Abstract
Purpose: Recently, new studies have brought to light the potential risks of low dose radiation (LDR) in cancer. In this review, we discuss in detail the detrimental effects of LDR in some model organisms and animal models, as well as potential risks to human beings from some routine medical screening procedures. Furthermore, cellular mechanisms by which LDR exerts its negative effects like endoplasmic reticulum stress, epigenetic changes and microRNAs are also reviewed. A few studies are discussed that have reported some benefits of LDR through changes in energy metabolism. Lastly, we focus on breast cancer, one of the predominant forms of cancer potentially affected by LDR and some of the benefits of n-3 polyunsaturated fatty acids (PUFA) as dietary compounds that offer protection against radiation effects on cancer cells and cancer progression.
Conclusions: Overall, LDR exerts mainly damaging effects through diverse cell and molecular mechanisms, with a few beneficial effects reported. In some cancers, surrounding adipose tissue of the breast may contribute to obesity-related cancer. Further, preclinical data suggest that anti-inflammatory dietary compounds such as PUFA and other dietary interventions may protect against radiation effects on cancer cells and cancer progression.
Introduction
Cancer is anticipated to cause over 609,000 deaths this year in the United States alone (Siegel et al. Citation2018). Radionuclides and radiation were added to the list of carcinogens by the national toxicology program in 2005 (Siemiatycki et al. Citation2004). This review summarizes the different types of radiation and focuses explicitly on low dose radiation (LDR) and studies performed in vitro and in vivo animals and human models over the last two decades for the first portion of the review. The remaining portion focuses on breast cancer as its diagnosis includes multiple LDR scans that pose potential health risks and the protective effects of n-3 polyunsaturated fatty acids (PUFA) in breast cancer treatment.
Types of radiation
Radiation is generally classified as either non-ionizing or ionizing based on the energy of the emitted particles. Non-ionizing radiation includes light and radio waves. Ionizing radiation is further classified as direct, or indirect with highly charged particles, electrons, protons and alpha particles being examples of direct ionizing radiation. X-rays and gamma rays are examples of indirect ionizing radiation as they interact with atoms causing ejection of secondary beta particles. Both direct and indirect ionizing types of radiation are used for treating/diagnosing malignant and non-malignant tumors. The purposes to which they are put primarily depend on the absorbed dose in Gray (Gy) or its equivalent dose in Sievert (Sv).
Low dose radiation
LDR is useful in diagnosing diseases through procedures such as computed tomography (CT) scans, which are critical in discovering potential cancers. Currently, more than 62 million scans are performed annually in the United States, including 4 million CT scans in children. More importantly, data published in 2009, showed that the number of scans to detect cancer had drastically increased, by 22-fold in the past three decades (Mettler et al. Citation2009). The dose of radiation in an average CT scan ranges from .03-.1 Gy. LDR was considered relatively harmless for decades; however, recent studies like that of Little et al. have raised concerns about risks to individuals exposed to LDR. LDR does not kill cells outright but may cause genomic alterations; and initiate cancer. A single procedure that utilizes LDR may be beneficial, but repeated doses increase risks due to the cumulative detrimental effects of radiation evident from various studies of atomic bomb survivors, nuclear workers and patients who receive repeated LDR (Little et al. Citation2009).
Detrimental effects of LDR
Humans, animals and in vitro studies using clonal cells have been performed to understand the effects of LDR. Most studies have found them harmful, but a few have demonstrated potential benefits. Most estimates of LDR have simply been extrapolated from epidemiological studies on subjects who received higher doses of radiation. Such extrapolations utilized a linear non-threshold (LNT) model and concluded that LDR possess risks similar to those of high dose radiation. Furthermore, these extrapolation models were based on assessing the totality of biological data, which does not hold well for LDR since the LNT hypothesis is based on the premise that damaging stochastic effects like cancer risk increase as a linear threshold function of absorbed radiation dose (Sokolov and Neumann Citation2014). Such a direct relationship, if accurate, implies that doubling the dose will also double the risk – a major pitfall of the LNT model (Sanders Citation2010). Finally, LNT models only consider total absorbed dose and not dose rates. Hence, these models are questionable and likely to underestimate the risk of LDR, especially in mammography studies as shown by a particularly elegant study (Heyes et al. Citation2009). The study demonstrated that extrapolated values are in reality different from the actual amount of absorbed LDR. The exact amount of radiation emitted by X-rays and absorbed by different organs was measured and found to be approximately .1289 Gy for the eye, and .0489 Gy for the thyroid, both lower than those predicted by extrapolation studies. These differences could perhaps be attributed to variations in the duration of radiation and the specific machine used for CT scan (Mettler et al. Citation2008; Kanagaraj et al. Citation2015). Hence, extrapolating epidemiological studies based on higher radiation doses may introduce serious errors in assessing the safety of LDR, and it is time for a new paradigm in the assessment of risks associated with LDR (Scott Citation2008). Despite extensive studies, it remains unclear whether LDR is harmful because of a lack of direct means to assess or understand its effects (Mullenders et al. Citation2009; Mobbs et al. Citation2011). It is harder to pinpoint if LDR causes cancer because the lag time between radiation exposure and diagnosis of cancer is at least 5 years (Berrington de Gonzalez et al. Citation2009). Long-term controlled dosimetry studies to determine the exact dose of harmful radiation could answer these critical questions, but they are not practical in humans, who would have to be studied over periods of many years.
LDR in human studies
The seriousness of the detrimental effects of LDR was revealed by a recent study (Abe et al. Citation2015) which reported chromosome cleavage by dicentric chromosome formation, a more sensitive measure of DNA damage. They found that even a single CT scan could cause chromosome cleavage, establishing the sensitivity of the technique and the harmful effects of LDR. The study described above clearly demonstrates that LDR may be detrimental, but the exact minimum dose of radiation that may have negative effects is still unclear. This is further complicated by long-term extensive epidemiological studies based on self-reported or claims data. Dosimetry studies in animals, however, can be structured to provide better-controlled measurements and more reliable conclusions than human epidemiological studies, as discussed below.
Exposure to LDR at a young age is more risky than in older age (adult) because children undergo rapid cell division, making them more sensitive to radiation than adults (IARC Citation2000). This risk was confirmed by a retrospective study which found that children who received ∼ .05 Gy radiation had a three times greater risk of developing leukemia and brain tumors (Pearce et al. Citation2012). Likewise, another study showed that children receiving .062 Gy irradiation on the scalp had significantly higher thyroid cancer risk compared to controls (Ron et al. Citation1989).
