Abstract
Purpose: The application of radiation therapy (RT) is not only used to treat cancer, in Germany, it is also an accepted and empirically established treatment of patients with benign diseases at low doses. The immune modulatory response generated by low-dose RT has a supporting anti-inflammatory effect within the treatment of inflammation-related diseases. The aim of this study was to investigate the effect of ionizing radiation (IR) on the expression and secretion of inflammatory mediators by endothelial cells (ECs) exposed to low and moderate doses.
Methods: Non-activated and activated EC were irradiated with doses between 0.01 Gy and 2 Gy with X-rays. Using a multiplex-assay, protein values of interleukin-8 (IL-8), granulocyte macrophage colony-stimulating factor (G-CSF) and platelet-derived growth factor (PDGF-BB) were measured in the supernatant at different time-points. To investigate possible differences between mRNA expression and protein secretion after IR, the mRNA expression of IL-8, G-CSF and PDGF-BB was determined by real-time quantitative PCR.
Results: Radiation treatment caused non-linear dose dependent effects on pro-inflammatory cytokine secretion of IL-8; G-CSF and PDGF-BB. The mRNA-expression levels of those cytokines were non-linear dose-dependent and differed from protein level in the culture supernatant.
Conclusions: This study provides deeper insights into the radiobiological effects of radiation doses below 0.3 Gy, in particular 0.05 Gy, and their significant immunomodulatory properties on EC, which is very important in order to assess the effect of LD-IR on EC.
Purpose
The application of radiation therapy (RT) is not only used to treat cancer, in Germany it is also an accepted and empirically established treatment of patients with benign diseases (Seegenschmiedt et al. Citation2004) at low doses. High and moderate doses of ionizing radiation (IR) are known to cause acute tissue injuries, organ failure or increase the risk of vascular diseases along with DNA strand breaks or programmed mitotic cell death, which is documented by several epidemiological (Azizova et al. Citation2010; Stewart et al. Citation2010) and experimental (Fajardo and Stewart Citation1970; Schultz-Hector et al. Citation1992; Hallahan et al. Citation1996) studies. The immune modulatory response generated by low-dose RT (LD-RT) has a supporting anti-inflammatory effect within the treatment of inflammation-related diseases.
LD-RT not only influences cells of the innate immune system, but also modulates the function of endothelial cells (ECs) (Seegenschmiedt and Micke Citation2012). These cells are sensitive for a variety of stimuli like IR or inflammatory markers as well, and therefore a potential target in RT. EC play a central role in inflammatory processes by recruiting leukocytes to the side of inflammation and, once activated by pro-inflammatory lipopolysaccharide (LPS) or tumor necrosis factor-alpha (TNF-α), are a source of a wide range of cytokines, chemokines, growth factors and adhesion molecules (Mantovani et al. Citation1992; Krishnaswamy et al. Citation1999; Sato Citation2001; Speyer and Ward Citation2011). Therefore, many studies examined the role of EC during inflammation in vitro and in vivo. Rödel et al. showed a biphasic activation of nuclear factor kappa B (NF-κB), one of the most important regulators of pro-inflammatory gene expression, and a discontinuous expression of the X chromosome-linked inhibitor of apoptosis (XIAP) after exposure to low-dose ionizing radiation (LD-IR) (Rödel et al. Citation2004, Citation2010). The enhanced expression of anti-inflammatory TGF-β1, a decreased E-selectin expression and a significantly reduced adhesion of leukocytes to EC after exposure to LD-IR are just a few of many observed effects in the range of 0.3–0.7 Gy (Kern et al. Citation2000; Hildebrandt et al. Citation2002; Roedel et al. Citation2002). On the basis of these and other studies, LD-IR seems to have anti-inflammatory properties. Further research, regarding the influence of IR below doses of 0.5 Gy, is necessary for understanding the immune modulating properties of radiation on cells of the immune system like EC.
The aim of this study was to investigate the effect of IR on the expression and secretion of inflammatory mediators by EC exposed to low and moderate doses of X-rays. There is a wide range of primary and immortalized EC lines which can be studied in vitro. Two common EC types used in the present study are the EA.hy926 cell line, derived from the fusion of human umbilical vein endothelial cells (HUVECs) with the adenocarcinoma cell line A549/8 (Edgell et al. Citation1983), and primary HUVEC. Endothelial cells were irradiated with doses of 0.01 Gy up to 2 Gy. The protein values of interleukin-8 (IL-8), granulocyte macrophage colony-stimulating factor (G-CSF) and platelet-derived growth factor (PDGF-BB) were measured in the supernatant by multiplex-assay at different time-points. To investigate possible differences between mRNA expression and protein secretion, the mRNA of IL-8, G-CSF and PDGF-BB was determined by real-time quantitative PCR (qRT-PCR). The present results will expand on the current knowledge about the immune modulatory response of EC after IR with doses in a range of very low (0.01 Gy) up to moderate doses (2 Gy).
