Abstract
In a similar way to high-dose exposures to low-LET radiations, cells show difficulties reaching mitosis after high-LET radiation exposure. For this reason, techniques have been proposed that are able to analyze chromosome aberrations in interphase by prematurely condensing the chromosomes (PCC-techniques). Few dose-effect curves for high-LET radiation types have been reported, and none for α-particles. The aim of this study was to evaluate, by chemically-induced PCC, the chromosome aberrations induced by several doses of α-particles. Monolayers of peripheral lymphocytes were exposed to an α-source of Americium-241 with a mean energy entering the cells of 2.7 MeV. Lymphocytes were exposed to 10 doses, from 0–2.5 Gy, and then cultured for 48 h. Colcemid and Calyculin-A were added at 24 and 1 h before harvesting, respectively. During microscope analysis, chromosome rings and extra chromosome pieces were scored in G2/M-PCC and M cells, while dicentric chromosomes were only scored in M cells. As the dose increased, fewer cells were able to reach mitosis and the proportion of G2/M-PCC cells increased. Chromosome rings were hardly observed in M cells when compared to G2/M-PCC cells. Extra fragments were more frequent than rings in both G2/M-PCC and M cells, but with lower frequencies than in G2/M-PCC cells. The distribution of dicentrics and extra fragments showed a clear overdispersion; this was not so evident for rings. The dose-effect curves obtained fitted very well to a linear model. Damaged cells after α-particle irradiation show more difficulties in reaching mitosis than cells exposed to γ-rays. After α-particle irradiation the frequency of all the chromosome aberrations considered increased linearly with the dose, and α-particles clearly produced more dicentrics and extra chromosome pieces with respect to γ-rays. After α-particle exposure, the existence of extra chromosome fragments in PCC cells seems to be a good candidate for use as a biomarker for dose assessment. However, the observed frequencies of different types of chromosomal aberrations could be influenced by some methodological aspects; for this reason, and in order to avoid possible methodological bias, standardization of the technique will be desirable.
Introduction
Dose assessment after exposures to ionizing radiation (IR) has long been performed by the analysis of chromosome aberrations in mitotic cells. The analysis of dicentric chromosomes in peripheral blood lymphocyte metaphases has allowed the development of biological dosimetry and is the method of choice to be used in suspected overexposures to IR (International Atomic Energy Agency [IAEA] Citation2011). Peripheral blood lymphocytes are usually in the resting phase G0, and must be stimulated to reach metaphase. During this process, the cells must overcome different cell cycle checkpoints that control adequate progression. When cells show DNA damage, the DNA repair machinery is activated with other signaling pathways, inducing cell progression delay and triggering programmed cell death if necessary. Among the checkpoints, the G2/M one ensures cells do not enter mitosis before they have the chance to repair the damaged DNA (Jeggo and Löbrich Citation2006; Niida and Nakanishi Citation2006). Considering the difficulty highly damaged cells have in overcoming the G2/M checkpoint and hence in reaching mitosis, for biological dosimetry purposes it is difficult to assess exposures higher than 4–5 Gy (IAEA Citation2011), because at higher doses only a few cells can reach mitosis. A delay in cell cycle progression, mainly due to a prolonged G2-arrest, has also been described for high-LET radiation exposures (Lee et al. Citation2011). For this reason, the standard dicentric analysis would considerably underestimate the effect of a high-LET irradiation (Lee et al. Citation2011).
In cases where dose-assessment at higher doses of low-LET radiation or after high-LET radiation is needed, the evaluation of chromosome aberrations in interphase cells using Premature Chromosome Condensation (PCC) techniques has been proposed. PCC-techniques can be carried out by fusing unstimulated peripheral blood lymphocytes with mitotic CHO cells, allowing an estimation of the dose immediately after blood collection (Pantelias and Maillie Citation1983). Another way to obtain interphase chromosomes is the chemical induction of PCC using Calyculin-A or okadaik acid (Durante et al. Citation1998; Kanda et al. Citation1999), although in this case cells need to be stimulated to growth. Both methods, fusion and chemically induced PCC, are now accepted for biological dosimetry (IAEA Citation2011). Chemically PCC has been applied in biodosimetry in cases of accidental exposures to a high dose of IR (Hayata et al. Citation2001; Yao et al. Citation2013) and several dose-effect calibration curves for low-LET radiation have been published using different biomarkers such as chromosome rings or extra chromosome pieces (Lamadrid et al. Citation2007; Balakrishnan et al. Citation2010; Lindholm et al. Citation2010; Puig et al. Citation2013). For high-LET radiation few dose-effect curves based on PCC have been published, and none for α-particle irradiation (Lamadrid et al. Citation2007; Citation2011; Wang et al. Citation2007; Lee et al. Citation2011). The aim of the present study was to analyze, by chemically-induced PCC, the chromosome aberrations induced at several doses of α-particle irradiation and to construct dose-effect curves.
