Abstract
Purpose: To analyze the role of mus-genes repair in system activation P-elements in Drosophila melanogaster induced by a chronic exposure to low doses.
Materials and methods: The materials were dysgenic individuals of Drosophila melanogaster with mutations in repair genes (mus101, mus205, mus304, mus308, mus309) and simultaneous transposition of mobile P-elements. The animals were exposed to a chronic irradiation in low doses (0.42 mGy/h). The reaction of animals was analyzed by the DNA damage rate in somatic cells (‘Comet assay’), level of dominant lethal mutations, fecundity, and survival rate.
Results: The combined action of the systems of post-replication, recombination repair (mus205, mus304), repair of DNA double-stranded breaks (mus304), and of the transposition activity of P-elements after a chronic irradiation in low doses was identified according to every study parameter. The other repair systems and their genes (mus101, mus308, and mus309) responded to action of only one factor (irradiation or mobile elements transposition).
Conclusion: The obtained data significantly contribute to the knowledge on a new reaction of the mechanisms of organisms to a chronic irradiation in low doses.
Introduction
One of the very significant problems of radiation genetics is the study of the molecular genetic mechanisms of reactions of organisms to the action of a low-intensity radiation. To date without the knowledge of the basic mechanisms (DNA damage repair, detoxification of free radicals, cell cycle control and apoptosis) manifestation of radiation-induced effects are known for certain regulatory processes of epigenetic reactions which are conditioned by the transposition activity of mobile elements (ME) (Slotkin and Martienssen Citation2007).
ME are found in all organisms including mammals and human. ME are basic inductors of spontaneous mutations. The genome of Drosophila melanogaster has more than 50 families of ME (Charlesworth and Langley Citation1989). It is known that some of the ME (P, hobo, mariner transposons) are moved by the mechanism of the ‘cut-paste’ forming of the chromosomal structures with DNA double-stranded breaks (DSB) (Engels et al. Citation1987; Gloor et al. Citation2000). These DNA damages are eliminated by cellular repair systems (Izsvák et al. Citation2008).
There are reports of a definite similarity of mechanisms in the initiation of DNA damage induced by radiation and the transposition activity of ME (Sobels and Eeken Citation1981; Banga et al. Citation1991; Bregliano et al. Citation1995; Koromyslov et al. Citation2004; Chmuzh et al. Citation2006). In response to the initiation of chromosomal damage induced both the radiation and activation of ME included repair systems and their genes. There is evidence pointing to genes (for example, mei-41) that may be involved in the repair of DNA damage after exposure to radiation and movement ME (Sobels and Eeken Citation1981; Koromyslov et al., 2004). Also the genes of repair can exhibit specificity and include in repair DNA damage only under the influence of any single factor (Banga et al., 1991).
This paper presents information on the role of the mus-repair genes in the system of radiation-induced activation of P-elements in Drosophila. The results are new in understanding what exactly the mus-genes of repair are involved in the recovery process of DNA damage breaks induced by a chronic irradiation in low doses and the transpositions of P-elements. This makes it possible to evaluate the mechanisms of interaction of the transposition of ME and cell repair systems in conditions of a chronic exposure to low doses.