Materials and methods
Endothelial cell culture
The experiments were performed with primary HUVECs and EA.hy926 cells. The HUVEC were obtained from PromoCell (Heidelberg, Germany) and cultured in Endothelial Cell Basal Medium (ECBM – PromoCell GmbH, Heidelberg, Germany) containing 2% fetal bovine serum (FBS), 0.1 ng/ml epidermal growth factor (EGF), 1 ng/ml basic fibroblast growth factor (bFGF), 90 µg/ml heparin and 1 µg/ml hydrocortisone. The EA.hy926 cells, derived from the fusion of HUVEC with a thioguanine-resistant clone of A549, were obtained from the American Type Culture Collection (ATCC, Manassas, VA). EA.hy926 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Lonza, Cologne, Germany) supplemented with 10% FBS (Merck Millipore, Darmstadt, Germany), 100 U/ml penicillin and 100 µl/ml streptomycin (Sigma-Aldrich, Hamburg, Germany) in 75 cm2 flasks at 37 °C and 5% CO2.
TNF-α stimulation
The cells were activated with TNF-α (R&D Systems, Wiesbaden, Germany) before IR to simulate an inflammatory environment and stimulate the secretion of inflammatory markers. Therefore, the cell medium was replaced 24 hours after seeding by serum-free medium with or without supplementation of 10 ng/ml TNF-α two hours before IR.
Ionizing radiation
EC were irradiated 26 hours after seeding with X-rays using an Xstrahl 200 therapy system (Xstrahl Ltd., Surrey, UK; dose rate 0.52 Gy/min; energy 200 kV) at room temperature. The irradiation was carried out with the following doses: 0.01 Gy, 0.05 Gy, 0.1 Gy, 0.3 Gy and 0.5 Gy. Cells irradiated with 2 Gy served as positive control. Sham irradiated samples (0 Gy) were kept at room temperature in the X-ray control room during irradiation. Throughout this paper, the following definitions for the radiation doses will apply as following:
Low doses – 0.01 and 0.05 Gy;
Medium doses – 0.1, 0.3 and 0.5 Gy;
High dose – 2 Gy.
Analysis of cytokine secretion
Sample collection for cytokine measurement
Cells (1 × 104) were seeded initially in 24-well plates and cultivated to confluence for 24 hours under standard conditions. The medium was replaced by serum-reduced medium with or without addition of TNF-α two hours before IR. Subsequently cells were exposed to radiation using doses ranged from 0.05 Gy to 2 Gy. Supernatants were collected 30 minutes, two hours, four hours, 24 hours and 48 hours after radiation procedure to examine a time-dependent secretion of inflammatory cytokines and stored at –80 °C until further measurement.
Measurement of inflammatory cytokines
Cytokine levels of 27 different inflammatory markers (IL-1β; IL-1ra; IL-2; IL-4; IL-5; IL-6; IL-7; IL-8; IL-9; IL-10; IL-12(p70); IL-13; IL-17; eotaxin; bFGF; G-CSF; GM-CSF; IFN-γ; IP-10; MCP-1 (MCAF); MIP-1α; IL-15; MIP-1β; PDGF-BB; RANTES; TNF-α; VEGF) were quantified (data not shown) in supernatants harvested from EC partially stimulated with TNF-α by using a 27-plex human cytokine/chemokine kit from BioRad Laboratories GmbH (Munich, Germany) according to the manufacturer protocol by using 50 µl of supernatant and performing the assay at room temperature. After measurement, three cytokines with the most promising changes in cytokine secretion levels were re-examined in more detail: IL-8, G-CSF and PDGF-BB with a customized x-plex human cytokine/chemokine kit (Bio-Rad Laboratories GmbH, Hercules, CA). Data were acquired using the Bio-Plex® 200 suspension array system and analyzed with the Bio-Plex Manager™ Software (version 4.1).
Analysis of mRNA expression
RNA-isolation
Total RNA from 5 × 105 cells was isolated 30 minutes, two hours, four hours, 24 hours and 48 hours post-IR from HUVEC and EA.hy926 by using the NucleoSpin® RNA kit (Macherey-Nagel, Duren, Germany) according to manufacturer’s protocol. The purity of the isolated RNA was verified using the Eppendorf BioPhotometer plus (Eppendorf AG, Hamburg, Germany) with 260/280 and 260/230 ratios as well as real-time PCR with RNA as template to proof the lack of genomic DNA contaminations causing false positive results during amplification.
Reverse transcription
Less than 1 µg of total RNA was reverse transcribed into cDNA using the RevertAid First Strand cDNA Synthesis Kit (Fermentas/Thermo Scientific, Schwerte, Germany) following manufacturer’s instructions.