Materials and methods
Peripheral blood lymphocytes isolation and culture
Peripheral blood samples were obtained by venipuncture from a 27-year-old female with no history of exposure to ionizing radiation or clastogenic agents. This project was approved by the Animal and Human Experimentation Ethics Committee of the Universitat Autònoma de Barcelona (Reference: 2624). Lymphocyte isolation, culture and irradiation were performed as previously described (Schmid et al. Citation1996). Briefly, lymphocytes were isolated from whole blood using the Percoll (Biochrom, Berlin, Germany) gradient centrifugation technique. Isolated cells were washed twice in Roswell Park Memorial Institute (RMPI)-1640 medium (Biochrom) and resuspended in the same medium at a final concentration of 5 × 106 lymphocytes per ml. A total of 0.5 ml of the lymphocyte suspension and 4.5 ml of RPMI-1640 medium, supplemented with 16% (v/v) of fetal calf serum (Biochrom), 27 μg of phytohaemagglutinin (PHA) (Biochrom) and (100 U.I. ml−1 penicillin, 100 mg·ml−1 streptomycin), were mixed and incubated for 3 h at 37 °C, in special dishes containing a stainless steel ring and a 2 μm thick Mylar foil (Goodfellow, Bad Nauheim, Germany), to allow lymphocyte attachment. Immediately before irradiation the residual medium was carefully drawn off, and monolayers of human peripheral lymphocytes were irradiated (Schmid et al. Citation1996; Regulla et al. Citation2002).
Irradiation procedure
The monolayers of attached lymphocytes were irradiated with α-particles from an Americium-241 source located at the Helmholtz Zentrum München (Neuherberg, Germany). The irradiation device was designed by Roos and Kellerer (Citation1989) and has already been employed in studies of chromosome analysis in human lymphocytes using fluorescence plus Giemsa staining or fluorescence in situ hybridization techniques, resulting in a high degree of accuracy and reproducibility (Schmid et al. Citation1996; Barquinero et al. Citation2004; Schmid and Roos Citation2009). Details of the source are fully described in Schmid et al. (Citation1996). During irradiations the most probable energy of α-particles entering the cells was 2.7 MeV with a dose-mean LET of 150 keV·μm−1. Monolayers of human peripheral blood lymphocytes were exposed at 10 doses: 0, 0.05, 0.1, 0.2, 0.5, 0.7, 1, 1.5, 2 and 2.5 Gy of α-particles at a dose-rate of 0.1 Gy·min−1. Although during the exposure the lymphocytes were kept in the chamber at 37 °C, remaining without medium during irradiation. Due to the dose-rate and the irradiation conditions it was not possible to obtain chromosome spreads at higher doses.
Cell culture and chromosome analysis
After irradiation, the lymphocytes were scraped off and cultured in supplemented RPMI-1640 medium for 48 h. To obtain metaphases and PCC spreads in the same sample, Colcemid (Boehringer Manheim, Manheim, Germany) was added after 24 h of culture at a final concentration of 0.1 μg·ml−1, and 1 h before harvesting Calyculin-A (LC Laboratories, Interlabo, Spain) was added at a final concentration 50 nM. The cells then were treated with hypotonic solution (KCl, 0.075 M) (Sigma-Aldrich, Munich, Germany) for 20 min at 37 °C and fixed with Carnoy’s solution (methanol:acetic acid, 3:1, v/v) (Sigma-Aldrich). Once cells were dropped onto slides and air-dried, the slides were placed for 24 h at 37 °C. The staining was done with Leishman’s stain (Merck, Madrid, Spain).
Chromosome spreads were analyzed with a Zeiss Axio Imager.Z2 microscope (Izasa, Barcelona, Spain) coupled to a Metafer® Slide Scanning System V3.8 (Izasa, Barcelona, Spain). Cytogenetic analysis was performed as previously described (Puig et al. Citation2013). In brief, each cell was classified as a G2/M-PCC cell or M cell according to the absence and presence of the centromeric constriction respectively, and it was assumed that G2/M-PCC cells were in G2 phase of the cell cycle and M cells in metaphase. Only cells with 46 or more pieces were considered. The number of chromosome rings and the number of extra chromosome fragments was recorded in both cell types. A chromosome ring was only considered when a corresponding fragment was present. Other fragments exceeding 46 and not related to a ring were considered as extra fragments. Dicentric chromosomes were only recorded in M cells.