Materials and methods
Drosophila stocks
Strains were obtained from Bloomington Drosophila Stock Center (USA). Harwich – inductive stock in Р-М system hybrid dysgenesis. It contains in genotype full copies P-transposons and has Р-cytotype (Castro and Carareto Citation2004). Сanton-S (CS) – reactive stock having I-cytotype. It is sensitive to the MR-factors (P, hobo) (Dimitri et al. Citation1997). DNA repair mutants mus (mutagen sensitive): strain mus101 (genotype: w[1]mus101[D1]) has mutation of the mus101 gene (allele D1) which take part in non-recombination postreplication DNA repair (de Buendia, Citation1998); strain mus205 (genotype: cn[1]bw[1]mus205[A1]) has the defect in the gene mus205 (allele A1) involving in post-replication repair on the mechanism of homologous recombination (Kane et al. Citation2012), in the repair of alkylation damage, excision repair of pyrimidine dimers and photorepair (Harris and Boyd Citation1993), homolog of mammal and human REV3L gene (Bhat et al. Citation2013); stock mus304 (genotype: st[1]mus304[D1]/TM3,Sb[1]Ser[1]) is defected in the gene mus304 (allele D1) which takes in post-replication DNA repair and recombination, in the repair DSB and excision repair of pyrimidine dimers (Harris and Boyd Citation1993; Brodsky et al. Citation2000; Chmuzh et al. Citation2007), homolog of mammal ATRIP gene (Oikemus et al. Citation2006); strain mus308 (genotype: ry[*]mus308[D5]/TM3,Sb[1]Ser[1]) has mutation of the mus308 gene (allele D5) which is involved in the repair of cross-links and of O-ethylpyrimidine DNA damage in an alternative end-joining system (Chan et al. Citation2010; Díaz-Valdés et al. Citation2010), homolog of mammal and human Polq gene (Sharief et al. Citation1999; Shima et al. Citation2004); stock mus309 (genotype: mus309[D3,ry]/CyO) (allele D3) has violation of the system repair DSB (Beall and Rio Citation1997; Portin Citation2010), homolog of mammal Kup70 and human XRCC6 genes (Beall and Rio Citation1996; Pei et al. Citation2013).
Experimental procedures
Some ME in particular P-elements is activated under certain hybridization conditions. These crosses are called dysgenic crossings. Inactive ME one of stock (for example, P-stock (Harwich)) become active only at crossing with stock (for example, M-stock (Canton-S, mus-stock)) which deprived of repressor protein of transposition. In such circumstances ME encode transposase enzyme that carries out the transposition ME genome. Thus, we conducted the induction of ME in the experiment. The term 'cytotype' reflects certain properties inherited through the maternal line and determine the presence/absence of the genotype of functional copies of certain ME-P, hobo and I. We also studied non-dysgenic individuals received as a result of intralinear crossings. Part of the individuals were subjected to a chronic γ-radiation from a radiation source 226Ra (56 mGy/h) at the radiated power of 0.42 mGy/h. The cumulative dose was 120 mGy (for the entire life cycle, 12 days). Another part was used as control. All investigated variants were contained in strictly controlled laboratory condition at photoperiodicity of 12 h light/12 h dark, at a temperature of 25 ± 0.1 °C, 60% humidity and a standard nutrient medium (Ashburner Citation1989). In genetic studies assessing the activity of ME in Drosophila required check-up the presence or absence of certain ME in genotypes studied stocks. This is due to the fact that some stocks of Drosophila for many generations of content their in the collection can lose full-length copies ME. Therefore conduct this molecular and genetic analysis (PCR analysis with primers for the open reading frame, test 'gonadal atrophy', etc.) (Kidwell et al. Citation1977; Marin et al. Citation2000; Zainullin and Yushkova Citation2012).
Dominant lethal mutations analysis
The analysis of dominant lethal mutations (DLM) was done according to the standard methods (Watti and Tikhomirova Citation1976; Aslanian et al. Citation1994). We selected males which were born due to dysgenic/non-dysgenic crossings. They were crossed with virgin females of the corresponding line. The final progeny at the egg stage was assessed for the presence of colored (brown) embryos. The latter are classified as later lethals or DLM and are caused by genetic defects, particularly by serious chromosome rearrangements (Watti and Tikhomirova Citation1976). The DLM frequency rate was calculated as a ratio of later lethals number to total number of laid embryos.
Survival analysis
The survival of individuals was evaluated by the known method (Engels et al. Citation1987). Individuals which pupated and flew from puparium were counted. The survival was evaluated reasoning from the imago-to-pupae ratio.
Estimation of the relative fecundity females
We selected 50 females from each experimental variant. Every day the number of eggs laid by the females was counted. The relative fecundity female was evaluated as the ratio of absolute fecundity (total number of offspring) females per day to their average absolute fertility during the whole observation period (10 days) in the experiment (Portin Citation2010). The fertility (amount embryo/female) was estimated by the average number of embryos per female.