Quantitative real-time PCR
The cDNA was used afterwards in a 20 µl real-time PCR containing TaqMan® Universal PCR Master mix and TaqMan® Gene Expression Assays for IL-8 (Hs00174103_m1), granulocyte-colony stimulating factor (G-CSF – Hs00738432_g1), PDGF-BB (Hs00966522_m1), as well as glyceraldehyde-3-phosphate dehydrogenase (GAPDH – Hs02758991_g1; Life Technologies, Darmstadt, Germany) as an internal control and 40 ng of cDNA as template. Quantitative real-time PCR was carried out using the 7300 Real Time PCR System (Applied Biosystems®, Life Technologies, Darmstadt, Germany). All reactions were performed in triplicates. The relative quantification of the mRNA expression was performed using the Delta Delta CT (2–ΔΔCT) method (comparative CT method).
Statistical analysis
All data are presented as means ± standard deviation (SD) on the basis of at least three independently performed experiments. The statistical significance of differences was assessed by Student’s t-test for measurements of viability and cytokine secretion. A value of p < .05 was considered as statistically significant. Using the one-sample t test, the statistical significance was calculated for the mRNA expression, therefore a value of p < .02 indicated a statistically significant difference.
Results
Effect of LD-IR on cytokine secretion
An initial screening of cytokines should ascertain which of the 27 inflammatory markers were secreted by EC with and without IR as well as treatment with TNF-α or not. From these 27 measured markers, 22 were secreted diversely in EA.hy926 or HUVEC so a direct comparison of primary cells with the cell line was not possible, the concentrations were below the detection limit of the assay or not detectable at all. Three out of 27 cytokines showed significant changes in cytokine secretion in both EC kinds after IR compared to non-irradiated and non-TNF-α treated control samples and therefore were examined more closely: IL-8, G-CSF and PDGF-BB ().
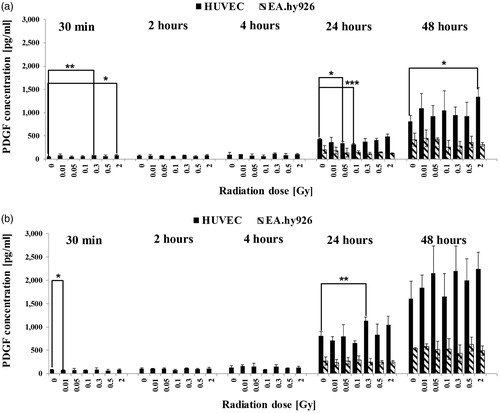
Figure 1. Released levels of the interleukin-8 (IL-8) in supernatant without TNF-α induction (a) and with TNF-α induction (b). The cytokine concentration was determined by multiplex assay at five time points after irradiation with low doses of X-rays. Changes in cytokine concentrations are presented as mean (pg/ml)±standard deviation (SD) from three independent experiments; Asterisks illustrate significance: *p < .05, **p < .01, ***p < .001.
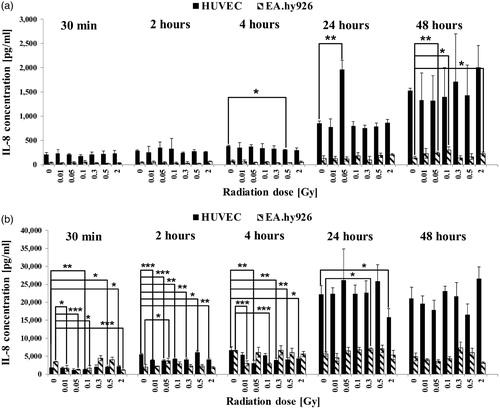
Figure 2. Released levels of granulocyte macrophage colony-stimulating factor (G-CSF) in supernatant without TNF-α induction (a) and with TNF-α induction (b). The cytokine concentration was determined by multiplex assay at five time points after irradiation with low doses of X-rays. Changes in cytokine concentrations are presented as mean (pg/ml)±standard deviation (SD) from three independent experiments; Asterisks illustrate significance: *p < .05, **p < .01, ***p < .001.
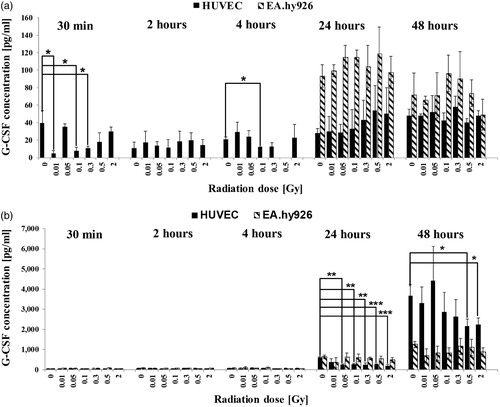
Figure 3. Released levels of platelet-derived growth factor (PDGF-BB) in supernatant without TNF-α induction (a) and with TNF-α induction (b). The cytokine concentration was determined by multiplex assay at five time points after irradiation with low doses of X-rays. Changes in cytokine concentrations are presented as mean (pg/ml)±standard deviation (SD) from three independent experiments; Asterisks illustrate significance: *p < .05, **p < .01, ***p < .001.
Effect of LD-IR on secretion of IL-8
Interleukin-8 is a cytokine with a pro-angiogenetic and anti-apoptotic effect on a variety of cells like monocytes or EC and promotes the migration of EC into the extracellular matrix (ECM).
