Abstract
Purpose
We aim to explore a potential treatment strategy for hair loss.
Materials and methods
A male 6-year-old child was diagnosed with hidrotic ectodermal dysplasia 2 (HED2) caused by GJB6 (p.G11R) mutations. He presented at our clinic with diffuse thinning and fine and brittle hair since birth. Additionally, the child exhibited abnormal development of teeth, fingernails, and toenails. The condition of the child’s hair had not improved significantly with age. He was treated with botanical extracts combined with Minoxidil.
Results
After one and a half months of treatment, the patient showed remarkable hair growth.
Conclusions
Our team has previously used botanical extracts in combination for the treatment of autosomal recessive wooly hair in children. In the present case, treatment with botanical extract combined with minoxidil was found to be equally efficacious. This case report provides valuable information for future studies on the use of botanical extracts in treating hair loss, as well as a safe and effective potential treatment strategy for children with congenital alopecia.
1. Introduction
Hidrotic ectodermal dysplasia 2 (HED2), also known as Clouston Syndrome, is an autosomal dominant disorder caused by a missense mutation in the gap junction beta 6 (GJB6) gene. Penetrance is close to 100%, with a prevalence of 1/100,000–9/100,000. The primary characteristics include discolored, short, thick, slow-growing, stunted or, in some cases, missing fingernails. Hair is fine and brittle, with localized hair loss or even absence. Usually, eyelashes, eyebrows, and armpit hair are also sparse or absent. Patients may also exhibit abnormal sweating, clubbed fingers, hyperkeratosis of the palms, and skin pigmentation, or they may have sensorineural deafness, cataracts, and abnormal mental development (Citation1–4).
To date, there is no available cure for HED2, and only symptomatic interventions are available. For palmoplantar keratosis, emollients, keratolytic drugs, and artificial nails are often used. It is important to avoid adverse effects and prevent infections (Citation5). Dental defects affecting chewing can be addressed with dentures (Citation6). Those with sparse or missing hair can consider using minoxidil for hereditary hair shaft disorders. According to a report, in four patients with monilethrix, a one-year treatment regimen with 2% minoxidil resulted in a significant increase in hair growth at 6 and 12 months without any adverse effects (Citation7). Other interventions, such as low-energy laser, fractional laser, microneedling, platelet-rich plasma injections, and many other adjunctive therapies, can also be used as traditional hair loss treatment strategies.
The use of botanicals in traditional medicine is effective in treating hair loss, with minimal side effects. A bibliometric study on botanicals for androgenetic alopecia identified three main mechanisms of action, namely, inhibition of 5-alpha-reductase, stimulation of hair growth, and inhibition of inflammation (Citation8). Although the application of botanical agents in congenital alopecia in children has yet to be investigated, the use of botanical extracts has certain advantages over synthetic drugs that are invasive or associated with extreme adverse effects (Citation9). Furthermore, some clinical studies have shown that botanical solutions combined with different concentrations of minoxidil are safe and effective in the treatment of female-pattern hair loss and male androgenetic alopecia (Citation10,Citation11). Our team has used botanical extracts in combination with minoxidil to treat pediatric HED2 patients with good efficacy, as reported below.
2. Case
On 5 July 2022, a 6-year-old male child presented at our clinic with diffuse thinning and fine and brittle hair since birth. The condition of the child’s hair had not improved significantly with age. Additionally, the child exhibited abnormal development of teeth, fingernails, and toenails. The main cause of concern was the abnormal development of temporal and occipital alopecia. The individual’s parents were not related, and while his father was in good health, his mother and maternal grandmother shared the same hair and nail phenotype as the patient. Nevertheless, they did not exhibit any significant dental abnormalities.
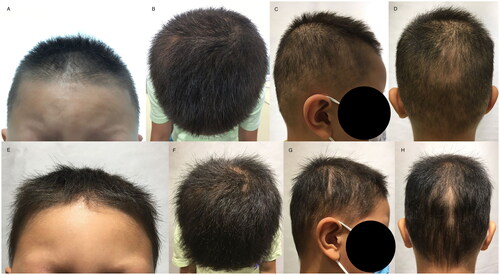
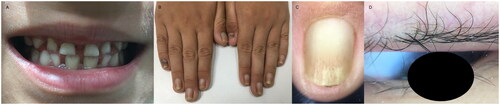
Physical examination of the patient revealed normal growth and development for their age. No ear deformities or hearing loss were detected, and their eyes and sweat secretion were normal. The palmoplantar skin showed no obvious abnormalities. Dermatological examination indicated that the patient had sparse and light brown eyebrows, diffuse thinning of hair, negative hair pull test, and nails that were brittle, thick, and black with heavy longitudinal lines on the surface. Additionally, the patient had poorly developed teeth ( and ).
