Abstract
Purpose
To provide an updated summary of recent advances in the application of gamma irradiation to elicit secondary metabolism and for induction of mutations in plant cell and organ cultures for the production of industrially important specialized metabolites (SMs).
Conclusions
Research on the application of gamma radiation with plants has contributed a lot to microbial decontamination of seeds, and the promotion of physiological processes such as seed germination, seedling vigor, plant growth, and development. Various studies have demonstrated the influence of gamma rays on the morphology, physiology, and biochemistry of plants. Recent research efforts have also shown that low-dose gamma (5–100 Gy) irradiation can be utilized as an expedient solution to alleviate the deleterious effect of abiotic stresses and to obtain better yields of plants. Inducing mutagenesis using gamma irradiation has also evolved as a better option for inducing genetic variability in crops, vegetables, medicinal and ornamentals for their genetic improvement. Plant SMs are gaining increasing importance as pharmaceutical, therapeutic, cosmetic, and agricultural products. Plant cell, tissue, and organ cultures represent an attractive alternative to conventional methods of procuring useful SMs. Among the varied approaches the elicitor-induced in vitro culture techniques are considered an efficient tool for studying and improving the production of SMs. This review focuses on the utilization of low-dose gamma irradiation in the production of high-value SMs such as phenolics, terpenoids, and alkaloids. Furthermore, we present varied successful examples of gamma-ray-induced mutations in the production of SMs.
Introduction
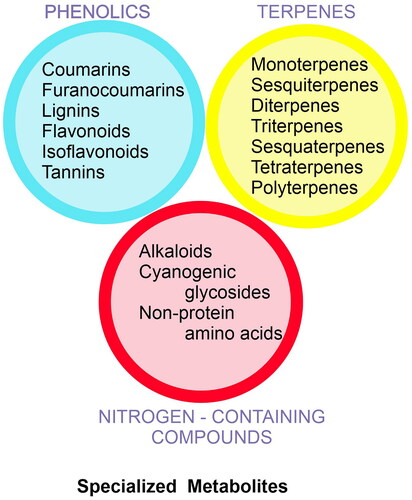
Plants synthesize a diverse group of metabolites both primary and secondary, which play crucial roles in the growth and survival of plant species (Taiz et al. Citation2015). The metabolites such as sugars, amino acids, nucleotides, and lipids are high-energy compounds that directly contribute to essential cellular processes for plant growth and development and are designated as primary metabolites. These metabolites are ubiquitous in plant cells, and their metabolic synthesis and regulation of mechanisms are relatively similar. In contrast, secondary metabolites are usually lineage-specific compounds including phenolics, terpenoids, and alkaloids which help plants interact with biological and non-biological environments (Taiz et al. Citation2015). Secondary metabolites play an important role in plant resistance to bacterial, fungal, and viral infections, and herbivores, and impart ultraviolet light resistance to plants. However, the classification boundary of primary metabolites and secondary metabolites is not very clear. Under the influence of complex and changeable environmental factors, plants synthesize several secondary compounds by utilizing shikimic acid, acetyl-coenzyme A, and pyruvate molecules. These metabolites are called specialized metabolites (SMs) in the recent past and their biosynthesis and storage are developmentally and temporally regulated and depend on abiotic and biotic factors. Specific cell types, subcellular organelles, microcompartments, and/or anatomical structures are often devoted to producing and storing these compounds (Huang and Dudareva Citation2023). It has been estimated that 200,000 to 1,000,000 compounds are on record in contrast to only about 8,000 primary metabolites in varied plants (Huang and Dudareva Citation2023). The alternative mechanism involved in the biosynthesis of SM leads to common products, such as phenolics, terpenoids, nitrogen-containing compounds such as alkaloids, and cyanogenic glycosides (). The shikimic acid pathway and Krebs cycle provide essential precursors for the production of phenolic compounds. The 2-C-methylerythritol-4-phosphate (MEP) and mevalonic acid (MVA) pathways are responsible for the biosynthesis of terpenes and other terpenoid compounds. Amino acids are precursors for nitrogenous compounds such as alkaloids and cyanogenic glycosides. Flavonoids are synthesized through the phenylpropanoid pathway.