Several human studies have also demonstrated the deleterious effects of LDR in adults. One found that effects of cumulative radiation occur at higher rates in women than in men, and that accumulation increases with age, possibly due to routine mammograms to detect breast cancer (Gargani and Picano Citation2015). According to another survey (Doody et al. Citation2000), women exposed to routine radiological examination for tuberculosis at an average dose of .108 Sv had a higher risk of breast cancer later in life. Similar effects were observed with lower doses ranging from .01-.09 Sv (Doody et al. Citation2000), suggesting that even lower-energy X-rays in routine diagnostic mammography, when carried out repeatedly, could cause damage. Along similar lines, data from death certificates of 531 workers who died from leukemia shows that 6% of those deaths could be attributed to radiation (Abbott Citation2015). Moreover, a study was performed using Poisson regression on 308,297 radiation workers who were exposed to a very low dose rate (1.1 mGy per year), this study showed a relative excessive risk of leukemia mortality of 2.96 per Gy (excluding chronic lymphocytic leukemia) (Leuraud et al. Citation2015). However, none of the studies were well controlled for radiation dose, and all were dependent on subjective, self-reported data. Studies performed using data from a large number of nuclear workers indicate a significant association between LDR and all-cause mortality (Cardis et al. Citation2007). All of these studies point toward the existence of deleterious effects due to LDR and the lack of well-controlled investigations.
In vivo animal studies
Mice exposed to both low (.075 Gy) and high doses (1.8 Gy) have alterations in genes much like those found in human cancer patients; in fact, one study identified 36 differentially expressed genes that were similar in both cancer patients and mice exposed to high dose radiation (Snijders et al. Citation2012). Interestingly, different pathways were affected by low vs. high doses, further confirming that the effects of high dose radiation could not be extrapolated to LDR (Snijders et al. Citation2012). In another study by Guo et al., mice were exposed to radiation ranging from .002 to .25 Gy daily for 1 month and were observed either immediately (acute exposure) or 3 months (chronic exposure) after irradiation. Doses over .01 Gy damaged the hematopoietic cells in these mice in a dose-dependent manner compared to their controls. These authors found that even the skeletal muscle stem cells had reduced proliferative capacity after LDR exposure. Their study suggested that LDR not only has adverse effects, but its effects vary on different tissues (Antosh et al. Citation2014; Guo et al. Citation2015; Masuda et al. Citation2015).
Beneficial effects of LDR
LDR, apart from causing harmful effects has also been shown to produce a few beneficial effects in some studies on smaller organisms such as Drosophila melanogaster and in animal and human studies. Drosophila demonstrated the adaptive response known as hormesis when exposed to LDR (Moskalev et al. Citation2011). When exposed to LDR in the range of .05-.4 Gy, lifespan increased compared to non-irradiated flies (Zhikrevetskaya et al. Citation2015). This study was consistent with other studies in Drosophila, which demonstrated beneficial effects of radiation and identified upregulation of genes such as Sirtuins 2, Jun kinases and others involved in the adaptive response to radiation (Moskalev et al. Citation2011). Furthermore, a genome-wide study conducted in flies, exposed to .8 Gy identified 39 differentially expressed genes that extended lifespan (Seong et al. Citation2011). The pathways that increased life span were related to protein synthesis and energy metabolism. Since the flies have a short life-span, it is worth noting that these adaptive responses could be transient. Furthermore, the exact amounts of absorbed energy were not known and had not been determined in Drosophila (Antosh et al. Citation2014). Although they were able to observe a threshold effect on the lifespan of flies, caution must be exercised when interpreting these effects in Drosophila or translating them to humans. Beneficial effects of LDR were also observed in a mouse study performed by Yoshida et al. (Citation1993). They reported that when mice were irradiated with ∼.05-.1 Gy, an adaptive response was triggered and mice became resistant to higher dose radiation. Further, the spleen of these mice had a higher capacity to repair itself as they were primed with LDR before receiving a high radiation dose (Sykes et al. Citation2006). Other studies have identified factors including polyadenylation, DNA binding protein and immune function as responsible factors for the adaptive response to radiation in mice (Wiencke et al. Citation1986; Ikushima Citation1987; Shadley et al. Citation1987).
A few mice studies have indicated that LDR may be beneficial in cancer, diabetes or kidney damage (Zhang et al. Citation2011). Additionally, even some human trials have shown positive effects of LDR in diabetes, arthritis and rheumatic diseases (Yamaoka and Komoto Citation1996; Falkenbach et al. Citation2005).
Mechanisms of LDR
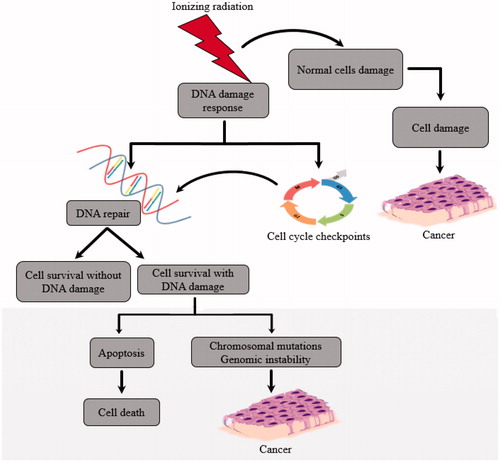
Under normal circumstances, ionizing radiation interacts with biological cells which are made up of 70% water and produces hydroxyl radicals in the body that might promote DNA damage (NRC Citation1990). Fortunately, most cells usually repair DNA if the damage is limited. However, all cells are not equally sensitive to radiation damage. When ionizing radiation strikes the nucleus of human or animal cells, DNA damage occurs by double strand breaks, potentially leading to uncontrolled cell replication and ultimately cancer (). 1 Gy of radiation results in approximately 20-40 double-strand breaks (Lomax et al. Citation2013). However, it is important to note that any number of DNA double-strand breaks is harmful (Lomax et al. Citation2013). When ionizing radiation interacts with water molecules in the body, it causes them to decompose into hydrogen (H) and hydroxide (OH) radicals, which are then converted to hydrogen peroxide thereby producing reactive oxygen species (ROS). ROS reduce the effectiveness of radiation, making tumor cells two or three times more resistant than healthy cells to radiation damage (Tominaga et al. Citation2004; Lomax et al. Citation2013). ROS produced by LDR promotes tumor progression by inducing genes such as hypoxia-inducible factor 1-alpha (Hif1A), a tumor promoter (Lall et al. Citation2014). Also, ROS promotes inflammation as seen by induction of genes such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), an inflammatory marker in chondrocytes.