DNA damage analysis
The frequency of DNA damage which resulted from the formation of DSB in cells of embryonic tissues of the Drosophila was determined by the Comet assay (neutral version pH) in some modification (Bilbao et al. Citation2002). For obtaining the cell suspensions the isolated nerve ganglia together with the imaginal discs from the third instar larvae as well as embryos (20 individuals per variant, per replication) were incubated in a solution of collagenase (type IV, 0.5 mg/ml PBS) at 37 °C. The resulting cell suspensions (10 μl) with 0.5% low-melting agarose (100 μl) were applied on glass slides coated by a layer of 1% norm-melting agarose and kept in a cold environment (4 °C) for 20 min. Further, the preparations with the agarose immobilized cells were lysed at 4 °C (2.5 M NaCl, 10 mM Na2EDTA, 20 mM TrisHCl, 1% Triton X-100, pH 10.0). Electrophoresis (30 mA, 0.7 V/cm, 4 °C, 20 min) was carried out in a refrigerated (4 °C) neutral buffer (0.9 M Trizma Base, 0.9 M Boric acid, 20 mM Na2EDTA, pH 8.2) and then the preparations were slightly dried and fixed in 70% ethanol for 15 min. DNA was stained using the solution SYBR Green I (0.2 μl/ml in TE-buffer, pH 7.5) (DNA-synthesis, Russia). The stained preparations were analyzed using a fluorescence microscope ‘Infiniti XS-148 FS’ at wave lengths of excitation and emission of 494 and 521 nm, respectively. The preview of the comets was processed using the program CometScore™ (version 1.5, TriTek Corp., Sumerduck, VA). The DNA DSB were assessed by the most informative and precise parameter OTM (Olive tail moment, the distance from the center of the head to the density center of the comets tail multiplied by tDNK[%]) (Olive Citation1999; Jha Citation2008). For each variant of each replicate 100 cells were counted (in total 300 cells per one variant were analyzed).
We used the following reagents: sodium chloride (NaCl), agarose, triton-X, boric acid, tris hydrochloride (Tris-HCl) (Panreac, Spain), collagenase type IV, phosphate buffered saline (PBS), tris(hydroxymethyl)aminomethane (Trizma Base) (Sigma, США), disodium EDTA (Na2EDTA) (AppliChem, Germany).
Statistical analyses of differences between the mean values of the parameters (the frequency OTM and DLM, the level survival) experimental variants were determined by Student’s t-test. The obtained data was processed with Statistica (version 7.0.61.0, StatSoft, Inc., Tulsa, OK). The significance of differences of the results of the fecundity was assessed by the Chi-square test in program R.
Results
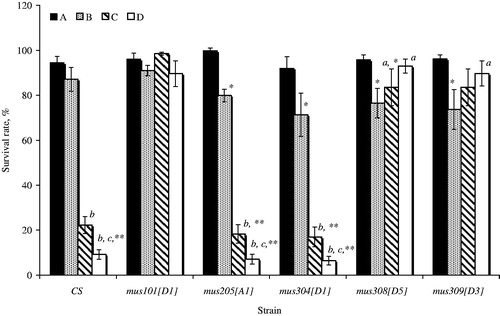
Effect of a chronic irradiation in low doses on survival
A chronic irradiation in low doses () truly decreases the survival for all mutant individuals (except for mus101 strain) without the transposition of P-elements. In dysgenic conditions we identified a high survival rate (∼90%) for irradiated individuals with mus205 and mus304 repair genes mutations. In wild-type strain Canton-S we also noted the low survival (22.3%). The mus308 and mus309 mutants demonstrated a reverse effect. The survival of irradiated dysgenic flies at the adult stage was truly higher than that of irradiated dysgenic individuals.