Statistical analysis
To test if there were differences in the frequencies of chromosome aberrations between G2/M-PCC and M cells, the Wilcoxon paired test was used. To check the Poisson distribution of rings and extra fragments in the cells, the u-test was used. Values of u outside the interval ±1.96 indicated that the aberrations did not follow a Poisson distribution (Papworth Citation1975). Dose-effect curves were fitted to a linear model by means of the weighted least-squares approximation. For rings the inverse of the mean was used as the weighting factor because departures from the Poisson distribution were not observed. For extra fragments and dicentrics the reciprocal of the estimated variance was used as the weighting factor because overdispersion was present at all doses. Differences in α coefficients were tested by means of the standard difference test (Barquinero et al. Citation1999).
Results
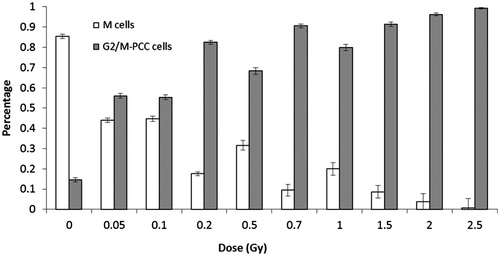
To evaluate the cytogenetic effect of α-particle irradiation by PCC, a total of 11,327 cells were analyzed, including cells in G2 phase (G2/M-PCC cells) and cells in metaphase (M cells). As can be seen in , the proportion of M cells in relation to total cells analyzed decreased as the dose increased, from 85% at 0 Gy to 1% at 2.5 Gy. Inversely, the proportion of G2/M-PCC cells increased with the dose, from 15% at 0 Gy to 99% at 2.5 Gy. At the lowest doses of irradiation, 0.05–0.1 Gy, percentages of M and G2/M-PCC cells were similar.
Figure 1. Percentages of M and G2/M-PCC cells at each dose. The error bars indicate the standard error of the mean.
For each dose and cell type the number of rings, and their distribution among cells, are shown in . At 0 and 0.05 Gy no rings were observed, and from 0.01–2.5 Gy the frequency of chromosome rings observed in G2/M-PCC cells was significantly higher than that observed in M cells (p = 0.0078). In fact in M cells rings were only observed after 0.5, 1 and 1.5 Gy. When G2/M-PCC cells and M cells were considered together as total cells, the frequency of rings increased with dose. Ring distribution among cells was in almost all cases in agreement with the Poisson distribution, and only at 1 and 2.5 Gy were u values indicative of a significant overdispersion. Dose-effect curves for ring chromosomes were established for G2/M-PCC and total cells (). The linear relationship and the coefficients obtained are shown in . Because ring chromosomes were hardly observed in M cells, the coefficients obtained for G2/M-PCC and total cells are almost the same. M cells did not show enough ring chromosomes to establish a dose-effect relationship.
Figure 2. Linear relationship for chromosome rings. Observed frequencies (±SE) in G2/M-PCC and total cells (G2/M-PCC and M cells together), error bars indicate the standard error of the mean. The values (±SE) of the coefficients are: y = 0.017(± 0.002)D for G2/M PCC cells, and y = 0.017(± 0.002)D for total cells.
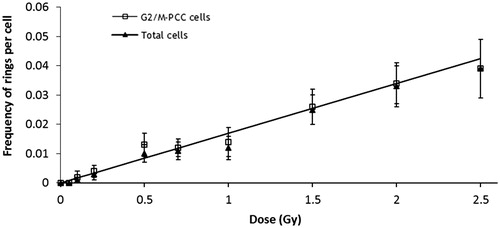
Table 1. Cell distribution of chromosome rings for each dose and cell type.
Extra fragments were scored in G2/M-PCC cells and M cells (). Their frequency clearly increased with dose. For all doses the extra fragments frequency was significantly higher in G2/M-PCC cells than in M cells (p = 0.002). As can be seen in , the distribution of extra fragments among cells for G2/M-PCC and M cells was overdispersed at all doses (u values > 1.96). For extra fragments, dose-effect curves were established for M cells, G2/M-PCC cells and total cells. The linear dose-effect relationships and the coefficients obtained are shown in . The α-coefficients of the dose-effect curves for extra fragments for G2/M-PCC and for total cells did not differ statistically, but both were significantly higher than that for M cells (p < 0.001). Dicentric chromosomes were only recorded in M cells. As can be seen in , their frequency increased with the dose and their distribution showed a clear overdispersion. Due to the low number of M cells observed after 2 and 2.5 Gy irradiation, 24 and 3 respectively, these two doses were not considered in calculating the linear coefficient of the dose-effect curve .
Figure 3. Linear relationships for extra fragments. Observed frequencies (±SE) of extra fragments in G2/M-PCC, M, and total cells (G2/M-PCC and M cells together), error bars indicate the standard error of the mean. The values (±SE) of the coefficients are: y = 0.865(± 0.018) + 1.527(± 0.025)D for G2/M-PCC cells, y = 0.018(± 0.03) + 0.2969(± 0.022)D for M cells, and y = 0.359(± 0.009) + 1.795(± 0.021)D for total cells.