Figure 2. The patient’s milk teeth were hypoplastic and trapezoidal in shape (A); the patient’s nails were brittle, thick, and black, with heavy, yellowish longitudinal lines on the nail surface (B,C); and the patient’s eyelashes were seen to be desquamated at the roots, with dilated capillaries in a branch-like pattern (D).
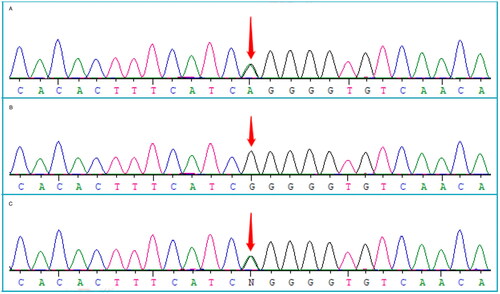
Trichoscopy revealed redness and flaking of the scalp skin, visible white spots, follicular orifice plugs, and a brown halo around the follicles. The majority of hairs were intermediate and had fine hair shafts. The hair loss site exhibited broken hairs, grey or white hairs, and vellus hairs. The scalp capillary morphology showed ring-shaped changes (). The skin at the root of the eyelashes was flaky, and the capillary expansion exhibited a branch-like pattern (). The patient was clinically diagnosed with HED2 and found to have a heterozygous mutation of c.31G > A (p.G11R) in the GJB6 gene. The patient’s mother also carried a heterozygous mutation of c.31G > A (p.G11R) in the GJB6 gene, while his father was wild-type (). These findings suggest a possible genetic cause for the patient’s condition.
Figure 3. Pretreatment trichoscopy findings (A–D). (A): frontal angle: obvious redness of the scalp with dandruff, fine and soft vellus hair, sparse distribution; (B): top of the head: marked redness of the scalp with flaking, heterogeneity of the hair shaft diameter >20%, dilated branching-like pattern of capillaries in the red circle, brown halo around the hair follicle in the blue circle, angular plugs at the follicle opening in the green circle; (C): hair spinning: flaking and white spot signs are visible on the scalp and hair distribution is sparse; green arrows indicate grey intermediate hair; (D): occipital: obvious broken hairs, short vellus hairs and white hairs can be seen, and the hairs are sparse; the blue arrow points to broken hairs, while the red arrow points to short vellus hairs. Trichoscopy findings one and a half months (E–H). (E): Frontal angle: redness of the scalp has subsided compared with before, flaking is slightly reduced, with more vellus hair than before; (F): Top of the head: reddened areas of the scalp are less red than before, flaking is reduced, and the density of the terminal hairs and other hairs has increased; (G): Hair spinning: terminal hair and hair density has increased, with more vellus hair observed; (H): Occipital: hair density has increased, the proportion of broken hair has decreased, and a large number of vellus hairs can be seen.
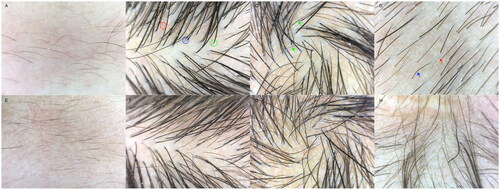
Figure 4. Patient with a heterozygous mutation c.31G > A (p.G11R) in the GJB6 gene (A); his father is wild-type (B); his mother carries a heterozygous mutation c.31G > A (p.G11R) in the GJB6 gene (C).
For treatment, the patient was given 2 tablets of compound glycyrrhizin (Mennen) orally twice a day, and 5% Minoxidil tincture (Mandy) 6:1 mixed with Hasinide solution was evenly applied to the area of the scalp exhibiting hair loss twice a day.
3. Result
On 23 August 2022, the patient revisited the clinic with positive hair growth and a reduction in local hair loss areas (). Trichoscopy revealed an increase in frontal angle vellus hair; a decrease in flaking; a reduction in the redness of the scalp at the crown, crown rotation, and occipital area; and an increase in hair density, with more terminal hairs. In areas of local hair loss, hair density had increased while hair breakage had decreased. Additionally, there was an increase in grey hairs and a decrease in the proportion of vellus hair ().
4. Discussion
This article reports a rare case of HED2 in a child with a heterozygous mutation c.31G > A (p.G11R) in the GJB6 gene. It includes the clinical presentation, results of familial genetic testing, and effective treatment options.