The secretion of IL-8 from EC into the supernatant was determined by using a multiplex analysis. In both EC kinds, IL-8 was detected at all tested time points (). In unstimulated primary HUVEC, the IL-8 concentration remained almost at the same level at the early time points 30 min to four hours and increased to a maximum of 2005 pg/ml 48 hours after IR with significant changes at four hours and 24 hours (). In EA.hy926, the IL-8 concentration at the early time points remained between 24 and 70 pg/ml and increased up to 300 pg/ml significant IL-8 concentrations 48 hours after IR but always remained up to 6.6-fold lower compared to primary EC. The changes in cytokine level were discontinuously dose-dependent. If cells were activated before IR, the IL-8 concentrations increased up to 13-fold (26,609 pg/ml) in primary HUVEC 24 hours after IR compared to non-activated samples with significant dose-dependent changes 30 min to 24 hours after IR (). In EA.hy926 cells, the activation before IR resulted in a 24-fold (7431 pg/ml) increase of IL-8 level in the supernatant compared to non-activated samples 24 hours after IR with significant dose-dependent changes from 30 min to 24 hours but remained up to 3.6-fold lower compared to primary EC.
Effect of LD-IR on secretion of G-CSF
G-CSF is a growth factor, which is synthesized by EC in response to pro-inflammatory cytokines and stimulates the generation of granulocytes during inflammation.
Unstimulated primary HUVEC secreted G-CSF at all tested time points with a slight increase in concentration 24 hours and 48 hours after IR and significant dose-dependent lower levels 30 min and four hours post-IR (). In unstimulated EA.hy926 cells, G-CSF was only detectable 24 hours and 48 hours after IR whereas the maximum concentration was measured at 24 hours. EA.hy926 secreted G-CSF 2-fold higher compared to primary HUVEC. The activation of HUVEC before LD-RT resulted in an increase of G-CSF in the supernatant, notably 24 hours and 48 hours after IR with a 73-fold higher concentration at 48 hours compared to non-activated samples (). Significant lower concentrations were detected 30 min and 24 hours after IR compared to the 0 Gy control at this time point as well as at 48 hours. The G-CSF levels were higher in primary HUVEC compared to the EC line. When EA.hy926 cells were stimulated with TNF-α before IR, the concentration of G-CSF was elevated approximately 10-fold at 48 hours.
G-CSF was detectable at the early time points 30 min to four hours after IR as well as later time points 24 hours and 48 hours, but with no significant dose-dependent change in cytokine level.
Effect of LD-IR on secretion of PDGF-BB
PDGF-BB is a homo-dimeric protein involved in cell proliferation, migration, wound healing or angiogenesis.
In the present study, the concentration of PDGF-BB in the culture supernatant of non-activated HUVEC ranged from 53 pg/ml to 120 pg/ml at the early time points 30 min to four hour and increased to a maximum of 1340 pg/ml 48 hours after IR (). Significant PDGF-BB changes were detected 30 min as well as 24 hours and 48 hours post-IR. Non-activated EA.hy926 cell secreted PDGF-BB in detectable concentrations from 120 pg/ml to 450 pg/ml only at the late time points 24 hours and 48 hours after IR, but the concentrations were in average threefold lower compared to HUVEC (). The activation of the cells with TNF-α before LD-IR resulted in an enhanced secretion of the cytokine in HUVEC at all tested time points (). At earlier time points from 30 min to four hours, a slight increase in concentration was detectable compared to non-activated samples whereas 24 hours and 48 hours the concentrations were in average twofold higher compared to non-activated samples with significant changes 30 min and 24 hours after IR. Activated EA.hy926 cells secreted PDGF-BB also merely 24 hours and 48 hours after IR in approximately 1.5-fold higher concentrations compared to non-activated samples (). In both, the non-activated and activated levels, HUVECs secreted PDGF-BB in higher concentrations compared to EA.hy926.