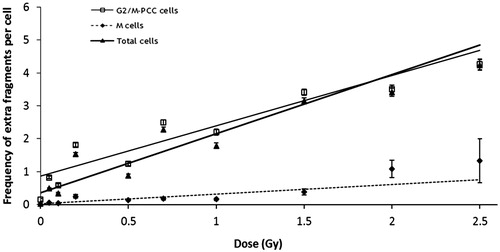
Figure 4. Linear relationship for dicentric chromosomes. Observed frequencies (±SE) of dicentric chromosomes in M cells, error bars indicate the standard error of the mean. The values (±SE) of the coefficients are: y = 0.373(± 0.074)D.
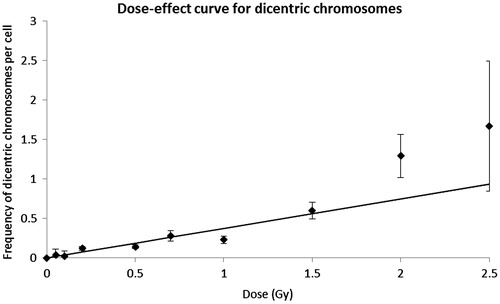
Table 2. Cell distribution of chromosome fragments for each dose and cell type.
Table 3. Cell distribution of dicentric chromosomes among cells in metaphase.
Discussion
By chemically-induced PCC several dose-effect curves for low-LET radiation have been published to date (Lamadrid et al. Citation2007; Balakrishnan et al. Citation2010; Lindholm et al. Citation2010; Puig et al. Citation2013), the main purpose of these curves being the dose-assessment after high-dose exposures. For high-LET radiation-types few curves have been reported using PCC (Lamadrid et al. Citation2007; Citation2011; Wang et al. Citation2007; Lee et al. Citation2011) and none of them for α-particles. In this sense the main purpose of this paper was to evaluate by PCC the chromosome aberrations induced by α-particle exposures and to establish dose-effect relationships using different cytogenetic biomarkers. The first observation of this study was the clear reduction in the proportion of M cells in respect to the G2/M-PCC cells as the dose increased. This situation is in accordance with previous studies done with low LET radiation (Kanda et al. Citation1999; Puig et al. Citation2013). For γ-rays, using blood samples from the same donor and with a similar protocol to chemically-induce PCC, the observed percentage of M cells with regard to total cells was 51.6% ± 1.1% and 3.4% ± 0.4% after 1 and 3 Gy of γ-irradiation, respectively (Puig et al. Citation2013). After α-particle irradiation the percentage of M cells observed at similar doses is 20% ± 1.4% and 0.6% ± 0.3% after 1 and 2.5 Gy irradiation. This result indicates that, when compared with γ-irradiation, cells irradiated at similar doses with α-particles show more difficulties in reaching mitosis. This is in agreement with previous results where a major effectiveness of α-particles in reducing the mitotic index was described (Lücke-Huhle et al. Citation1983; Ritter et al. Citation2002). This delay seems to be linked to the presence of cells with complex aberrations (George et al. Citation2003a). In fact, after different heavy-ion exposures and applying FISH chromosome painting to distinguish between simple and complex exchange type aberrations, George et al. (Citation2003b) reported higher frequencies of chromosome exchanges after Calyculin-A treatment with regard to those after Colcemid treatment. This result was also consistent with a previous study from the same group (George et al. Citation2001) indicating that chromosome exchanges, particularly complex type exchanges, were affected by the cell cycle delay. In our group we have seen that after α-particle irradiation complex aberrations are induced at low doses, whereas for low-LET radiation complex aberrations appeared from doses of 2–3 Gy (Barquinero et al. Citation1999, Citation2004; Rodríguez et al. Citation2009).
By the chemically-induced PCC technique, chromosome spreads show different morphologies according to the presence or absence of the centromeric constriction and chromatids alignment (Lamadrid et al. Citation2007; Puig et al. Citation2013). In the present study two cell morphologies were observed, G2/M-PCC cells and M cells. Another morphology described in a previous work with low-LET radiation (Puig et al. Citation2013) was the M/A-PCC cells, without the centromere constriction and with the two chromatids separated. This cell type was not observed in the present study. In the study with low-LET radiation, G2/M-PCC and M cells proportions were clearly dose-dependent, whereas the proportion of M/A-PCC was constant, indicating that their presence could result from a methodological aspect linked to the use of Calyculin-A (Puig et al. Citation2013). A possible explanation for an absence of M/A-PCC cells in the present study is again methodological. Although Calyculin-A treatment was also performed, here isolated lymphocytes were stimulated to growth to obtain a monolayer of lymphocytes attached to the Mylar foil for irradiation, whereas in the previous study whole peripheral blood was irradiated and then cultured.