The GJB6 gene encodes connexin 30, a crucial member of the connexin family that is highly expressed in epithelial cells—particularly in hair follicles, nail beds, and mesenchymal structures of the inner ear (Citation12). Among the GJB6 missense mutations, the p.G11R mutation is the most commonly causatively associated with HED2 (Citation13). The patient in this case report also harbored this causative mutation. Interestingly, abnormal dental development was observed in the patient, although his mother’s teeth appeared normal. This manifestation has been reported in other studies (Citation14). Additionally, a previous report described an adult female patient with HED2 who had a missing incisor, although the rest of her permanent teeth were normal. Mutations in GJB6 may have a more significant impact on the development of deciduous dentition than on permanent teeth (Citation15).
The clinical phenotype of HED varies. Diagnosis requires a combination of assessment of clinical manifestations and genetic testing. In this case, abnormalities of hair, teeth, and fingernails are the main symptoms. Thinning and discoloration of hair can be easily confused with hereditary hypertrichosis. Therefore, a careful differential diagnosis is necessary. Hereditary hypertrichosis encompasses several conditions, including simple hereditary hypertrichosis, autosomal dominant hereditary wooly hair, autosomal recessive hereditary wooly hair, and monilethrix. The causative genes for these conditions include LIPH, LPAR6, KRT25, and others. The clinical manifestation of hereditary hypertrichosis is sparse or curly hair at birth or soon after, with slow or no hair growth after shaving. This may be accompanied by sparse or missing hair throughout the body, as well as hypopigmentation and follicular keratotic papules. However, patients typically have normal toenails, teeth, and hearing (Citation16,Citation17).
Previously, our team has treated a child with autosomal recessive hereditary wooly hair using a combination of botanical extracts, namely, compound glycyrrhizin tablets and total glucosides of peony capsules, for internal use, and hair growth tincture for topical use. The patient’s hair growth was significantly improved after 6 months of treatment. At the 4-year follow-up, the density of hair was maintained, and the liver and kidney function tests were normal, indicating a safe and satisfactory therapeutic effect (Citation18). In the present case report, to address the patient’s symptoms of hair thinning and hair loss, we administered a combination of the botanical extract compound glycyrrhizin tablets for oral use and external application of 5% minoxidil for one and a half months. This treatment yielded positive results, and the patient’s parents confirmed the sustained therapeutic effect via telephone follow-up one year later.
The main ingredient of the compound glycyrrhizin tablets is glycyrrhizin, extracted from the traditional Chinese medicine (TCM) licorice. Licorice is a traditional medicine with a long history of use dating back to ancient Egyptian, European, Indian, and Assyrian civilizations, as well as in China (Citation19). According to the Pharmacopeia of the People’s Republic of China (2020 edition), licorice has the following effects: tonifying the spleen and qi, clearing heat and removing toxins, expectorating phlegm and relieving cough, relieving pain, and moderating the nature of other herbs, among others (Citation20). Modern pharmacological studies have confirmed its anti-inflammatory, antioxidant, and immunomodulatory effects (Citation21). In addition, our team’s study showed that the administration of compound glycyrrhizin alone for the treatment of severe alopecia areata in children had higher clinical efficacy than that observed in the control group, and no adverse events occurred during 12 consecutive months of treatment (Citation22). This reflects the feasibility of licorice in the treatment of hair loss, although the exact therapeutic mechanism remains to be investigated. It has been speculated that hair follicles are susceptible to oxidative stress, which may explain why botanical extracts with antioxidant effects ameliorate hair loss (Citation23). In particular, licorice inhibits the JAK pathway, which is involved in the regulation of alopecia areata (Citation24). Furthermore, there is a case report of a patient with hypohidrotic ectodermal dysplasia and severe atopic dermatitis who showed continuous improvement of skin symptoms and the appearance of vellus hairs on the forehead within 6 months of administration of a JAK inhibitor, without adverse events (Citation25). This suggests that the therapeutic action of oral compound glycyrrhizin tablets against hair loss, observed in the present case, may be closely related to the inhibition of the JAK pathway. The exact mechanism by which Minoxidil, a piperidine derivative, promotes hair growth is unclear at this time. Some experiments have shown that it can play a key role in early-stage cell proliferation by increasing cellular DNA synthesis and enhancing cell proliferation (Citation26). It has also been suggested that the mechanism of action of Minoxidil may involve enhancing blood supply to the hair follicle (Citation27). To alleviate skin irritation caused by propylene glycol in the Minoxidil excipient, a solution of Hasinide mixed in Minoxidil at a ratio of 1:6 is used, owing to its anti-inflammatory, antipruritic and vasoconstrictive properties. However, it is not recommended for long-term use.