The genetic responses to LDR have been determined by studies involving unbiased global profiling methods, such as microarray and transcriptome profiling, which identified key genes modified by LDR (Kruse et al. Citation2004). The altered genes are involved in immunological processes, (Luzhna and Kovalchuk Citation2014), cell-mediated cytotoxicity and chemokine signalling, indicating potential damages caused by ionizing radiation. Furthermore, LDR also downregulates fatty acid metabolism (Luzhna and Kovalchuk Citation2014). Additional studies using gene expression profiling showed induction of tumorigenesis and obesity with LDR in liver (Uehara et al. Citation2010). Deleterious genetic/genomic consequences of radiation were also observed in a microarray profiling, which showed upregulation of vascular injury-related genes in kidney (Kruse et al. Citation2004).
Additional mechanisms found to mediate the effects of radiation in cell culture studies includes triggering of senescence which could be dependent on a prior induction of autophagy (Alessio et al. Citation2015). Human mesenchymal stem cells (HMSC) exposed to .04-2 Gy were rendered senescent; the authors showed that there was a significant effect of LDR exposure on autophagic flux and its reduction contributed to inducing senescence (Alessio et al. Citation2015). This was further corroborated by results from lymphoblastic cells irradiated with 1 Gy, which induced apoptosis. LDR has also been shown to influence apoptosis by regulating miRNAs (microRNAs), small non-coding RNAs (consisting of about 22 nucleotides) that are capable of regulating metabolic diseases and cancers via RNA degradation or translation inhibition. LDR induced the expression levels of miRNAs including miR-144, miR-200a, miR-598 and miR 650, that are involved in regulating apoptotic genes such as BBC3, TP53I3, ZMAT3, caspase-3 and FDXR (Cha et al. Citation2009; Girardi et al. Citation2012). Mice exposed to .01 Gy of LDR with 2 Gy of subsequent gamma irradiation had alterations in apoptosis genes such as Bal1, Birc2 and Birc3 in the liver (Gridley et al. Citation2013).
Another interesting mechanism identified was the inherited epigenetic changes which alter the function of the genome by alterations in DNA methylation and also by post translational histone modifications (Ma et al. Citation2010). These epigenetic changes could be caused by radiation, one of the best examples of which was the Chernobyl accident which showed dose dependent DNA methylation resulting in genomic instability (Kovalchuk and Baulch Citation2008). Similar results were also obtained from plant and animal studies with radiation causing DNA hypomethylation which could be a potential contributor to genomic instability (Pogribny et al. Citation2004, Citation2005). However, comprehensive studies linking radiation to epigenetics are scarce, and this could open a whole new array of treatment options once it is deciphered.
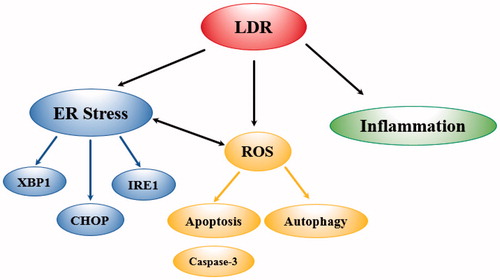
Another mechanism which has been proposed to mediate cellular effects of radiation is endoplasmic reticulum (ER) stress (Wang et al. Citation2013). The ER is found mostly in eukaryotic cells, maintaining normal functions and cell survival (Xu et al. Citation2005). Unfolded or misfolded proteins disturbed calcium ion homeostasis, nutrition deprivation, DNA damage or virus infection are associated with ER stress (Wang et al. Citation2013). Indeed, ER stress was shown to be involved in apoptosis of testicular cells induced by LDR (Wang et al. Citation2013). Mice exposed to LDR ranging from .015-.2 Gy for ∼1 h showed alterations in ER stress-related genes in testicular cells, with the highest number of gene alterations at doses from .1 to .2 Gy (Wang et al. Citation2013). The primary genes altered included IRE1, XBP1, PERK, Eif2 and ATF6. Increased Chop expression by LDR also indicated the presence of apoptosis. ER stress increases ROS and disturbs calcium homeostasis, which could induce apoptosis. The mechanisms of action are summarized in . These findings indicate the importance of ER stress and calcium homeostasis in LDR-induced apoptosis suggesting potential benefits of therapeutic approaches targeting ER stress when radiation is used. LDR effects are quite evident with routine multiple scans for diagnosis of breast cancer which is the focus in the next part of this review.
Figure 2. Schematic Illustration of Radiation Mechanisms That Might Contribute to Breast Cancer Development. Endoplasmic Reticulum stress (ER stress). Reactive Oxygen Species (ROS). Genes altered (IRE1 (the ribonuclease inositol-requiring protein-1), XBP1 (X-Box-binging protein), CHOP (the transcription factor C/EBP homologous protein).
LDR and breast cancer
Breast cancer has been identified as the second leading cause of mortality in women after heart disease. It accounted for 29% of all newly diagnosed cancers in women in 2016 (Siegel et al. Citation2016). It has a multiplicity of causal factors/agents, among which are obesity, socioeconomic status, genetics and radiation. Women over age 40 receive X-ray radiation during routine mammogram screening to diagnose early-stage breast cancer. According to the American Cancer Society, each image requires an exposure (dose) in the amount of .001-.002 Gy to the breast and may be affected by factors such as the X-ray instrument output and breast size. The benefit of the mammogram procedure is regarded to outweigh the probability of developing cancer from the radiation exposure. While some experts do not consider low dose X-ray radiation from mammograms sufficient to increase the risk for breast cancer, it is nonetheless well accepted that due to its stochastic effects, there can be no threshold for a safe dose of LDR (Apostolova and Paskalev Citation2001). Indeed, a National Research Council (NRC) report warned that “the risk of cancer proceeds in a linear fashion at lower doses of ionizing radiation without a threshold and the smallest dose has the potential to cause a small increase in cancer risk to humans” (National Research Council Citation2006). It has also been suggested that cumulative radiation effects on DNA damage occur over a lifetime and repeated low-dose exposures might have the same harmful effects as a single high-dose exposure (Preston et al. Citation2002). Furthermore, the projected breast cancer risk in women is almost double that of men who have undergone chest X-rays (Smith-Bindman et al. Citation2009). More importantly, a higher incidence of breast cancer has been observed in women who received X-rays for the diagnosis of spinal cord disorders and tuberculosis, or treatment of Hodgkin’s disease (Howe and McLaughlin Citation1996; Preston et al. Citation2002). These findings were validated by a systemic review which analyzed 11 retrospective studies and 3 case-controlled studies and concluded that chest irradiation received, mainly by women, increased the risk of breast cancer at a young age and continued to have long-term effects compared to the general population (Henderson et al. Citation2010). These women received irradiation mostly as a part of treatment for other cancers such as lymphoma, leukemia or bone cancer.
