interpreted with caution. The PMDA conducted a safety assessment of the risk of neutropenia with anti-IL-23 antibodies based on all available information including this study results, case reports and related literature, and concluded that no additional safety measures were necessary at present.
Conclusion
Overall, no clear increased risk of neutropenia from anti-IL-23 antibodies was observed compared with that from anti-IL-17 antibody, adalimumab, or apremilast based on real world data from MID-NET®.
Ethics approval and consent to participate
As this study was conducted as an official activity of the PMDA under the Pharmaceuticals and Medical Devices Agency Law (Articles 15–5–(c) and (f)), it was not subject to a review through an institutional review board (Citation30, Citation31) and the requirement for informed consent was waived. However, the opportunity of a patient to opt out from the MID-NET® was ensured by all MID-NET® cooperative hospitals.
Authors’ contributions
All authors designed the study. YO and KK performed the research and analyzed the data. All the authors wrote the manuscript.
Supplementary file_final240606.docx
Download MS Word (503.9 KB)Acknowledgments
The authors thank all project members of MID-NET® for their continuous efforts to maintain its reliability.
Disclosure statement
All authors declared no competing interests for this work.
Data availability statement
The dataset generated in the study is not publicly available due to the terms of use for MID-NET® to which we adhered when conducting this study; the accessibility of the dataset used for this analysis is restricted to specific authors including the corresponding author in a predetermined secure environment. No outside researchers are allowed to access the dataset.
Additional information
Funding
References
- Ghoreschi K, Balato A, Enerbäck C, et al. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397(10275):1–8. doi: 10.1016/S0140-6736(21)00184-7.
- Jeon C, Sekhon S, Yan D, et al. Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis. Hum Vaccin Immunother. 2017;13(10):2247–2259. doi: 10.1080/21645515.2017.1356498.
- Eshwar V, Kamath A, Shastry R, et al. A review of the safety of interleukin-17A inhibitor secukinumab. Pharmaceuticals (Basel). 2022;15(11):1365. doi: 10.3390/ph15111365.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824.
- Mease P, Roussou E, Burmester GR, et al. Safety of ixekizumab in patients with psoriatic arthritis: results from a pooled analysis of three clinical trials. Arthritis Care Res (Hoboken). 2019;71(3):367–378. doi: 10.1002/acr.23738.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. doi: 10.1056/NEJMoa1109017.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(1):83–98.e4. doi: 10.1016/j.jaad.2016.03.024.
- Witowski J, Pawlaczyk K, Breborowicz A, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000;165(10):5814–5821. doi: 10.4049/jimmunol.165.10.5814.
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246.
- Stark MA, Huo Y, Burcin TL, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–294. doi: 10.1016/j.immuni.2005.01.011.
- Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25(1):821–852. doi: 10.1146/annurev.immunol.25.022106.141557.
- Smith E, Zarbock A, Stark MA, et al. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol. 2007;179(12):8274–8279. doi: 10.4049/jimmunol.179.12.8274.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. doi: 10.1016/j.jaad.2016.11.041.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391(10136):2213–2224. doi: 10.1016/S0140-6736(18)30952-8.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4.
- Yamaguchi M, Inomata S, Harada S, et al. Establishment of the MID-NET® medical information database network as a reliable and valuable database for drug safety assessments in Japan. Pharmacoepidemiol Drug Saf. 2019;28(10):1395–1404. doi: 10.1002/pds.4879.
- Yamada K, Itoh M, Fujimura Y, et al. The utilization and challenges of Japan’s MID-NET® medical information database network in post-marketing drug safety assessments: a summary of pilot pharmacoepidemiological studies. Pharmacoepidemiol Drug Saf. 2019;28(5):601–608. doi: 10.1002/pds.4777.
- Pharmaceuticals and Medical Devices Agency. Information for studies approved through a discussion by the expert committee of MID-NET®; 2024. [in Japanese]. https://www.pmda.go.jp/safety/mid-net/0010.html.
- Andrès E, Villalba NL, Zulfiqar AA, et al. State of art of idiosyncratic drug-induced neutropenia or agranulocytosis, with a focus on biotherapies. J Clin Med. 2019;8(9):1351. doi: 10.3390/jcm8091351.
- Andrès EVN, Zulfiqar A-A, Maouche Y, et al. TNF-alpha inhibitors and neutropenia: current state of art. J Cell Immunol. 2020;2(4):157–164.
- Dogra S, Khullar G. Tumor necrosis factor-α antagonists: side effects and their management. Indian J Dermatol Venereol Leprol. 2013;79 Suppl 7(7):S35–S46. doi: 10.4103/0378-6323.115526.
- Bessissow T, Renard M, Hoffman I, et al. Review article: non-malignant haematological complications of anti-tumour necrosis factor alpha therapy. Aliment Pharmacol Ther. 2012;36(4):312–323. doi: 10.1111/j.1365-2036.2012.05189.x.
- The package insert of Humira® Syringe and Humira® Pen. 2024. [in Japanese]. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/112130_3999426G3027_1_10.
- Carrascosa JM, Del-Alcazar E. Apremilast for psoriasis treatment. G Ital Dermatol Venereol. 2020;155(4):421–433. doi: 10.23736/S0392-0488.20.06684-5.
- The package insert of Otezla® Tablets. 2024. [in Japanese]. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/112292_3999042F1025_3_03
- Pharmaceuticals and Medical Devices Agency. The review report of Otezla® tablets. 2024. [in Japanese]. https://www.pmda.go.jp/drugs/2016/P20161220001/380809000_22800AMX00729000_A100_1.pdf.
- National Cancer Institute. Common terminology criteria for adverse events (CTCAE) Version 5.0. 2024. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- Japan Clinical Oncology Group. JCOG common reference intervals. 2024. [in Japanese]. https://jcog.jp/assets/JCOG_kyouyoukijunchi_50_20201221.pdf.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356. doi: 10.1056/NEJMoa1512711.
- Pharmaceuticals and Medical Devices Agency. Act on the pharmaceuticals and medical devices agency (act no.192 of 2002). [in Japanese]. https://elaws.e-gov.go.jp/document?lawid=414AC0000000192.
- Pharmaceuticals and Medical Devices Agency. Pharmacoepidemiological studies for drug safety assessment under MIHARI framework. 2024. [in Japanese]. https://www.pmda.go.jp/safety/surveillance-analysis/0045.html.