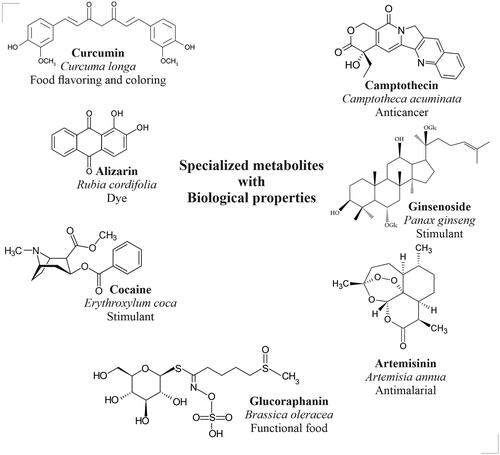
The plant-based SMs are widely used in pharmaceutical, therapeutic, cosmetic, and agricultural products. For example, curcumin (Curcuma longa) is an accepted food flavoring and coloring agent, alizarin (Rubia cordifolia) is a natural dye, cocaine (Eryroxylem coca) acts as a central nervous system stimulant, glucoraphanin (Brassica oleracea) is a popular functional food ingredient, artemisinin (Artemisia annua) is a popular antimalarial drug, ginsenoside (Panax ginseng) is an excellent immunostimulant, and camptotheicin (Camptotheca acuminata) is a potent anticancer drug (). However, the plants will accumulate these SMs in meager quantities (<1%). The production of specialized metabolites is highly dependent on the developmental stages and physiological conditions of plants (Reshi et al. Citation2023). On the other hand, in vitro cell and organ cultures have emerged as alternatives for the large-scale production of valuable specialized metabolites (Murthy et al. Citation2014, Citation2016, Citation2023a, Citation2023b, Citation2023c, Citation2023d, Citation2023e). Varied strategies such as selection cell or organ lines, optimization of medium parameters (nutrient medium, salt strength, carbohydrates, and minerals), and optimization of culture environment (temperature, light, agitation, aeration) have been applied for the enhancement of production of SMs in plant cell and organ cultures (Murthy et al. Citation2014, Citation2016, Citation2023a, Citation2023b, Citation2023c, Citation2023d, Citation2023e). Increasing the productivity of target compounds could be achieved by applying various bioreactor cultures (Murthy et al. Citation2022, Citation2023a, Citation2023b, Citation2023c). Again, varied bioprocess parameters including nutrient feeding, precursor feeding, permeabilization, immobilization, and selective adsorption of metabolites have been applied after the selection of suitable bioreactor systems for the propagation/cultivation of plant cells and organs (Murthy et al. Citation2023a, Citation2023b, Citation2023c, Citation2023d, Citation2023e).
Plants are known to synthesize and accumulate SMs during varied abiotic and biotic stress conditions in nature. For instance, abiotic factors include salinity, nutrient deficiency, radiation (UV, X-ray, and gamma rays), drought, water logging, extreme temperature, and anthropogenic pollutants that influence the accumulation of SMs in plants. Similarly, the invasion of plants by microbes like bacteria, fungi, and viruses also causes SMs to build up in natural plants (Taiz et al. Citation2015). Therefore, elicitors of abiotic and biotic origin are widely used in stimulating defense response in plant cells and organ cultures for the production of SMs. Inorganic salts, metal ions such as Ni, Ag, Fe, Co, Cr, Zn, and Mn, irradiation by visible light sources, UV, and gamma radiations have been used as abiotic elicitors to enhance secondary metabolism in cultured cells and organs (Zhao et al. Citation2005). Biotic elicitors including microbial enzymes, fungal and bacterial lysates, yeast extracts, and microbial cell wall polysaccharides (e.g. chitin and glucans) have been also successfully used as elicitors for enhancing the biotechnological production of SMs (Narayani and Srivastava Citation2017; Ramirez-Estrada et al. Citation2016). Recently nanomaterials have been tested successfully as elicitor production of SMs in vitro (Murthy et al. Citation2023d).