Novel role of adipose tissue in breast cancer
The breast tissue contains different types of cells including epithelial, glandular and adipose or fat cells. Radiologists and physicians estimate the risk of radiation in the breast based on the amount of absorbed dose in the glandular tissue. However, the breast also contains adipose tissue that varies depending on the women’s body mass index (BMI) and should be taken into consideration. There is evidence of the influential role of adipose tissue in inducing breast cancer (Ligibel Citation2011). People with higher BMI or who are suffering from obesity are more susceptible to higher radiation doses from exposure to radiation for either diagnostic or treatment purposes. Radiation, apart from targeting cancerous cells, also affects adjacent cells and damages noncancerous cells. Accordingly, the probability of the adipose tissue in the breast being affected is high since the breast has a dense network of adipose tissue. Given the high content of adipose tissue within the breast, especially in obese women, it is critical to assess the effects of mammography X-ray exposure on adipocytes and how irradiated adipocytes alter other breast tissue cell types, possibly via secreted cytokines and hormones. Furthermore, radiating the adipose tissue may indirectly affect other breast cells through direct cell to cell communication or factors secreted by adipose tissue, which may affect normal epithelial or cancerous cells and vice versa. Factors secreted by adipose tissue in response to radiation include inflammatory cytokines, levels of which are higher following ionizing radiation (Siriwardhana Citation2012). The inflammatory cytokines then stimulate a cascade of events that damage surrounding tissues and organs (Siriwardhana Citation2012). Because irradiation causes inflammation, and adipose tissue is well established as an endocrine tissue that secretes hormones and other inflammatory molecules, irradiated adipocytes may increase breast cancer cell growth via this mechanism. One study has addressed the sensitivity of adipose tissue to radiation (Poglio et al. Citation2009), but it was conducted for acute exposure. The risk of LDR has been studied in the breast, but to our knowledge, no studies to date have addressed effects of LDR on adipose tissue surrounding breast cancer micro-tumors. Only a few studies have focused on breast epithelial cells (Soler et al. Citation2009; Hernandez et al. Citation2013).
Modeling radiation exposure: Monte Carlo method
The interaction of ionizing particles (photons, electrons, protons, neutrons or heavy ions) with atoms in cells is random. When an organism or tissue is irradiated, the number of particles striking electrons or the nucleus inside the atoms that compose the cells is very large. Radiation transport models such as the discrete ordinates method (DOM), finite volume method (FVM), spherical harmonics approximation (SHA), zonal method and deterministic approaches solve the radiation transport equation and obtain average particle behavior. The stochastic nature and extremely large numbers of particle interactions involved, however, are especially well-suited to probabilistic Monte Carlo methods and random sampling-based codes such as MCNPX, MCNP6, EGS4, Geant4, PENELOPE or FLUKA that simulate radiation interaction with matter by the use of mathematical modelling and use repeated random sampling to solve the Boltzmann transport equation, and thus evaluate the average behavior (interactions) of the ionizing particles. In each simulation, millions of random histories (ionizing particles) are used (Goorley et al. Citation2013); each history (ionizing particle) is tracked in the region of interest (cells or tissues) and its interactions with atoms are based on probability theory; each interaction results in partial energy deposition of the primary particle or its secondary (secondary particles result from the interaction of the primary particle with matter, generating photons, electrons, proton, neutrons or fragments). Once the primary particles or their secondary particles emerge from the region, the partial deposition of energies is summed and another history is created. The final results are the sums of the energy deposition from all histories (particles and its secondary) normalized to the number of histories used. The Monte Carlo codes have proven to be a very useful technique to derive energy deposition in tissue (dose) from low and high energy ionized particles passing through it. Information obtained by this method has provided data needed for epidemiological studies to improve the various risk models introduced in the Biological Effects of Ionizing Radiation (BEIR V and VII) reports (Benevides Citation2005; National Research Council Citation2006).
Monte Carlo transport codes are the most accurate methods for estimating dose distributions in radiotherapy. Their usefulness in clinical applications is, however, hampered by limited computer power and consequently long run-times (Ziegenhein et al. Citation2015). Each Monte Carlo code contains trade-offs (e.g. the storage limitations for MCNPX code, its input line limitation and so on) that limit its application in particular situations, so users must choose the one best suited to solve the problem at hand (Pelowitz Citation2011).
Minimizing the risks of radiation
Performing imaging only when clinically relevant or essential for diagnosis could reduce the risks associated with radiation exposures. Information regarding previous scans/images should be verified to prevent undue imaging and radiation accumulation, an important consideration made clear by a renal colic imaging study that identified exposures of up to .05 Sv of cumulative radiation in 3 years (Stein et al. Citation2010). Even more unsafe is multiscan CT imaging, where the organ is scanned from different angles, resulting in radiation exposure up to 4 times higher than a regular CT, an indication that routine multiscan CT imaging should be performed only in extenuating circumstances (Smith-Bindman et al. Citation2009). Other factors such as patient weight must be considered for imaging studies. Obese versus lean women should be screened with different radiation doses. Also, pregnant women should be handled with extreme caution as the fetus may be affected by the radiation.