Effect of low-dose IR on IL-8, G-CSF and PDGF-BB mRNA expression
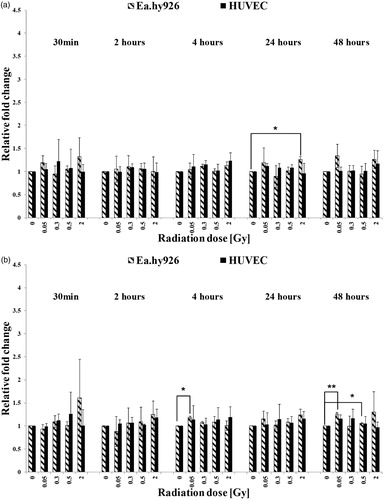
In general, the mRNA-expression levels were non-linear dose-dependent and differed from protein level in the culture supernatant (). HUVEC with no TNF-α activation before IR expressed mRNA coding for IL-8 at each tested time point in a biphasic non-dose-dependent pattern (). A significant increased expression was detected 24 hours at 0.5 Gy following IR. Once activated, HUVEC expressed IL-8 mRNA in no distinctive higher level compared to non-activated samples with significant decreased at 24 hours with 0.5 Gy. Non-activated EA.hy926 also expressed IL-8 mRNA at each tested time point with significant higher level two hours after IR with 2 Gy, (). If activated, the mRNA level of IL-8 in EA.hy926 cells was not distinctively higher compared to the level of non-activated samples. Significant lower mRNA levels were detected four hours after IR with 0.3 Gy and 0.5 Gy, always compared to the sham-irradiated sample at each time point. HUVEC with no activation before IR expressed mRNA coding for G-CSF at all tested time points with enhanced level compared to the 0 Gy controls, significant for 0.5 Gy (24 hours) and 2 Gy (24 and 48 hours) (). The activation of the primary EC resulted in no distinctive higher expression of G-CSF, but with significantly higher expression level two hours, after IR with 0.3 Gy. Non-activated EA.hy926 cells also expressed G-CSF non-linear dose-dependent at each time point, significant higher levels were reached at 30 min with 2 Gy and at two hours with 0.3 Gy (). Activated EA.hy926 cells expressed no distinct higher or lower mRNA level of G-CSF when compared to non-activated samples. Regardless of the activation, primary HUVEC expressed PDGF-BB at all tested time points with no significant changes in mRNA levels (). The expression of the PDGF-BB coding mRNA expression occurred at all five tested time points and revealed a non-linear dose-dependent trend in both EC kinds. In non-activated EA.hy926 cells, the mRNA level for PDGF-BB was significantly elevated. After activation, significant changes revealed four hours with 0.05 Gy and 48 hours after IR with 0.05 Gy and 0.5 Gy.
Figure 4. Expression levels of IL-8 in cultured endothelial cells without TNF-α induction (a) and with TNF-α induction (b). The mRNA-expression was determined by qRT-PCR and the relative quantification to GAPDH was performed by using the Delta Delta CT (2–ΔΔCT) method. Asterisks illustrate significance: *p < .05, **p < .01.
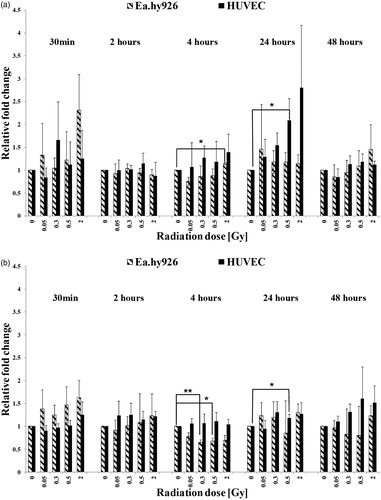
Figure 5. Expression levels of G-CSF in cultured endothelial cells without TNF-α induction (a) and with TNF-α induction (b). The mRNA-expression was determined by qRT-PCR and the relative quantification to GAPDH was performed by using the Delta Delta CT (2–ΔΔCT) method. Asterisks illustrate significance: *p < .05, **p < .01.
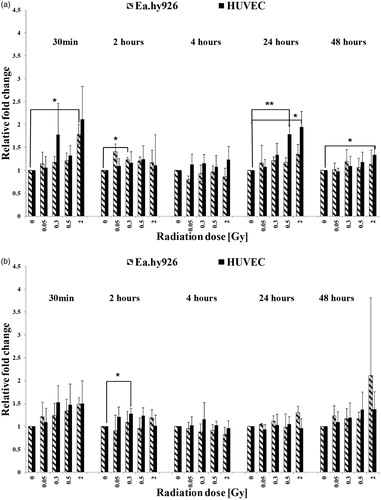
Figure 6. Expression levels of PDGF-BB in cultured endothelial cells without TNF-α induction (a) and with TNF-α induction (b). The mRNA-expression was determined by qRT-PCR and the relative quantification to GAPDH was performed by using the Delta Delta CT (2–ΔΔCT) method. Asterisks illustrate significance: *p < .05, **p < .01.
Conclusions
In order to investigate the effects of LD-IR on human EC, the present study examined the effects of LD-IR especially in the range of very low doses of X-rays. The doses ranged from 0.05 Gy up to 2 Gy. Using HUVEC and EA.hy926 as two reliable cell lines within endothelium research (Bouïs et al. Citation2001), we examined the secretion and mRNA expression of three cytokines IL8, G-CSF and PDGF-BB. As the composition of the FBS and the potential concentration of cytokines are unknown, all experiments were performed with serum-reduced culture media to guarantee that the results are not affected only by the serum content. Currently, there are limited studies on the effects of X-ray exposure at doses lower than 0.3 Gy.
The aim of the present research was to investigate the immune modulatory properties of low doses of IR on ECs with respect to an early response to inflammatory stimuli solely and in combination with irradiation. Do cells correspond to the stimuli in an anti- or pro-inflammatory way? Which proteins will be secreted? Does the expression of these proteins correlate with the secretion? And furthermore, is the effect transient or long-term?