Figure 1. The level of survival (%) of mutant on repair individuals Drosophila melanogaster developing in conditions induction of the transposition P-elements and the influence of a chronic radiation in low doses. (A) Non-dysgenic individuals (control); (B) non-dysgenic individuals (γ-radiation); (C) dysgenic individuals having in the genotype of active copies P-elements (control); (D) dysgenic individuals (γ-radiation). The differences are statistically significant at *p < 0.05, **p < 0.01 with own control; ap < 0.05, bp < 0.01 with irradiated non-dysgenic individuals; cp < 0.05, dp < 0.01 with unexposed dysgenic individuals; errors indicate the standard error of the mean (± SEM) for n = 4 independent experiments.
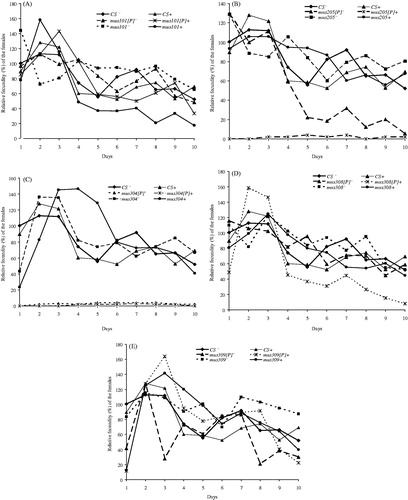
Effect of a chronic irradiation in low doses on fecundity
Irradiated/non-irradiated dysgenic adult individuals (imago) of Canton-S and mus304 strains, as well as irradiated mus205 individuals were practically sterile. The number of embryos per female varied within 2–4% (). Dysgenic females of mus308 and mus309 genotypes had a low relative fecundity index during the first day, especially having been chronically irradiated (). The fecundity index of females towards the second and third days reached its maximum and then sharply decreased. The mean number of embryos per female for mus309 was almost half for mus308. For strain mus101, the effect of chronic irradiation and the induction of the transposition activity of P-elements were equal ().
Figure 2. Effect of a chronic irradiation in low doses on the relative fecundity of dysgenic/non-dysgenic females of mus101 (A), mus205 (B), mus304 (C), mus308 (D) and mus309 (E) strains. Frequencies in percentages and their standard deviations of the number of offspring compared to the mean number of offspring in each procedure in 10 consecutive daily broods of the dysgenic/non-dysgenic mutant females in control and after low-dose irradiation.
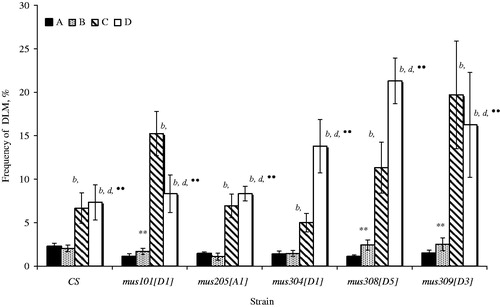
Effect of a chronic irradiation in low doses on DLM frequency
High DLM frequency was observed for every strain of Drosophila on condition of simultaneous action of a chronic irradiation and the induction of the transposition of P-elements (). In the absence of active copies of P-elements, a chronic irradiation in low doses caused high DLM frequency only for mus101, mus308 and mus309. Canton-S dysgenic individuals and mus205, mus304 and mus308 individuals with gene mutations were highly sensitive to irradiation in parameter of DLM. The reaction to the effects of radiation of mus101 and mus309 dysgenic individuals was opposite, i.e. radiation-induced DLM frequency was below the mutation level of non-irradiated individuals.