Polyunsaturated fatty acids/chemoprevention, radiation and cancer
Several chemo-preventive approaches have been used in conjunction with major cancer therapy, such as dietary bioactive compounds, antioxidant diets and supplements used in a few clinical trials. PUFAs are considered essential fatty acids as humans are unable to synthesize them (Anderson and Ma Citation2009). PUFA are subdivided into two major groups (n-3 and n-6) based on the position of the first double bond from the methyl end of the chain. N-6 PUFAs include arachidonic acid (AA), while n-3 PUFAs include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Predominant sources of n-3 PUFAs include fish and vegetable oils such as safflower and sunflower oil. PUFAs are also found in flaxseed, walnuts and fish, especially trout, salmon and herring (AHA Citation2015). The western diet is abundant with n-6, but deficient in n-3 PUFAs, with current intake in a ratio of about 16:1, respectively, while the ideal ratio is 2-3:1 (Simopoulos Citation2002). Furthermore, n-3 PUFAs impart cardiovascular benefits by lowering triglycerides and improving insulin sensitivity (Simopoulos Citation2002). n-6 PUFAs have demonstrated tumorigenic properties, while n-3 PUFAs have anti-cancer properties. Increased consumption of fish or n-3 PUFAs has been extensively shown to prevent breast cancer (Mandal et al. Citation2010), but protective qualities they may have in reducing the harmful effects of radiation exposure have not been well studied. The use of n-3 PUFAs was proposed as an accessory to cancer therapies as it could increase the therapeutic effect of radiation therapy, demonstrated by increasing radio-sensitivity in both in vitro and in vivo models (Cai et al. Citation2014) as well as in human colorectal cancer radiotherapy as an nutritional adjuvant (Benais-Pont et al. Citation2006). N-3 PUFAs increase tumor sensitivity to anticancer drugs and irradiation by increasing the rate of apoptosis of cancer cells (Conklin Citation2002). Furthermore, the cytotoxic action of n-3 PUFA is assumed to be mediated mainly via the generation of free radicals, and lipid peroxidation since n-3 PUFA incorporation into tumor cells leads to an increased potential for oxidative stress and raises the susceptibility of the cells to therapies generating ROS (Vartak et al. Citation1997).
A few animal studies have explored the protective effects on PUFAs concerning irradiation ( and ). Injury of the gastrointestinal (GI) tract caused by irradiation reduces villi to crypt height ratio and increases pro-inflammatory cytokines in the GI tract (Sun et al. Citation2014). PUFAs are known to protect against radiation-induced GI disorders such as ulcerative colitis or short bowel syndrome by reducing inflammation (Sun et al. Citation2014). In the study by Sun et al., mice were supplemented with or without PUFAs (Omegaven) and then subjected to 4 Gy/min rate of irradiation. They also exhibited increased villi to crypt ratio depth and reduced mortality rates. The authors suggested the possible mechanisms are through reduced inflammation and lipid peroxidation, as indicated by observed higher levels of superoxide dismutase. n-3 PUFAs have been found to inhibit the migration of metastatic breast cancer cells (Mandal et al. Citation2010). Furthermore, PUFA supplementation to mice implanted with clonal breast cancer cells MDA-MB-231 brought about significant decreases in tumor volume and weight compared to control animals (Wu et al. Citation2005). Another study tested the effects of n-3 PUFA enriched diet on human breast cancer xenograft growth and angiogenesis with and without radiation in mice (Hardman et al. Citation2005); the study showed that incorporating n-3 into the diet increased production of anti-oxidant enzymes in normal cells, thereby protecting them from radiation damage. Furthermore, n-3 protected the bone marrow and the small intestine (Hardman et al. Citation2005). A few clinical trials have been conducted with n-3 PUFAs (Pilkington et al. Citation2013). An interesting role of n-3 PUFAs was identified in mitigating the effects of ultraviolet (UV) low dose radiation exposure (Pilkington et al. Citation2013). The experimental data suggest that n-3 PUFA might protect against skin cancer by suppressing cell-mediated immunity, reducing tumor multiplicity and raising tumor latency (Pilkington et al. Citation2013). PUFA supplementation has been shown to improve chemotherapy and radiation efficacy. However, these effects were dependent on whether PUFAs were administrated in combination as fish oil or as single n-3 fatty acid (Wynter et al. Citation2004). These effects are due to changes in membrane lipid fluidity and increases in lipid peroxidation (Corsetto et al. Citation2017).
Table 1. Effects of n-3 PUFAs in animals exposed to radiation.
Table 2. Mechanisms of n-3 PUFAs in different types of cells exposed to radiation.
Conclusion
LDR has long been considered safe as it involves such a relatively small amount of radiation but recent discoveries have thrown light on its harmful effects. The risk of cancer due to radiation depends on the type of cancer, age, sex, magnitude and duration of the dose to a particular organ, and the presence of other carcinogens and promoters interacting with the effects of radiation. People develop cancers when exposed to similar doses of radiation depending on their unique individual characteristics. Our current level of understanding is still insufficient to predict the exact outcomes of LDR in irradiated cells, but scientists have begun to identify the intracellular processes initiated by radiation and the epigenetic changes that may result in cancer. Even though radiation exposure can contribute to cancer development and damage normal adjacent tissues, it is still the cornerstone of cancer diagnosis. Nonetheless, during radiation exposure, other tissues or cells in the irradiated organ must be considered. Specifically, in obese breast cancer patients, adipose tissue plays a major role in cancer development through secretion of inflammatory markers. Hence additional studies are required in that area. LDR could also affect other types of cancers since some of the cellular molecules affected are involved in other diseases such as diabetes. Thus, such investigations could lead to better diagnosis of other cancers and prevention of other diseases as well.
Apart from harmful effects, increasing evidence has suggested that LDR could also induce some beneficial effects such as extended life span, enhanced immunity and improved DNA repair. Hence this might also be helpful in some cases of chemotherapy.
Dietary interventions using n-3 PUFA might be a helpful approach in protecting normal cells from radiation damage. Although several studies have highlighted the role of n-3 PUFA in enhancing chemotherapy and patient’s survival, there are several factors that must be taken into consideration before incorporating n-3 PUFA as part of clinical practices. These factors include concentration of n-3 PUFA in combination as fish oil or as individual single fatty acids (EPA or DHA), duration of supplementation, type of chemotherapeutic agent given to the patient with n-3 PUFA and individual patient variability with n-3 PUFA interventions.
Notes on contributors
Al Maqsudur Rashid is a Ph.D. student of Mechanical Engineering at Texas Tech University. He has a Bachelor and Masters in Mechanical Engineering. His research interests are radiation dosimetry, radiation and cancer, simulation of radiation transport and modelling.
Dr. Latha Ramalingam is a Research Assistant Professor of Nutritional Sciences at Texas Tech University. Her research interest is to investigate effects of bioactive compounds in maternal obesity. She has a Bachelor in Pharmacy, M.S. in Biotechnology, Ph.D. in Biochemistry and Molecular Biology, and a postdoc in obesity and diabetes research.
Arwa Al-Jawadi’s doctoral research was on dissecting the relationship between obesity and breast cancer. She has a Bachelor in Medical Engineering, and both M.S. and Ph.D. in Nutritional Sciences from Texas Tech University.