Here, we demonstrated that LD-IR alone already resulted in a release of pro-inflammatory markers in the supernatant of primary EC. Within the 27-plex human cytokine/chemokine kit also, anti-inflammatory markers were included: IL-1ra, IL-1, IL-4, IL-6, IL-10, IL-13, IFNγ and TGF-β. For all these markers, the cytokine concentration was not detectable or not significantly changed compared to the respective control samples (0 Gy, no TNF-α) and therefore excluded from this study. The increase of the pro-inflammatory markers occurred in a time-dependent manner from 30 min up to 48 hours after IR and the stimulation of the cells with TNF-α enhanced the release considerably for all tested proteins. In general, our study demonstrated a higher cytokine release after LD-IR for primary EC compared to the immortalized cell line EA.hy926. Our results clearly point out that LD-IR causes significant dose-dependent changes in cytokine release into the supernatant of primary as well as immortalized EC. The fact, that irradiation alone is sufficient to induce cytokine secretion in EC was already shown by others. Meeren et al. (Citation1997) published a study showing the enhanced IL-6 and IL-8 production by human EC via IR. A dose of 2 Gy already induced a significant increase in cytokine production with a time-dependent increase over a period of six days. They also demonstrated that the effects of γ irradiation and TNF-α were synergistic (Meeren et al. Citation1997). We could demonstrate this effect in both EC kinds as well, but in contrast to the study of Meeren et al. with lower irradiation doses from 0.05 Gy to 0.5 Gy and 2 Gy. Another difference between the studies is the pattern of cytokine release in relation to the radiation dose. In our study with LD-IR, the cytokine secretion was non-linear dose-dependent whereas IL-6 and IL-8 were dose-dependently increasing in the experiments of Meeren et al. (Citation1997). In recent studies, a radiation induced bystander effect caused by low dose and targeted radiation has been observed. This effect describes the response of non-irradiated cells to the irradiation of the neighboring cells and has been observed in apoptosis, DNA-damage (Nagasawa and Little Citation1992), cell proliferation (Shao et al. Citation2002) or genomic instability (Moore et al. Citation2005). These processes saturated at low doses and showed a non-linear dose response and were often cell type and radiation type specific (Jacob et al. Citation2010; Shuryak et al. Citation2011; Butterworth et al. Citation2013).
Similarly, Riquier et al. investigated the secretion of IL-8 after IR (X-rays and α-particles) on HUVEC and A549 cells. Analyzing the mRNA expression, they revealed a strong increase of IL-8 but no changes of PDGF-BB, following irradiation of much higher dose (10 Gy) compared to our study. Their study only used one single time point for measurements. For IL-8 secretion, they showed a significant increase for A549 cells and a significant for primary EC after IR (Riquier et al. Citation2013). Despite the high differences in the dose range, we could demonstrate a broad trend of expression and secretion of IL-8 over a time period of 48 hours consisting of five specific time points with or without activation due to TNFα-addition. As another example, we consider the study of Girdhani et al. (Citation2012) in a lower dose range only up to 2 Gy, human microvascular ECs and A549 cells showed depending on the radiation type (proton vs. photon) an up- and downregulation of IL-8 mRNA expression following irradiation (Girdhani et al. Citation2012). Consistent with our data, they showed an upregulation of IL-8 after photon irradiation. They used an early time point of six hours after IR, which is comparable to our third time point of four hours. In addition by Pluder et al. (Citation2011), it was shown that IR with a dose of 200 mGy cause alterations in proteome of EA.hy926 (Pluder et al. Citation2011). However, less is known about the radiation response of EC after irradiation doses below 0.3 Gy, particularly under 0.1 Gy. The expression pattern of IL-8 was found to be noticeably different than protein secretion at the same time points and applied doses and does not correlate at all. Our findings suggest that IL-8 underlies different post-transcriptional and post-translational mechanisms, as also demonstrated for the expression and secretion of PDGF-BB in the present study. Stimuli for inducing the expression are distinct and dependent on the cytokine (Anderson Citation2010). Possible mechanisms were summarized by Keene (Citation2007) where ribonucleoproteins (RNPs) play a key role in mRNA processing, from transcription to protein synthesis (Keene Citation2007).
It is also interesting that the continuous expression pattern of PDGF-BB in EA.hy926 cells was found to be noticeably different to the non-existent protein secretion at the early time points. Our findings indicate that PDGF-BB underlies different post-transcriptional and post-translational mechanisms, which could explain the diverging results obtained on protein and mRNA level. In 2010, Shebl et al. analyzed various mRNA–protein correlations in PBMCs by testing 22 various cytokines. They illustrated a wide range of correlation between proteins secreted by the cells and the expression of the gene varying from marker to marker and also assumed the regulatory mechanisms after transcription and translation (Shebl et al. Citation2010). The transcription rates, splicing mechanisms, protein processing or degradation or message turnover are just a few of many regulation mechanisms that can influence cytokine gene expression. This study provides a deeper insight into the radiobiological effects of radiation doses below 0.3 Gy, in particular 0.01 Gy and 0.05 Gy, and their significant immunomodulatory properties on EC, which is very important in order to assess the effect of LD-IR on EC.