Irrespective of the cell type, the frequency of chromosome aberrations considered showed a clear increase with the dose. Among the chromosome aberrations recorded, chromosome rings showed the lowest frequency, and their frequency in M cells was clearly lower than in G2/M-PCC cells. In fact, rings were hardly found in M cells, being unobservable at most doses. The poor induction of chromosome rings when compared with the other chromosome aberrations is widely described, even after α-particle irradiation (Schmid et al. Citation1996; Barquinero et al. Citation2004). With Calyculin-A induced PCC and after exposures to mixed gamma-neutron radiation, a saturation has been described in the induction of chromosome rings at doses around 5 Gy with a frequency of 0.33 per cell (Lamadrid et al. Citation2011). However, another possible explanation is the absence of M/A-PCC cells in the present study; as was observed in our previous study, chromosome rings are much easier to detect when both chromatids are separated (Puig et al. Citation2013). On the other hand, extra fragments were detected at all doses, but in a similar way to rings, the frequency in G2/M-PCC cells was always significantly higher than in M cells. Higher frequencies of extra fragments in G2/M-PCC cells to those in M-cells were also observed after X- or γ-ray irradiation (Kovacs et al. Citation1994; Kanda et al. Citation2004; Puig et al. Citation2013). Using pan-telomeric and pan-centromeric PNA probes it was reported that the G2/M checkpoint negatively selects cells with incomplete chromosome aberrations (Rodríguez et al. Citation2009). When the cell distribution of extra fragments was considered a clear overdispersion was observed. However, it is interesting to note that whereas for G2/M-PCC cells the u values are relatively constant, for M cells the u values increased from 7.9 at 0.05 Gy to 40.4 at 0.2 Gy, and then decreased. This result seems to indicate that although the induction of extra fragments by α-particle irradiation results in a non-dose dependent overdispersion, the suitability of different damaged cells in entering mitosis modifies the distribution of extra acentric fragments in M cells.
The distribution of dicentric chromosomes in M cells showed an initial increase in overdispersion (between 0.05 and 0.1 Gy), followed by a gradual decrease up to 2.5 Gy. A similar variation in the overdispersion was also reported by Schmid et al. (Citation1996). The frequencies of dicentrics observed in the present study are similar to those reported previously using the same methodology of irradiation and the same source (Schmid et al. Citation1996; Barquinero et al. Citation2004; Schmid and Roos Citation2009), indicating the reproducibility of the method. This result indicates that the frequency of dicentrics observed after the conventional Colcemid treatment is similar to the one that can be observed in M cells after the co-treatment with Colcemid and Calyculin-A.
Regarding dose-effect relationships, the linear coefficient obtained for ring chromosomes (0.017 ± 0.02 Gy−1, the same for G2/M-PCC and for total cells) was lower than one reported for neutrons (0.059 ± 0.02 Gy−1) with a similar dose range (from 0–3.8 Gy) using the same methodology to induce PCC (Lamadrid et al. Citation2011). One explanation could be that α-particles are less efficient than 0.49 MeV neutrons in inducing chromosome rings. However, the linear coefficients obtained here are similar to that obtained by analyzing G2/M-PCC cells after γ-ray irradiation (0.019 ± 0.002 Gy−1) and clearly lower for total cells after γ-rays (0.027 ± 0.002 Gy−1) (Puig et al. Citation2013). If only G2/M-PCC cells are considered the low number of chromosome rings observed in the present study makes any comparison difficult. But when total cells are considered it is difficult to argue that α-particles are less efficient than γ-rays in producing chromosome rings. Probably the low linear coefficient obtained could be attributed to the absence of M/A-PCC cells. In this cell type chromosome rings are more easily detected (Puig et al. Citation2013). Another possible explanation for the lack of chromosome rings observed in the present study would be due to the irradiation conditions. In the present study lymphocytes were exposed to α-particles as monolayers of attached cells. It has been demonstrated that nucleus morphology in such situation is flattened in respect to settled lymphocytes that maintain a round shape morphology (Schmid et al. Citation2011). Taking into account that individual chromosomes occupy localized domains in the nucleus (Cremer and Cremer Citation2001), and that DSB occurring at the periphery of these chromosome domains will be more prone in producing chromosome interchanges than DSB occurring in the inner territory, which will tend to produce more chromosome intrachanges (Cremer et al. Citation1993). Irradiating flattened nucleus (i.e. enlarging chromosome territory surface) would result in a decrease of chromosome intrachanges such as chromosome rings. In fact, with the same device used here to irradiate with α-particles, Schmid et al. (Citation2011) described that the ratio between dicentric and ring chromosomes, the F-ratio, was clearly lower after irradiating settled lymphocytes than after irradiating attached lymphocytes, indicating a lesser induction of chromosome rings in relation to the induction of dicentric chromosomes. Taking into account that the proportion of M/A-PCC cells as well as the relationship between different types of radiation-induced chromosome aberrations could depend on some methodological aspects, if dose-effect curves for ring chromosomes using chemically-induced PCC are starting to be produced among biological dosimetry laboratories a harmonized protocol seems desirable.