A case report has indicated that prenatal ultrasonography may offer useful indications of ectodermal dysplasia (Citation28). This highlights the importance of sufficient prenatal screening and genetic testing in families with a history of the condition. Furthermore, alopecia, being a disfiguring skin disease, can significantly affect the mental health of patients. Studies have shown that patients with alopecia areata have a higher prevalence of depression and unspecified anxiety disorders relative to the general population. Additionally, there is a bi-directional correlation between major depression and baldness (Citation29,Citation30). Therefore, it is important to implement early and positive interventions to address the hair problems experienced by HED2 patients, in order to safeguard their psychological well-being as well as that of their parents, when the affected individuals are children.
This case report indicates that botanical extracts, when used in combination with minoxidil, may promote hair growth in patients with HED2. Furthermore, based on previous cases treated by our team, no significant adverse effects were observed. However, there are some limitations in this study. In case of hair fragility, a trichogram can be used to assess the hair shaft at the microscopic level of the hair and aid in diagnosis. However, because this involves the removal of multiple hairs, it is not easy to obtain consent from the child with alopecia and his parents (Citation31). Therefore, only trichoscopy and genetic testing were performed as a basis for diagnosis in the case report. In addition, more evidence is necessary to clarify the mechanism of action of the licorice extract used in this paper for the treatment of hair loss. Overall, this case report provides valuable information for future studies on the use of botanical extracts in treating hair loss, as well as a safe and effective potential treatment strategy for children with congenital alopecia.
Informed consent
The patient’s family has signed informed consent.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hassed SJ, Kincannon JM, Arnold GL. Clouston syndrome: an ectodermal dysplasia without significant dental findings. Am J Med Genet. 1996;61(3):274–276. doi: 10.1002/(SICI)1096-8628(19960122)61:3<274::AID-AJMG13>3.0.CO;2-Q.
- Stevens HP, Choon SE, Hennies HC, et al. Evidence for a single genetic locus in Clouston’s hidrotic ectodermal dysplasia. Br J Dermatol. 1999;140(5):963–964. doi: 10.1046/j.1365-2133.1999.02837.x.
- Cammarata-Scalisi F, Rinelli M, Pisaneschi E, et al. Novel clinical features associated with Clouston syndrome. Int J Dermatol. 2019;58(8):e143–e146. doi: 10.1111/ijd.14507.
- Kibar Z, Der Kaloustian VM, Brais B, et al. The gene responsible for Clouston hidrotic ectodermal dysplasia maps to the pericentromeric region of chromosome 13q. Hum Mol Genet. 1996;5(4):543–547. doi: 10.1093/hmg/5.4.543.
- Avshalumova L, Fabrikant J, Koriakos A. Overview of skin diseases linked to connexin gene mutations. Int J Dermatol. 2014;53(2):192–205. doi: 10.1111/ijd.12062.
- Montanari M, Grande F, Lepidi L, et al. Rehabilitation with implant-supported overdentures in preteens patients with ectodermal dysplasia: a cohort study. Clin Implant Dent Relat Res. 2023;25(6):1187–1196. doi: 10.1111/cid.13258.
- Rossi A, Iorio A, Scali E, et al. Monilethrix treated with minoxidil. Int J Immunopathol Pharmacol. 2011;24(1):239–242. doi: 10.1177/039463201102400129.
- Rondanelli M, Perna S, Peroni G, et al. A bibliometric study of scientific literature in Scopus on botanicals for treatment of androgenetic alopecia. J Cosmet Dermatol. 2016;15(2):120–130. doi: 10.1111/jocd.12198.
- Youssef A, Al-Mahdy DA, Sayed RH, et al. A comprehensive review of natural alternatives for treatment of alopecia with an overview of market products. J Med Food. 2022;25(9):869–881. doi: 10.1089/jmf.2021.0156.
- McMichael A, Pham H, von Grote E, et al. Efficacy and safety of minoxidil 2% solution in combination with a botanical hair solution in women with female pattern hair loss/androgenic alopecia. J Drugs Dermatol. 2016;15(4):398–404.
- Keaney TC, Pham H, von Grote E, et al. Efficacy and safety of minoxidil 5% foam in combination with a botanical hair solution in men with androgenic alopecia. J Drugs Dermatol. 2016;15(4):406–412.