Dr. Naima Moustaid-Moussa is Professor in Nutritional Sciences and Founding Director of the Obesity Research Cluster at Texas Tech University. Her research program focuses on the role of adipose tissue expansion and inflammation in obesity and related diseases including breast cancer and diabetes; and the mechanisms mediating protective effects of botanicals and dietary bioactive compounds in reducing obesity-associated inflammation. She has a Bachelor in Cell Biology and Physiology, an M.S. and Ph.D. in Endocrinology and a postdoc in molecular nutrition with more than 25 years of independent academic research devoted to the areas of nutrient-gene interactions, adipocyte biology, obesity and related diseases.
Dr. Hanna Moussa is an Assistant Professor of Mechanical Engineering at Texas Tech University. His research interests are mainly focused on radiation safety/control, dosimetry, risk assessment and radiation transport modelling and the relation between low dose radiation and cancer, especially breast cancer; and radiotherapy in cancer. He has a Bachelor’s in Health Physics, a Master’s in Radiological Sciences and Ph.D. in Nuclear and Radiological Engineering with extensive experience in health and medical physics and radiation protection.
Disclosure statement
The authors reported no potential conflict of interest
Additional information
Funding
References
- Abbott A. 2015. Researchers pin down risks of low-dose radiation. Nature. 523:17–18.
- Abe Y, Miura T, Yoshida MA, Ujiie R, Kurosu Y, Kato N, Katafuchi A, Tsuyama N, Ohba T, Inamasu T, Shishido F, Noji H, Ogawa K, Yokouchi H, Kanazawa K, Ishida T, Muto S, Ohsugi J, Suzuki H, Ishikawa T, Kamiya K, Sakai A. 2015. Increase in dicentric chromosome formation after a single CT scan in adults. Sci Rep. 5:13882.
- AHA. 2015. Polyunsaturated Fats. [accessed 2018 April 2] https://healthyforgood.heart.org/Eat-smart/Articles/Polyunsaturated-Fats.
- Alessio N, Del Gaudio S, Capasso S, Di Bernardo G, Cappabianca S, Cipollaro M, Peluso G, Galderisi U. 2015. Low dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process. Oncotarget. 6:8155–8166.
- Anderson BM, Ma DW. 2009. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 8:33
- Antosh M, Fox D, Hasselbacher T, Lanou R, Neretti N, Cooper LN. 2014. Drosophila melanogaster show a threshold effect in response to radiation. Dose Response. 12:551–581.
- Apostolova D, Paskalev Z. 2001. Ionizing radiation used in medical diagnostics as a source of radiation exposure of the patient with occupational diseases. Analysis and problems. International Conference for Radiological Protection of Patients in Diagnostic and Interventional Radiology, Nuclear Medicine and Radiotherapy. Vienna, Austria: International Atomic Energy Agency (IAEA). p. 35. https://inis.iaea.org/search/search.aspx?orig_q=RN:32039821.
- Benais-Pont G, Dupertuis YM, Kossovsky MP, Nouet P, Allal AS, Buchegger F, Pichard C. 2006. ω-3 Polyunsaturated fatty acids and ionizing radiation: combined cytotoxicity on human colorectal adenocarcinoma cells. Nutrition. 22:931–939.
- Benevides L. 2005. Breast dosimetry in clinical mammography. A dissertation presented to the graduate school of the University of Florida in partial fulfillment of the requirements for the degree of doctor of philosophy. University of Florida.
- Berrington de Gonzalez AM, Mahesh KP, Kim M, Bhargavan R, Lewis F, Mettler C. Land 2009. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 169:2071–2077.
- Cai F, Sorg O, Granci V, Lecumberri E, Miralbell R, Dupertuis YM, Pichard C. 2014. Interaction of ω-3 polyunsaturated fatty acids with radiation therapy in two different colorectal cancer cell lines. Clin Nutr. 33:164–170.
- Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Schubauer-Berigan M, et al. 2007. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: Estimates of radiation-related cancer risks. Radiat Res. 167:361–416.
- Cha HJ, Shin S, Yoo H, Lee E-M, Bae S, Yang K-H, Lee S-J, Park I-C, Jin Y-W, An S. 2009. Identification of ionizing radiation-responsive microRNAs in the IM9 human B lymphoblastic cell line. Int J Oncol. 34:1661.
- Conklin KA. 2002. Dietary polyunsaturated fatty acids: impact on cancer chemotherapy and radiation. Altern Med Rev. 7:4–21.
- Corsetto PA, Colombo I, Kopecka J, Rizzo AM, Riganti C. 2017. Omega-3 long chain polyunsaturated fatty acids as sensitizing agents and multidrug resistance revertants in cancer therapy. Ijms. 18:2770.
- Doody MM, Lonstein JE, Stovall M, Hacker DG, Luckyanov N, Land CE. 2000. Breast cancer mortality after diagnostic radiography: findings from the US Scoliosis Cohort Study. Spine. 25:2052–2063.
- Falkenbach A, Kovacs J, Franke A, Jörgens K, Ammer K. 2005. Radon therapy for the treatment of rheumatic diseases–review and meta-analysis of controlled clinical trials. Rheumatol Int. 25:205–210.
- Gargani L, Picano E. 2015. The risk of cumulative radiation exposure in chest imaging and the advantage of bedside ultrasound. Crit Ultrasound J. 7:4.
- Girardi C, De Pitta C, Casara S, Sales G, Lanfranchi G, Celotti L, Mognato M. 2012. Analysis of miRNA and mRNA expression profiles highlights alterations in ionizing radiation response of human lymphocytes under modeled microgravity. Plos One. 7:e31293.
- Goorley J, T M, James T, Booth F, Brown J, Bull L, Cox A. Zukaitis 2013. MCNP6 User's Manual. Los Alamos, New Mexico: Los Alamos National Laboratory, Manual LA-CP-13-00634.
- Gridley DS, Mao XW, Cao JD, Bayeta EJM, Pecaut MJ. 2013. Protracted low-dose radiation priming and response of liver to acute gamma and proton radiation. Free Radic Res. 47:811–820.
- Guo C-Y, Luo L, Urata Y, Goto S, Huang W-J, Takamura S, Hayashi F, Doi H, Kitajima Y, Ono Y, et al. 2015. Sensitivity and dose dependency of radiation-induced injury in hematopoietic stem/progenitor cells in mice. Sci Rep. 5:8055.
- Hardman WE, Sun L, Short N, Cameron IL. 2005. Dietary omega-3 fatty acids and ionizing irradiation on human breast cancer xenograft growth and angiogenesis. Cancer Cell Int. 5:12.