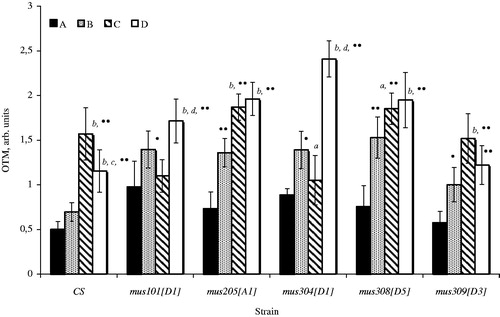
Effect of a chronic irradiation in low doses on DNA damage frequency
Every strain (except for Canton-S) was observed for a high amount of cells with damaged DNA as response to irradiation (). On simultaneous action of irradiation and the induced transposition activity of P-elements, the frequency of DNA damage significantly varied. The variation depended on the presence of a particular mutation in the genotype of animals and the availability of radiation factor. In comparison with non-irradiated dysgenic and irradiated non-dysgenic individuals, mus101 and mus304 dysgenic individuals demonstrated a true rise in DNA damage frequency after action of a chronic low-intensive irradiation. The same reaction was characteristic of the other mus-mutants (except for mus309) but only in comparison with irradiated animals which did not have active copies of P-elements in genotype. The data agree with the above results on DLM and fecundity.
Discussion
The biological efficiency of irradiation in low doses may be higher than the exposure of acute irradiation (Grosovsky Citation1999; Nagasawa and Little Citation1999; Ma et al. Citation2010). The principle genetic effect of an irradiation in low doses is radiation-induced genetic instability which lasts during numerous cell divisions after the initial cell has been irradiated (Holmberg et al. Citation1998; McIlrath et al. Citation2003). Genetic instability is favored by changes of repair activity, divergences in cell cycle control, and apoptosis (Murnane Citation1996). Genetic instability can also be related to the activity of ME (Qüesta et al. Citation2013). After irradiation, active ME may produce several transposition cycles which cause the formation of instable mutations and numerous DNA damages in cells. Although ME under certain conditions (dysgenesis, inbreeding, impact of radiation, temperature, chemical factors) cause irreversible damages of genetic material, they are highly prevalent and invasive in a natural population and are important for adaptation (Khurana et al. Citation2011). The interaction between repair and induction of the transposition activity systems (hybrid dysgenesis and insertion mutagenesis systems) plays an important role in the manifestation of adequate reaction of organisms to stress.
The reaction of the studied ‘mutagen-sensitive’ genotypes to joint and separate action of an irradiation and P-elements-induced activity system is different. It is well illustrated by the analysis of DLM which characterize action of mus-mutations in different conditions. The DLM frequency caused by a chronic γ-radiation is significantly different from the DLM level induced by the transposition activity of P-elements. This is evidenced by high DLM frequency in the case of dysgenesis (5–19.7%) which largely exceeds the level of radiation-induced DLM (1.09–2.51%). In conditions of ME induction, high DLM levels were found in mus101 and mus309 genotypes (compared with Canton-S). This suggested specificity of involving of post-replication and repair DSB. High radiation-induced DLM frequency is found for mus308 and mus309. This points to the involved repair of interstrand links, O-ethylpyrimidine DNA damages and DSB in response to a chronic irradiation. By survival and fecundity rates, mus308 and mus309 strains are the most sensitive to irradiation, and Canton-S, mus205, mus304 strains are the most sensitive to active P-elements. The obtained data visualize specific action of each factor. Irradiation greatly damages proliferating cells at preimaginal stages (embryonal, larval, pupal stages) but the effects depend not only on repair processes but also on specific development features of Drosophila, i.e. compensatory mechanisms and critical value of histoblasts damages which play a key role in new growth of tissues in the organism of imago insect and in the process of puparium leaving. Moving of the autonomic ME, including P-factor, possibly produce not total but local damages of genetic structures in separate cells and the impact of these damages on organism survival is mostly unknown.