A major effectiveness of α-particles with respect to low-LET radiation types, like γ-rays, in producing chromosome damage is clearly observed when dicentrics or extra chromosome pieces are considered. The linear coefficient for dicentrics chromosomes in M cells is 0.373 ± 0.074 Gy−1, slightly higher than the other two coefficients obtained using the same source and blocking division with a Colcemid treatment only (0.27 ± 0.02 Gy−1 and 0.29 ± 0.02 Gy−1 for Schmid et al. (Citation1996) and Barquinero et al. (Citation2004), respectively. The slight, but non-significant, increase could be due to the combined use of Colcemid and Calyculin A here used to obtain chromosome spreads. Other reported linear coefficients after α-particles irradiation are in the same range: 0.37 ± 0.02 Gy−1 from Purrott et al. (Citation1980), 0.29 ± 0.02 Gy−1 from Edwards et al. (Citation1980) and 0.24 ± 0.02 Gy−1 from Moquet et al. (Citation2001).
The linear coefficient for dicentrics is 17-fold higher than that obtained after γ-rays (0.021 + 0.005 Gy−1) (Barquinero et al. Citation1995) and also clearly higher than that obtained using PCC after γ-rays (Puig et al. Citation2013), 0.072 ± 0.008 Gy−1, where the dose range analyzed was from 0–20 Gy. Similarly, α-particles are also more efficient than γ-rays in producing extra chromosome fragments. For total cells the linear coefficient observed here (1.795 ± 0.021 Gy−1) is 4- to 5-fold higher than the other two obtained for γ-rays (0.393 ± 0.029 Gy−1 and 0.41 ± 0.03 Gy−1 by Puig et al. (Citation2013) and Balakrishnan et al. (Citation2010), respectively. It is interesting to note that although α-particles are more efficient in producing extra chromosome fragments than γ-rays, the G2/M checkpoint seems to reduce their presence in M cells to a similar proportion. After α-particle irradiation the linear coefficient in G2/M cells is 1.53 ± 0.02 Gy−1 5-fold higher than that obtained in M cells 0.30 ± 0.02 Gy−1. A similar reduction is observed after γ-rays from 0.41 ± 0.02 Gy−1 in G2/M cells to 0.07 ± 0.01 Gy−1 in M cells (Puig et al. Citation2013).
Considering that exchange type aberrations result from the misrepair of different DSB and that the initial damage after high-LET exposure, like the α-particles analyzed here, shows a higher degree of complexity (Brenner and Ward Citation1992) that results in an increase of the half-life of rejoining (Heilmann et al. Citation1993; Asaithamby et al. Citation2008), one would expect the presence of a quadratic component for some types of exchange type aberrations. A possible explanation for the absence of this quadratic component is the method used to analyze the aberrations. It it is not possible to distinguish between dicentrics or rings coming from simple or complex exchanges by analyzing solid stained chromosomes. Additionally, it has been described that after α-particle exposures, although the degree of complexity in the exchange type aberrations is higher than that observed after low-LET radiation types, the proportion of complex aberrations in respect to the total is constant with the dose (Barquinero et al. Citation2004), this would make the observation of a quadratic component difficult when all exchanges are considered together. It is interesting to note that in the study from George et al. (Citation2003b) for all the particles evaluated, whereas simple aberrations fitted to linear curves, when complex exchanges alone were considered the obtained curves fitted to a linear quadratic model.
In conclusion, when the proportion of PCC and M cells was compared, the results indicated that damaged cells after α-particle irradiation have more difficulties entering mitosis than cells exposed to γ-rays. After α-particle irradiation the frequency of all chromosome aberrations considered increased linearly with the dose. However, ring chromosomes were not very informative due to the smaller amounts observed in the two cells types analyzed, G2/M-PCC and M cells; dicentric chromosomes observed in M cells gave similar results to those obtained by the classical method to obtain metaphase spreads blocking cell division with Colcemid; and the frequency of extra chromosome pieces was clearly reduced in M cells with regard to G2/M-PCC cells. The amount of extra chromosome pieces observed in all cell types analyzed suggests that this type of aberration should be a good candidate to be used as a biomarker of exposure after high-LET radiation types if chemically-induced PCC techniques are used. However, in order to avoid possible methodological bias, standardization of the technique will be desirable.