- Henderson TO, Amsterdam A, Bhatia S, Hudson MM, Meadows AT, Neglia JP, Diller LR, Constine LS, Smith RA, et al. 2010. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 152:444–455. W144-454.
- Hernandez L, Terradas M, Martin M, Feijoo P, Soler D, Tusell L, Genesca A. 2013. Increased mammogram-induced DNA damage in mammary epithelial cells aged in vitro. PLoS One. 8:e63052.
- Heyes GJ, Mill AJ, Charles MW. 2009. Mammography-oncogenecity at low doses. J Radiol Prot. 29:A123–A132.
- Howe GR, McLaughlin J. 1996. Breast cancer mortality between 1950 and 1987 after exposure to fractionated moderate-dose-rate ionizing radiation in the Canadian fluoroscopy cohort study and a comparison with breast cancer mortality in the atomic bomb survivors study. Radiat Res. 145:694–707.
- IARC 2000. Ionizing Radiation Part 1: X-ray, and Gamma-radiation, and Neutron. International Agency for Research on Cancer (Monographs on the Evaluation of the Carcinogenic Risk to Humans). 75.
- Ikushima T. 1987. Chromosomal responses to ionizing radiation reminiscent of and adaptive response in cultured Chinese hamster cells. Mutat Res Fundam Mol Mech Mutag. 180:215–221.
- Kanagaraj K, Abdul Syed Basheerudeen S, G TS, M.T J, Ozhimuthu A, S PS, Pattan S, Perumal V. 2015. Assessment of dose and DNA damages in individuals exposed to low dose and low dose rate ionizing radiations during computed tomography imaging. Mutat Res Genet Toxicol Environ Mutag. 789-790:1–6.
- Kovalchuk O, Baulch JE. 2008. Epigenetic changes and nontargeted radiation effects-is there a link? Environ Mol Mutag. 49:16.
- Kruse JJ, te Poele JA, Russell NS, Boersma LJ, Stewart FA. 2004. Microarray analysis to identify molecular mechanisms of radiation-induced microvascular damage in normal tissues. Int J Radiat Oncol Biol Phys. 58:420–426.
- Lall R, Ganapathy S, Yang M, Xiao S, Xu T, Su H, Shadfan M, Asara JM, Ha CS, Ben-Sahra I, et al. 2014. Low-dose radiation exposure induces a HIF-1-mediated adaptive and protective metabolic response. Cell Death Differ. 21:836–844.
- Leuraud K, Richardson DB, Cardis E, Daniels RD, Gillies M, O’Hagan JA, Hamra GB, Haylock R, Laurier D, Moissonnier M, Schubauer-Berigan MK, Thierry-Chef I, Kesminiene A. 2015. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2:e405–E281.
- Ligibel J. 2011. Obesity and breast cancer. Oncology (Williston Park, N.Y.) 25:994.
- Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington de Gonzalez A. 2009. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology. 251:6–12.
- Lomax M, Folkes L, O’Neill P. 2013. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol. 25:578–585.
- Luzhna L, Kovalchuk O. 2014. Low dose irradiation profoundly affects transcriptome and microRNAme in rat mammary gland tissues. Oncoscience. 1:751–762.
- Ma S, Liu X, Jiao B, Yang Y, Liu X. 2010. Low-dose radiation-induced responses: focusing on epigenetic regulation. Int J Radiat Biol. 86:517–528.
- Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N. 2010. Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun. 402:602–607.
- Masuda S, Hisamatsu T, Seko D, Urata Y, Goto S, Li TS, Ono Y. 2015. Time‐and dose‐dependent effects of total‐body ionizing radiation on muscle stem cells. Physiol Rep. 3:e12377.
- Mettler FA, Jr., Thomadsen BR, Bhargavan M, Gilley DB, Gray JE, Lipoti JA, McCrohan J, Yoshizumi TT, Mahesh M. 2008. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 95:502–507.
- Mettler FA, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG, et al. 2009. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources—1950–2007. Radiology. 253:520–531.
- Mobbs SF, Muirhead CR, Harrison JD. 2011. Risks from ionising radiation: an HPA viewpoint paper for Safegrounds. J Radiol Prot. 31:289–307.
- Moskalev AA, Plyusnina EN, Shaposhnikov MV. 2011. Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: the role of cellular stress-resistance mechanisms. Biogerontology. 12:253–263.
- Mullenders L, Atkinson M, Paretzke H, Sabatier L, Bouffler S. 2009. Assessing cancer risks of low-dose radiation. Nat Rev Cancer. 9:596–604.
- Siriwardhana N, Layman R, Karwandyar A. 2012. Inflammatory cytokines link obesity and breast cancer. J Metabolic Synd. 01:1–6.
- National Research Council. 2006. Committee to assess health risks from exposure to low levels of ionizing radiation, health risks from exposure to low levels of ionizing radiation (BEIR VII Phase 2). Washington, DC: National Academies Press, Date 16: 406.
- NRC (National research council). 1990. BEIR V: Health effects of exposure to low levels of ionizing radiation. Washington, DC: The National Academies Press. www.nap.edu.
- Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, et al. 2012. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 380:499–505.
- Pelowitz DB. 2011. MCNPX user manual, version 2.7.0. Los Alamos National Laboratory report LA-CP011-00438.
- Pilkington SM, Massey KA, Bennett SP, Al-Aasswad NM, Roshdy K, Gibbs NK, Friedmann PS, Nicolaou A, Rhodes LE. 2013. Randomized controlled trial of oral omega-3 PUFA in solar-simulated radiation-induced suppression of human cutaneous immune responses. Am J Clin Nutr. 97:646–652.
- Poglio S, Galvani S, Bour S, Andre M, Prunet-Marcassus B, Penicaud L, Casteilla L, Cousin B. 2009. Adipose tissue sensitivity to radiation exposure. Am J Pathol. 174:44–53.
- Pogribny I, Koturbash I, Tryndyak V, Hudson D, Stevenson SM, Sedelnikova O, Bonner W, Kovalchuk O. 2005. Fractionated low-dose radiation exposure leads to accumulation of DNA damage and profound alterations in DNA and histone methylation in the murine thymus. Mol Cancer Res. 3:553–561.
- Pogribny I, Raiche J, Slovack M, Kovalchuk O. 2004. Dose-dependence, sex-and tissue-specificity, and persistence of radiation-induced genomic DNA methylation changes. Biochem Biophys Res Commun. 320:1253–1261.