The DNA damage frequency in somatic cells (neuroblasts, cells of imaginal discs) highlights precise repair processes of DNA breaks which are caused by both ME transpositions and irradiation. Subsequent to the results of cytogenetic analysis, all the studied mus-genes respond to action of a chronic irradiation. Irradiation-induced damages are different and include damaged DNA bases, DNA-DNA and DNA-protein cross-linkage, DNA single-stranded breaks and DSB (Price Citation1993). This means that restoration of different radiation-induced DNA damages is done by different mechanisms and by means of a large number of repair genes. However, the P-elements’ excisions involve the mus205, mus308, and mus309 genes into repair of damaged DNA. The role of the mus205 and mus308 genes in the repair of such DNA damage is not clear and requires further experimental research. Participation of the mus205 gene in this process may be explained by the fact that excisions of P-elements proceed after DNA replication at G2 phase of cell cycle (Weinert et al. Citation2005). Consequently, final DNA damages are possibly repaired by post-replication or excision repair (mus205). High frequency of DNA damage for mus309 strain which has active copies of P-elements are proven by the results of Beall and Rio on participation of the mus309 gene in repair of P-induced DSB (Beall and Rio Citation1996).
The specificity of switching on the study of the repair genes is also visual on joint interaction of irradiation and dysgenesis. In this case the mus304 gene plays a key role. Its mutation truly increases the frequency of genetic defects (by DLM and DSB) and decreases the vitality index (by survival and fecundity parameters). The mus304 mutants can have high frequency of recombination due to P-transposase. A specific feature of such recombination is the appearance of quite long deletions and duplications (Preston et al. Citation1996). The increase of such damages can cause cell and even organism death. The mus304 is a homolog of the mammalian and human ATRIP gene and a central recombination protein. Its biological role is similar to that of the mei-41 gene (homolog of human ATM gene). The mei-41 is known to participate in the repair of DSB initiated by the transpositions of P-elements by mechanism homolog recombination (Koromyslov et al. Citation2004; Chmuzh et al. Citation2006). A similar reaction is demonstrated by mus205 but cytogenetically we do not observe true differences between non-irradiated and irradiated dysgeniс individuals.
Conclusion
The formation of radiation effects depends on interaction between the repair genes and ME-induced activity systems. By the obtained data post-replication and recombination repair (the mus205 and mus304 genes) as well as DSB repair (mus304) is involved into genetic material restoration in response to joint action of intracellular (P induction) and exterior (a chronic low-dose γ-radiation) factors. Since the P-elements transpositions lead to the formation of DSB, we cannot exclude the fact that the studied mus-genes can possibly have additional functions on repair of such DNA damage.
Use of the discussed approach in radiobiological studies allows for the additional possibilities to discover a new formation of the mechanisms of organism response to a chronic irradiation in low doses.
Disclosure statement
The authors report no conflicts of interest. The authors alone are response for the content and writing of the paper.
Funding
This work was supported by a grant from the Presidium of the Russian Academy of Science [No. 0414-2015-0024].
References
- Ashburner M. 1989. Drosophila: a laboratory handbook. Cold Spring Harbor Laboratory Press.
- Aslanian MM, Kim AI, Magomedova MA, Fatkulbaianova NL. 1994. Analysis of dominant and recessive sex-linked lethal mutations induced by low radiation doses in genetically different strains of Drosophila melanogaster w and MS. Russian J Genet. 30:1220–1223.
- Banga SS, Velazquez A, Boyd JB. 1991. P transposition in Drosophila provides a new tool for analyzing postreplication repair and double-strand break repair. Mutat Res. 255:79–88.
- Beall EL, Rio DC. 1996. Drosophila IRBP/Ku p70 corresponds to the mutagen-sensitive mus309 and is involved in P-element excision in vivo. Genes Develop. 10:921–933.
- Beall EL, Rio DC. 1997. Drosophila P-element transposase is a novel site-specific endonuclease. Genes Develop. 11:2137–2151.
- Bhat A, Andersen PL, Qin Z, Xiao W. 2013. Rev3, the catalytic subunit of Polzeta, is required for maintaining fragile site stability in human cells. Nucleic Acids Res. 41:2328–2339.
- Bilbao С, Ferreiro JA, Comendador MA, Sierra LM. 2002. Influence of mus201 and mus308 mutations of Drosophila melanogaster on the genotoxicity of model chemicals in somatic cells in vivo measured with the Comet assay. Mutat Res. 503:11–19.