Acknowledgements
The authors express their appreciation to Sabine Wenzel for her technical support and Ulrike Kulka for her considerations. This work received financial support from the Consejo de Seguridad Nuclear. The sponsor had no role in the study design, data collection, analysis or interpretation. M.R.C., L.B. and J.F.B. belong to a consolidated research group of the Generalitat de Catalunya (2014 SGR 354).
Disclosure statement
The authors report no conflicts of interest. The authors alone are response for the content and writing of the paper.
References
- Asaithamby A, Uematsu N, Chatterjee A, Story MD, Burma S, Chen DJ. 2008. Repair of HZE-particle-induced DNA double-strand breaks in normal human fibroblasts. Radiat Res. 169:437–446.
- Balakrishnan S, Shirsath K, Bhat N, Anjaria K. 2010. Biodosimetry for high dose accidental exposures by drug induced premature chromosome condensation (PCC) assay. Mutat Res. 699:1–2.
- Barquinero JF, Barrios L, Caballín MR, Miró R, Ribas M, Subias A, Egozcue J. 1995. Establishment and validation of a dose-effect curve for gamma-rays by cytogenetic analysis. Mutat Res. 326:65–69.
- Barquinero JF, Cigarrán S, Caballín MR, Braselmann H, Ribas M, Egozcue J, Barrios L. 1999. Comparison of X-ray dose-response curves obtained by chromosome painting using conventional and PAINT nomenclatures. Int J Radiat Biol. 75:1557–1566.
- Barquinero JF, Stephan G, Schmid E. 2004. Effect of americium-241 alpha-particles on the dose-response of chromosome aberrations in human lymphocytes analysed by fluorescence in situ hybridization. Int J Radiat Biol. 80:155–164.
- Brenner DJ, Ward JF. 1992. Constraints on energy deposition and target size of multiply damaged sites associated with DNA double-strand breaks. Int J Radiat Biol. 61:737–748.
- Cremer T, Kurz A, Zirbel R, Dietzel S, Rinke B, Schröck E, Speicher MR, Mathieu U, Jauch A, Emmerich P, Scherthan H, Ried T, Cremer C, Lichter P. 1993. Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harb Symp Quant Biol. 58:777–792.
- Cremer T, Cremer C. 2001. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2:292–301.
- Durante M, Furusawa Y, Gotoh E. 1998. A simple method for simultaneous interphase-metaphase chromosome analysis in biodosimetry. Int J Radiat Biol. 174: 457–462.
- Edwards AA, Purrott RJ, Prosser JS, Lloyd DC. 1980. The induction of chromosome aberrations in human lymphocytes by alpha-radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 38:83–91.
- George K, Wu H, Willingham V, Furusawa Y, Kawata T, Cucinotta FA. 2001. High- and low-LET induced chromosome damage in human lymphocytes: a time-course of aberrations in metaphase and interphase. Int J Radiat Biol. 77:175–183.
- George K, Durante M, Wu H, Willinghan V, Cucinotta FA. 2003a. In vivo and in vitro measurements of complex-type chromosomal exchanges induced by heavy ions. Adv Space Res. 31:1525–1535.
- George K, Durante M, Willingham V, Wu H, Yang TC, Cucinotta FA. 2003b. Biological effectiveness of accelerated particles for the induction of chromosome damage measured in metaphase and interphase human lymphocytes. Radiat Res. 160:425–435.
- Hayata I, Kanda R, Minamihisamatsu M, Furukawa M, Sasaki S. 2001. Cytogenetical dose estimation for 3 severely exposed patients in the JCO criticality accident in Tokai-Mura. J Radiat Res. 42:S149–S155.
- Heilmann J, Rink H, Taucher-Scholz G, Kraft G. 1993. DNA strand break induction and rejoining and cellular recovery in mammalian cells after heavy-ion irradiation. Radiat Res. 135:46–55.
- International Atomic Energy Agency (IAEA). 2011. Cytogenetic dosimetry: Applications in preparedness for and response to radiation emergencies. Vienna: IAEA.
- Jeggo PA, Löbrich M. 2006. Contribution of DNA repair and cell cycle checkpoints arrest to maintenance of genomic stability. DNA Repair. 5:1192–1198.
- Kanda R, Hayata I, Lloyd DC. 1999. Easy biodosimetry for high-dose radiation exposures using drug-induced, prematurely condensed chromosomes. Int J Radiat Biol. 75:441–446.
- Kanda R, Yamagishi Y, Hayata I. 2004. Sister chromatid exchanges in ring chromosomes following X-irradiation of human lymphocytes. Int J Radiat Biol. 80:363–368.