- Preston DL, Mattsson A, Holmberg E, Shore R, Hildreth NG, Boice JD, Jr. 2002. Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat Res. 158:220–235.
- Ron E, Modan B, Preston D, Alfandary E, Stovall M, Boice JD. Jr 1989. Thyroid neoplasia following low-dose radiation in childhood. Radiat Res. 120:516–531.
- Sanders CL. 2010. Radiation Hormesis and the Linear-No-Threshold Assumption. Radiation Hormesis and the Linear-No-Threshold Assumption. Berlin Heidelberg, New York: Springer-Verlag. 1–217.
- Scott BR. 2008. It’s time for a new low-dose-radiation risk assessment paradigm-one that acknowledges hormesis. Dose Response. 6:333–351.
- Seong KM, Kim CS, Seo SW, Jeon HY, Lee BS, Nam SY, Yang KH, Kim JY, Kim CS, Min KJ, Jin YW. 2011. Genome-wide analysis of low-dose irradiated male Drosophila melanogaster with extended longevity. Biogerontology. 12:93–107.
- Shadley JD, Afzal V, Wolff S. 1987. Characterization of the adaptive response to ionizing radiation induced by low doses of X rays to human lymphocytes. Radiat Res. 111:511–517.
- Siegel RL, Miller KD, Jemal A. 2016. Cancer statistics, 2016. CA Cancer J Clin. 66:7–30.
- Siegel RL, Miller KD, Jemal A. 2018. Cancer statistics, 2018. CA Cancer J Clin. 68:7.
- Siemiatycki J, Richardson L, Straif K, Latreille B, Lakhani R, Campbell S, Rousseau MC, Boffetta P. 2004. Listing occupational carcinogens. Environ Health Perspect. 112:1447–1459.
- Simopoulos AP. 2002. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 56:365–379.
- Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, de Gonzalez AB, Miglioretti DL. 2009. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 169:2078–2086.
- Snijders AM, Marchetti F, Bhatnagar S, Duru N, Han J, Hu Z, Mao J-H, Gray JW, Wyrobek AJ. 2012. Genetic differences in transcript responses to low-dose ionizing radiation identify tissue functions associated with breast cancer susceptibility. PloS One. 7:e45394.
- Sokolov M, Neumann R. 2014. Effects of low doses of ionizing radiation exposures on stress-responsive gene expression in human embryonic stem cells. Int J Mol Sci. 15:588–604.
- Soler D, Pampalona J, Tusell L, Genescà A. 2009. Radiation sensitivity increases with proliferation-associated telomere dysfunction in nontransformed human epithelial cells. Aging Cell. 8:414–425.
- Stein EG, Haramati LB, Bellin E, Ashton L, Mitsopoulos G, Schoenfeld A, Amis ES. 2010. Radiation exposure from medical imaging in patients with chronic and recurrent conditions. J Am Coll Radiol. 7:351–359.
- Sun M, Pang L, Ju X, Sun H, Yu J, Zhao H, Yao W, Wei M. 2014. Attenuating effects of omega-3 fatty acids (Omegaven) on irradiation-induced intestinal injury in mice. Food Chem Toxicol. 64:275–280.
- Sykes PJ, Day TK, Swinburne SJ, Lane JM, Morley AA, Hooker AM, Bhat M. 2006. In vivo mutagenic effect of very low dose radiation. Dose-Response. 4:309–316.
- Tominaga H, Kodama S, Matsuda N, Suzuki K, Watanabe M. 2004. Involvement of reactive oxygen species (ROS) in the induction of genetic instability by radiation. J Radiat Res. 45:181–188.
- Uehara Y, Ito Y, Taki K, Nenoi M, Ichinohe K, Nakamura S, Tanaka S, Oghiso Y, Tanaka K, Matsumoto T, et al. 2010. Gene expression profiles in mouse liver after long-term low-dose-rate irradiation with gamma rays. Radiat Res. 174:611–617.
- Vartak S, Robbins ME, Spector AA. 1997. Polyunsaturated fatty acids increase the sensitivity of 36B10 rat astrocytoma cells to radiation-induced cell kill. Lipids. 32:283–292.
- Wang ZC, Wang JF, Li YB, Guo CX, Liu Y, Fang F, Gong SL. 2013. Involvement of endoplasmic reticulum stress in apoptosis of testicular cells induced by low-dose radiation. J Huazhong Univ Sci Technol [Med Sci]. 33:551–558.
- Wiencke JK, Afzal V, Olivieri G, Wolff S. 1986. Evidence that the [3H] thymidine-induced adaptive response of human lymphocytes to subsequent doses of X-rays involves the induction of a chromosomal repair mechanism. Mutagenesis. 1:375–380.
- Wu M, Harvey KA, Ruzmetov N, Welch ZR, Sech L, Jackson K, Stillwell W, Zaloga GP, Siddiqui RA. 2005. Omega‐3 polyunsaturated fatty acids attenuate breast cancer growth through activation of a neutral sphingomyelinase‐mediated pathway. Int J Cancer. 117:340–348.
- Wynter MP, Russell ST, Tisdale MJ. 2004. Effect of n-3 fatty acids on the antitumour effects of cytotoxic drugs. In Vivo. 18:543–547.
- Xu C, Bailly-Maitre B, Reed JC. 2005. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 115:2656–2664.
- Yamaoka K, Komoto Y. 1996. Experimental study of alleviation of hypertension, diabetes and pain by radon inhalation. Physiol Chem Phys Med Nmr. 28:1–5.
- Yoshida N, Imada H, Kunugita N, Norimura T. 1993. Low dose radiation-induced adaptive survival response in mouse spleen T-lymphocytes in vivo. J Radiat Res. 34:269–276.
- Zhang C, Jin S, Guo W, Li C, Li X, Rane MJ, Wang G, Cai L. 2011. Attenuation of diabetes-induced cardiac inflammation and pathological remodeling by low-dose radiation. Radiat Res. 175:307–321.
- Zhikrevetskaya S, Peregudova D, Danilov A, Plyusnina E, Krasnov G, Dmitriev A, Kudryavtseva A, Shaposhnikov M, Moskalev A. 2015. Effect of low doses (5-40 cGy) of gamma-irradiation on lifespan and stress-related genes expression profile in Drosophila melanogaster. Plos One. 10:e0133840.
- Ziegenhein P, Pirner S, Ph Kamerling C, Oelfke U. 2015. Fast CPU-based Monte Carlo simulation for radiotherapy dose calculation. Phys Med Biol. 60:6097.