- Bregliano JC, Laurençon A, Degroote F. 1995. Evidence for an inducible repair-recombination system in the female germ line of Drosophila melanogaster. I. Induction by inhibitors of nucleotide synthesis and by gamma rays. Genetics. 141:571–578.
- Brodsky MH, Sekelsky JJ, Tsang G, Hawley RS, Rubin GM. 2000. Mus304 encodes a novel DNA damage checkpoint protein required during Drosophila development. Genes Develop. 14:666–678.
- Castro JP, Carareto CMA. 2004. Canonical P elements are transcriptionally active in the saltans groups of Drosophila. J Molec Evolut. 59:31–40.
- Chan SH, Yu AM, McVey M. 2010. Dual roles for DNA polymerase theta in alternative end-joining repair of double-strand breaks in Drosophila. PLoS Genet. 6:e1001005.
- Charlesworth B, Langley CN. 1989. The population genetics of Drosophila transposable elements. Ann Rev Genet. 23:251–287.
- Chmuzh EV, Shestakova LA, Volkova VS, Zakharov IK. 2006. Diversity of mechanisms and functions of enzyme systems of DNA repair in Drosophila melanogaster. Russian J Genet. 42:363–375.
- Chmuzh EV, Shestakova LA, Volkova VS, Zakharov IK. 2007. Highly sensitive systems for experimental insertional mutagenesis in repair-deficient genetic environment in Drosophila melanogaster: New opportunities for studying postreplication repair of double-stranded DNA breaks and mechanisms of transposable element migration. Russian J Genet. 43:41–47.
- de Buendia PG. 1998. Search for DNA repair pathways in Drosophila melanogaster. Mutat Res. 407:67–84.
- Díaz-Valdés N, Comendador MA, Sierra LM. 2010. Mus308 processes oxygen and nitrogen ethylation DNA damage in germ cells of Drosophila. J Nucleic Acids. 2010:1–7.
- Dimitri P, Arca B, Beghella L. 1997. High genetic instability of heterochromatin after transposition of LINE-like I factor in Drosophila melanogaster. Proc Natl Acad Sci USA. 94:8052–8057.
- Engels WR, Benz WK, Preston CR, Graham PL, Phillis RW, Robertson HM. 1987. Somatic effects of P element activity in Drosophila melanogaster: Pupal lethality. Genetics. 117:745–757.
- Gloor GB, Moretti J, Mouyal J, Keeler KJ. 2000. Distinct P-element excision products in somatic and germline cells of Drosophila melanogaster. Genetics. 155:1821–1830.
- Grosovsky AJ. 1999. Radiation-induced mutations in unirradiated DNA. Proc Natl Acad Sci USA. 96:5346–5347.
- Harris PV, Boyd JB. 1993. Re-evaluation of excision repair in the mus304, mus306 and mus308 mutants of Drosophila. Mutat Res. 301:51–55.
- Holmberg K, Mejer AE, Harms-Rindahl M, Lambert B. 1998. Chromosomal instability in human lymphocytes after low doze rate irradiation and delayed mitogen stimulation. Int J Radiat Biol. 73:21–34.
- Jha AN. 2008. Ecotoxicological applications and significance of the comet assay. Mutagenesis. 23:207–221.
- Izsvák Z, Wang Y, Ivics Z. 2008. Interactions of transposons with the cellular DNA repair machinery. In: Lankenau D-H, Volff J-N, editors. Transposons and the dynamic genome. Berlin Heidelberg: Springer-Verlag. pp. 133–176.
- Kane DP, Shusterman M, Rong Y, McVey M. 2012. Competition between replicative and translesion polymerases during homologous recombination repair in Drosophila. PLoS Genet. 8:e1002659.
- Khurana JS, Wang J, Xu J, Koppetsch BS, Thomson TC, Nowosielska A, Li S, Zamore PD, Weng Z, Theurkauf WE. 2011. Adaptation to P element transposon invasion in Drosophila melanogaster. Cell. 147:1551–1563.