- Kovacs MS, Evans JW, Johnstone IM, Brown JM. 1994. Radiation-induced damage, repair and exchange formation in different chromosomes of human fibroblasts determined by fluorescence in situ hybridization. Radiat Res. 137:34–43.
- Lamadrid AI, García O, Delbos M, Voisin P, Roy L. 2007. PCC-ring induction in human lymphocytes exposed to gamma and neutron irradiation. J Radiat Res. 48:1–6.
- Lamadrid AI, González JE, García O, Voisin P, Roy L. 2011. Prematurely condensed chromosome rings after neutron irradiation of human lymphocytes. J Radiat Res. 52:531–535.
- Lee R, Nasonova E, Hartel C, Durante M, Ritter S. 2011. Chromosome aberration measurements in mitotic and G2-PCC lymphocytes at the standard sampling time of 48 h underestimate the effectiveness of high-LET particles. Radiat Environ Biophys. 50:371–381.
- Lindholm C, Stricklin D, Jaworska A, Koivistoinen A, Paile W, Arvidsson E, Deperas-Standylo J, Wojcik A. 2010. Premature chromosome condensation (PCC) assay for dose assessment in mass casualty accidents. Radiat Res. 173:71–78.
- Lücke-Huhle C, Hiebert L, Wegner RD. 1983. Caffeine-mediated release of alpha-radiation-induced G2 arrest increases the yield of chromosome aberrations. Int J Radiat Biol. 43:123–132.
- Moquet JE, Fernández JL, Edwards AA, Lloyd DC. 2001. Lymphocyte chromosomal aberrations and their complexity induced in vitro by plutonium-239 alpha-particles and detected by FISH. Cell Mol Biol (Noisy-le-grand). 47:549–556.
- Niida H, Nakanishi M. 2006. DNA damage checkpoints in mammals. Mutagenesis. 21:3–9.
- Pantelias GE, Maillie HD. 1983. A simple method for premature chromosome condensation induction in primary human and rodent cells using polyethylene glycol. Somat Cell Genet. 9:533–547.
- Papworth D. 1975. Curve fitting by maximum likelihood. Appendix to paper by J.K.R. Savage. Radiation induced chromosomal aberrations in the plant Tradescantia. Dose-response curves. Radiat Bot. 15:81.
- Puig R, Barrios L, Pujol M, Caballín MR, Barquinero JF. 2013. Suitability of scoring PCC rings and fragments for dose assessment after high-dose exposures to ionizing radiation. Mutat Res. 757:1–7.
- Purrott RJ, Edwards AA, Lloyd DC, Stather JW. 1980. The induction of chromosome aberrations in human lymphocytes by in vitro irradiation with alpha-particles from plutonium-239. Int J Radiat Biol Relat Stud Phys Chem Med. 38:277–284.
- Regulla D, Schmid E, Friedland W, Panzer W, Heinzmann U, Harder D. 2002. Enhanced values of the RBE and H ratio for cytogenetic effects induced by secondary electrons from an X-irradiated gold surface. Radiat Environ Biophys. 158:505–515.
- Ritter S, Nasonova E, Furusawa Y, Ando K. 2002. Relationship between aberration yield and mitotic delay in human lymphocytes exposed to 200 MeV/u Fe-ions or X-rays. J Radiat Res. 43:S175–S179.
- Rodríguez P, Barquinero FJ, Duran A, Caballín MR, Ribas M, Barrios L. 2009. Cells bearing chromosome aberrations lacking one telomere are selectively blocked at the G2/M checkpoint. Mutat Res. 670:53–58.
- Roos H, Kellerer AM. 1989. Design criteria and performance parameters of and alpha-irradiation device for cell studies. Phys Med Biol. 34:1823–1832.
- Schmid E, Hieber L, Heinzmann U, Roos H. 1996. Analysis of chromosome aberrations in human peripheral lymphocytes induced by in vitro alpha-particle irradiation. Radiat Environ Biophys. 35:179–184.
- Schmid E, Roos H. 2009. Influence of the bystander phenomenon on the chromosome aberration pattern in human lymphocytes induced by in vitro α-particle exposure. Radiat Environ Biophys. 48:181–187.
- Schmid TE, Oestreicher U, Molls M, Schmid E. 2011. Alpha particles induce different F values in monocellular layers of settled and attached human lymphocytes. Radiat Res. 176:226–233.
- Wang ZZ, Li WJ, Zhi DJ, Jing XG, Wei W, Gao QX, Liu B. 2007. Biodosimetry estimate for high-LET irradiation. Radiat Eviron Biophys. 46:229–235.
- Yao Y, Li Y, Liu G, Guo M, Bai J, Man Q, Qiu L, Ai H. 2013. Estimation of the biological dose received by five victims of a radiation accident using three different cytogenetic tools. Mutat Res. 751:66–72.