- Reyes-Reali J, Mendoza-Ramos MI, Garrido-Guerrero E, et al. Hypohidrotic ectodermal dysplasia: clinical and molecular review. Int J Dermatol. 2018;57(8):965–972. doi: 10.1111/ijd.14048.
- Khatter S, Puri RD, Mahay SB, et al. Mutation-proved Clouston syndrome in a large Indian family with a variant phenotype. Indian J Dermatol. 2019;64(2):143–145. doi: 10.4103/ijd.IJD_510_17.
- Kantaputra PN, Intachai W, Carlson BM, et al. Clouston syndrome with dental anomalies, micropores of hair shafts and absence of palmoplantar keratoderma. J Dermatol. 2020;47(3):e90–e91. doi: 10.1111/1346-8138.15236.
- Zhan Y, Luo S, Pi Z, et al. A recurrent mutation of GJB6 in a big Chinese family with Hidrotic ectodermal dysplasia. Hereditas. 2020;157(1):34. doi: 10.1186/s41065-020-00148-8.
- Ahmad W, Faiyaz Ul Haque M, Brancolini V, et al. Alopecia universalis associated with a mutation in the human hairless gene. Science. 1998;279(5351):720–724. doi: 10.1126/science.279.5351.720.
- Akiyama M. Isolated autosomal recessive woolly hair/hypotrichosis: genetics, pathogenesis and therapies. J Eur Acad Dermatol Venereol. 2021;35(9):1788–1796. doi: 10.1111/jdv.17350.
- Qu B, Meng S, Yang C, et al. Botanical extracts in combination improve autosomal recessive woolly hair/hypotrichosis caused by LIPH mutations. J Cosmet Dermatol. 2022;21(10):5255–5258. doi: 10.1111/jocd.14880.
- Armanini D, Fiore C, Mattarello MJ, et al. History of the endocrine effects of licorice. Exp Clin Endocrinol Diabetes. 2002;110(6):257–261. doi: 10.1055/s-2002-34587.
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: China Pharmaceutical Science and Technology Press; 2020.
- Chen M, Zhu J, Kang J, et al. Exploration in the mechanism of action of licorice by network pharmacology. Molecules. 2019;24(16):2959. doi: 10.3390/molecules24162959.
- Yang D, Zheng J, Zhang Y, et al. Total glucosides of paeony capsule plus compound glycyrrhizin tablets for the treatment of severe alopecia areata in children: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:378219–378215. doi: 10.1155/2013/378219.
- Dell’Acqua G, Richards A, Thornton MJ. The potential role of nutraceuticals as an adjuvant in breast cancer patients to prevent hair loss induced by endocrine therapy. Nutrients. 2020;12(11):3537. doi: 10.3390/nu12113537.
- Li Y, Chu F, Li P, et al. Potential effect of Maxing Shigan decoction against coronavirus disease 2019 (COVID-19) revealed by network pharmacology and experimental verification. J Ethnopharmacol. 2021;271:113854. doi: 10.1016/j.jep.2021.113854.
- Li X, Wu X, Elston DM, et al. Hypohidrotic ectodermal dysplasia with c.28delG mutation in ectodysplasin A gene and severe atopic dermatitis treated successfully with tofacitinib. Acta Derm Venereol. 2021;101(1):adv00352. doi: 10.2340/00015555-3693.
- Suchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des Devel Ther. 2019;13:2777–2786. doi: 10.2147/DDDT.S214907.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–194. doi: 10.1111/j.1365-2133.2004.05785.x.
- Li L, Zhou Y, Tian R, et al. Prenatal ultrasound findings of ectodermal dysplasia: a case report. BMC Pregnancy Childbirth. 2022;22(1):100. doi: 10.1186/s12884-022-04430-7.
- Lauron S, Plasse C, Vaysset M, et al. Prevalence and odds of depressive and anxiety disorders and symptoms in children and adults with alopecia areata: a systematic review and meta-analysis. JAMA Dermatol. 2023;159(3):281–288. doi: 10.1001/jamadermatol.2022.6085.
- Vallerand IA, Lewinson RT, Parsons LM, et al. Assessment of a bidirectional association between major depressive disorder and alopecia areata. JAMA Dermatol. 2019;155(4):475–479. doi: 10.1001/jamadermatol.2018.4398.
- Bhat YJ, Trumboo T, Krishan K. Hair shaft disorders in children – An update. Indian Dermatol Online J. 2023;14(2):163–171. doi: 10.4103/idoj.idoj_7_22.