- Kidwell MG, Kidwell JP, Sved JA. 1977. Hybrid dysgenesis in Drosophila melanogaster. A syndrome of aberrant traits including mutation, sterility and male recombination. Genetics. 36:813–833.
- Koromyslov YA, Chmuzh EV, Shestakova LA. 2004. Mutability of unstable sex-linked alleles of Drosophila melanogaster and their interaction with mutations of the genes of the repair system. Drosophila Informat Serv. 86:35.
- Ma S, Liu X, Jiao B, Yang Y, Liu X. 2010. Low-dose radiation-induced responses: focusing on epigenetic regulation. Int J Radiat Biol. 86:517–528.
- Marin L, Lehmann M, Nouaud D, Izaabel H, Anxolabéhère D, Ronsseray S. 2000. P-element repression in Drosophila melanogaster by a naturally occurring defective telomeric P copy. Genetics. 155:1841–1854.
- McIlrath J, Lorimore SA, Coates PJ, Wright EG. 2003. Radiation-induced genomic instability in immortalized haemopoietic stem cells. Int J Radiat Biol. 79:27–34.
- Murnane JP. 1996. Role of induced genetic instability in the mutagenic effects of chemicals and radiation. Mutat Res. 367:11–23.
- Nagasawa H, Little JB. 1999. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 52:6394–6396.
- Oikemus SR, Queiroz-Machado J, Lai KJ, McGinnis N, Sunkel C, Brodsky MH. 2006. Epigenetic telomere protection by Drosophila DNA damage response pathways. PLoS Genet. 2:e71.
- Olive PL. 1999. DNA damage and repair in individual cells: applications of the comet assay in radiobiology. Int J Radiat Biol. 75:395–405.
- Pei JS, Lee YM, Lo HH, Hsu YN, Lin SS, Bau DT. 2013. Association of X-ray repair cross-complementing-6 genotypes with childhood leukemia. Anticancer Res. 33:5395–5399.
- Portin P. 2010. Evidence based on studies of the mus309 mutant, deficient in DNA double-strand break repair, that meiotic crossing over in Drosophila melanogaster is a two-phase process. Genetica. 138:1033–1045.
- Preston CR, Sved JA, Engels WR. 1996. Flanking duplications and deletions associated with P-induced male recombination in Drosophila. Genetics. 44:1623–1638.
- Price A. 1993. The repair of ionizing radiation-induced damage to DNA. Semin Cancer Biol. 4:61–71.
- Qüesta J, Walbot V, Casati P. 2013. UV-B radiation induces Mu element somatic transposition in maize. Mol Plant. 6:2004–2007.
- Sharief FS, Vojta PJ, Ropp PA, Copeland WC. 1999. Cloning and chromosomal mapping of the human DNA polymerase θ (POLQ), the eighth human DNA polymerase. Genomics. 59:90–96.
- Shima N, Munroe RJ, Schimenti JC. 2004. The mouse genomic instability mutation chaos1 is an allele of Polq that exhibits genetic interaction with Atm. Mol Cell Biol. 24:10381–10389.
- Slotkin RK, Martienssen R. 2007. Transposable elements and the epigenetic regulation of the genome. Nature Rev Genet. 8:272–285.
- Sobels FH, Eeken JCJ. 1981. Influence of the MR (mutator) factor on X-ray-induced genetic damage. Mutat Res. 83:201–206.
- Watti KV, Tikhomirova MM. 1976. Spontaneous and radiation induced dominant lethal mutations in male and female Drosophila. Res Genet. 6:32–43.
- Weinert BT, Min B, Rio DC. 2005. P element excision and repair by non-homologous end joining occurs in both G1 and G2 of the cell cycle. DNA Repair. 4:171–181.
- Zainullin VG, Yushkova EA. 2012. Estimation of the levels of radiation-induced P-element transposition in Drosophila melanogaster experimental populations and laboratory strains. Russian J Genet. 48:473–476.