Even at low doses of IR, the discontinuous secretion of inflammatory markers was observable which is well known to be present after IR with high doses. The effects of LD-IR identified in this study were obtained over a time period of 48 hours, but to understand the underlying processes of a long-term effect, the time periods should be extended in future research approaches.
| Abbreviations | ||
| bFGF | = | Basic fibroblast growth factor |
| DMEM | = | Dulbecco’s modified Eagle’s medium |
| EC | = | Endothelial cells |
| ECBM | = | Endothelial cell basal medium |
| EGF | = | Epidermal growth factor |
| FBS | = | Fetal bovine serum |
| GAPDH | = | Glyceraldehyde-3-phosphate dehydrogenase |
| G-CSF | = | Granulocyte-colony stimulating factor |
| GM-CSF | = | Granulocyte macrophage colony-stimulating factor |
| HUVECs | = | Human umbilical vein endothelial cells |
| IFNγ | = | Interferon gamma |
| IP-10 | = | Interferon gamma inducible protein 10 |
| IL | = | Interleukin |
| IR | = | Ionizing radiation |
| LD-IR | = | Low-dose ionizing radiation |
| LD-RT | = | Low-dose radio therapy |
| LPS | = | Lipopolysaccharide |
| NF-κB | = | Nuclear factor kappa B |
| MIP-1α | = | Macrophage inflammatory protein 1alpha |
| MIP-1β | = | Macrophage inflammatory protein 1 beta |
| MCP-1 | = | Monocyte chemotactic protein 1 |
| PDGF-BB | = | Platelet-derived growth factor |
| RT | = | Radiation therapy |
| qRT-PCR | = | Real-time quantitative PCR |
| TNF-α | = | Tumor necrosis factor-alpha |
| VEGF | = | Vascular endothelial growth factor |
| XIAP | = | X chromosome-linked inhibitor of apoptosis |
Disclosure statement
The authors declare that there are no conflicts of interest.
Additional information
Funding
Notes on contributors
Sabine Schröder
Sabine Schröder is a PhD student at the University of Rostock. After receiving a diploma degree in biology at the University of Rostock, she studies the inflammatory response after radiation on endothelial cells at the department of radiotherapy and radiation oncology in Rostock.
Dajana Juerß
Dajana Juerß is a PhD student at the University of Rostock. After receiving a diploma degree in biology at the University of Rostock in 2011, she studies the radiation response of normal tissue cells at the department of radiotherapy and radiation oncology in Rostock.
Stephan Kriesen
Stephan Kriesen is a medical physicist of the Department of Radiotherapy and Radiation Oncology at the University Medical Center Rostock since 2000. He received his diploma in physics from the University of Rostock in 2000 and his PhD in radiobiology in 2013.
Katrin Manda
Katrin Manda has been the leader of the radiation biology laboratory at Department of Radiotherapy and Radiation Oncology at the University Medical Center Rostock since 2007. She received her diploma in biology from the Ernst-Moritz-Arndt University of Greifswald in 2006 and her PhD in microbiology in 2006.
Guido Hildebrandt
Guido Hildebrandt, director of the Department of Radiotherapy and Radiation Oncology at the University Medical Center Rostock since 2009, received his MD in 1996 at the University of Leipzig and his MSc in radiation biology from the University of London in 1997.
References
- Anderson P. 2010. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat Rev Immunol. 10:24–35.
- Azizova TV, Muirhead CR, Druzhinina MB, Grigoryeva ES, Vlasenko EV, Sumina MV, O'Hagan JA, Zhang W, Haylock RGE, Hunter N. 2010. Cerebrovascular diseases in the cohort of workers first employed at Mayak PA in 1948–1958. Radiat Res. 174:851–864.
- Bouïs D, Hospers GAP, Meijer C, Molema G, Mulder NH. 2001. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis. 4:91–102.
- Butterworth KT, McMahon SJ, Hounsell AR, O'Sullivan JM, Prise KM. 2013. Bystander signalling: exploring clinical relevance through new approaches and new models. Clin Oncol. 25:586–592.
- Edgell CJ, McDonald CC, Graham JB. 1983. Permanent cell line expressing human factor {VIII}-related antigen established by hybridization. Proc Natl Acad Sci USA. 80:3734–3737.
- Fajardo LF, Stewart JR. 1970. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol. 59:299–316.
- Girdhani S, Lamont C, Hahnfeldt P, Abdollahi A, Hlatky L. 2012. Proton irradiation suppresses angiogenic genes and impairs cell invasion and tumor growth. Radiat Res. 178:33–45.
- Hallahan D, Kuchibhotla J, Wyble C. 1996. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 56:5150–5155.
- Hildebrandt G, Maggiorella L, Rodel F, Rodel V, Willis D, Trott KR. 2002. Mononuclear cell adhesion and cell adhesion molecule liberation after X-irradiation of activated endothelial cells in vitro. Int J Radiat Biol. 78:315–325.
- Jacob P, Meckbach R, Kaiser JC, Sokolnikov M. 2010. Possible expressions of radiation-induced genomic instability, bystander effects or low-dose hypersensitivity in cancer epidemiology. Mutat Res. 687:34–39.
- Keene JD. 2007. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 8:533–543.
- Kern PM, Keilholz L, Forster C, Hallmann R, Herrmann M, Seegenschmiedt MH. 2000. Low-dose radiotherapy selectively reduces adhesion of peripheral blood mononuclear cells to endothelium in vitro. Radiother Oncol. 54:273–282.
- Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. 1999. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res. 19:91–104.
- Mantovani A, Bussolino F, Dejana E. 1992. Cytokine regulation of endothelial cell function. FASEB J. 6:2591–2599.
- Meeren AV, Bertho JM, Vandamme M, Gaugler MH. 1997. Ionizing radiation enhances IL-6 and IL-8 production by human endothelial cells. Mediat Inflamm. 6:185–193.
- Moore SR, Marsden S, Macdonald D, Mitchell S, Folkard M, Michael B, Goodhead DT, Prise KM, Kadhim MA. 2005. Genomic instability in human lymphocytes irradiated with individual charged particles: involvement of tumor necrosis factor alpha in irradiated cells but not bystander cells. Radiat Res. 163:183–190.
- Nagasawa H, Little JB. 1992. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 52:6394–6396.
- Pluder F, Barjaktarovic Z, Azimzadeh O, Mörtl S, Krämer A, Steininger S, Sarioglu H, Leszczynski D, Nylund R, Hakanen A, et al. 2011. Low-dose irradiation causes rapid alterations to the proteome of the human endothelial cell line EA.hy926. Radiat Environ Biophys. 50:155–166.
- Riquier H, Wera A-C, Heuskin A-C, Feron O, Lucas S, Michiels C. 2013. Comparison of X-ray and alpha particle effects on a human cancer and endothelial cells: survival curves and gene expression profiles. Radiother Oncol. 106:397–403.
- Rödel F, Frey B, Capalbo G, Gaipl U, Keilholz L, Voll R, Hildebrandt G, Rödel C. 2010. Discontinuous induction of X-linked inhibitor of apoptosis in EA.hy.926 endothelial cells is linked to NF-κB activation and mediates the anti-inflammatory properties of low-dose ionising-radiation. Radiother Oncol. 97:346–351.
- Rödel F, Hantschel M, Hildebrandt G, Schultze-Mosgau S, Rödel C, Herrmann M, Sauer R, Voll RE. 2004. Dose-dependent biphasic induction and transcriptional activity of nuclear factor kappa B (NF-kappaB) in EA.hy.926 endothelial cells after low-dose X-irradiation. Int J Radiat Biol. 80:115–123.
- Roedel F, Kley N, Beuscher HU, Hildebrandt G, Keilholz L, Kern P, Voll R, Herrmann M, Sauer R. 2002. Anti-inflammatory effect of low-dose X-irradiation and the involvement of a TGF-beta1-induced down-regulation of leukocyte/endothelial cell adhesion. Int J Radiat Biol. 78:711–719.
- Sato Y. 2001. Current understanding of the biology of vascular endothelium. Cell Struct Funct. 26:9–10.
- Schultz-Hector S, Böhm M, Blöchel A, Dominiak P, Erdmann E, Müller-Schauenburg W, Weber A, Bohm M, Blochel A, Muller-Schauenburg W. 1992. Radiation-induced heart disease: morphology, changes in catecholamine synthesis and content, beta-adrenoceptor density, and hemodynamic function in an experimental model. Radiat Res. 129:281–289.
- Seegenschmiedt MH, Micke O, Willich N. 2004. Radiation therapy for nonmalignant diseases in Germany: current concepts and future perspectives. Strahlenther Onkol 80:115–123.
- Seegenschmiedt MH, Micke O. 2012. Strahlentherapie Nichtmaligner Erkrankungen – Vergangenheit, Gegenwart Und Zukunft. Strahlenther Onkol. Special issue 3:1–19.
- Shao C, Furusawa Y, Aoki M, Matsumoto H, Ando K. 2002. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int J Radiat Biol. 78:837–844.
- Shebl FM, Pinto LA, García-Piñeres A, Lempicki R, Williams M, Harro C, Hildesheim A. 2010. Comparison of mRNA and protein measures of cytokines following vaccination with human papillomavirus-16 L1 virus-like particles. Cancer Epidemiol Biomark Prevent. 19:978–981.
- Shuryak I, Brenner DJ, Ullrich RL. 2011. Radiation-induced carcinogenesis: mechanistically based differences between gamma-rays and neutrons, and interactions with DMBA. PLoS One. 6:e28559.
- Speyer CL, Ward PA. 2011. Role of endothelial chemokines and their receptors during inflammation. J Investig Surg. 24:18–27.
- Stewart FA, Hoving S, Russell NS. 2010. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res. 174:865–869.