Gamma radiation (γ-rays) is a form of electromagnetic ionizing radiation and upon exposure of plants to gamma radiation, it interacts with atoms or molecules to produce free radicals in cells (Jan et al. Citation2012). These radicals can damage or modify important components of plant cells and have been reported to affect the morphology, anatomy, and physiology of plants differentially, depending on the radiation level. Modulation of the antioxidative system, dilation of thylakoid membranes, alteration in photosynthesis, and accumulation of secondary metabolites are some of the effects reported due to exposure of plants to gamma irradiation (Kim et al. Citation2009; Wi et al. Citation2005). Plant radiation sensitivity varies by species and is also dependent on radiation dosage levels. Typically, ionizing radiation, such as γ-rays, interacts with biological organisms to provide energy. The quantity of energy absorbed per unit weight of tissue or organ is represented in gray (Gy), which is the absorbed dosage. One gray dose is equivalent to one joule of radiation absorbed by a kilogram of tissue or organ weight and one gray is equivalent to 100 roentgen (R). Usually, low doses of γ-rays (1–20 Gy) are reported to have stimulating effects on plant processes such as the promotion of seed germination, seedling, and plant growth. It has been also utilized as a potential tool for the mitigation of abiotic stresses like heat, salinity, flood, heavy metal, and high radiance in plants (Katiyar et al. Citation2022). While greater doses of γ-rays (> 100 Gy) are mutagenic, carcinogenic, and deadly (Gudkov et al. Citation2019), careful application of γ-rays to induce mutation and create mutant types in a variety of crop plants can tolerate salt, low temperature, drought, and heat stresses (Katiyar et al. Citation2022). The development of crop plants, vegetables, tree species, and ornamentals with exceptional agronomic traits can also be accomplished through induced mutagenesis utilizing γ-rays (Katiyar et al. Citation2022; Riviello-Flores et al. Citation2022; Vardhan and Shukla Citation2017).
Varied researchers have utilized a low dose γ-irradiation as an elicitor to trigger the production of specialized metabolites in cell, tissue, and organ cultures. Induced mutant cell and organ lines have also been developed by several researchers, and these lines may be employed as raw materials for the commercial manufacturing of plant SMs because of their enhanced capacity to synthesize specific metabolites. This review has therefore covered the significance of γ-irradiation as an elicitor on in vitro cell, tissue, and organ cultures for the synthesis of SMs. Additionally, successful instances of induced mutants that are helpful in the hyperaccumulation of plant SMs have been described, along with the potential mechanisms.
The effect of gamma irradiation on the accumulation of phenolic compounds in cell and organ cultures
γ-irradiation has been successfully used for the stimulation of the accumulation of phenolic compounds in cell, callus, adventitious root, and plantlet cultures of varied species (). Azeez et al. (Citation2017) have investigated the effect of gamma irradiation on biomass formation and yield of pharmaceutically important phenolic compounds including p-OH-benzoic acid, chlorogenic acid, and epicatechin and naphthodianthrones viz. hypericin and pseudohypericin in leaf, stem, and root-derived callus cultures of Hypericum triquetrifolium. They induced callus from leaf, stem, and root explants H. triquetrifolium on MS medium containing 0.5 mg L−1 indole acetic acid and 0.4 mg L−1 thidiazuron, and subsequently callus was irradiated with γ-rays at doses 10, 20, 30 and 40 Gy with an average dose rate of 0.5 Gy min−1 for 90 s. Then callus cultures were maintained on MS medium with similar concentrations of hormonal combination explained as above by sub-culturing at an interval of 21 days. They reported a low-dose irradiation i.e. 10 Gy dose was beneficial in increasing fresh callus biomass compared to control (nonirradiated callus), whereas, higher doses of gamma (40 Gy) treatments were responsible for a significant reduction in callus growth. They noticed a differential accumulation of SMs in 10 Gy gamma irradiated callus and reported 4.35 mg 100 g−1 DW of p-OH-benzoic acid in leaf-derived callus (as against 3.55 mg 100 g−1 DW in non-treated callus) and 12.91 mg 100 g−1 DW of chlorogenic acid (10.22 mg 100 g−1 DW in control) in root-derived callus. Their experimental results also showed that the 20 Gy γ-irradiation doses stimulate the accumulation of epicatechin in the leaf-derived callus (126.39 mg 100 g−1 DW in gamma-treated callus and 98.81 mg 100 g−1 DW in non-treated callus) and stem-derived callus (148.80 mg 100 g−1 DW in gamma-treated callus and 101.72 mg 100 g−1 DW in non-treated callus). The callus induced from the leaf and irradiated with 10 Gy showed higher amounts of hypericin (gamma treated vs. control: 0.29 mg 100 g−1 DW vs. 0.25 mg 100 g−1 DW) and pseudohyperincin (4.01 mg 100 g−1 DW vs. 3.57 mg 100 g−1 DW) content compared to the non-irradiated callus. In another study, Chung et al. (Citation2006) demonstrated the effects of γ-irradiation on the production of shikonin derivates in callus cultures of Lithospermum erythrorhizon. They raised callus cultures of L. erythrorhizon from root tissue and selected superior callus lines for shikonin accumulation, they treated selected callus lines with gamma irradiation of 2, 16, and 36 Gy and subsequently established cell suspension cultures in small-scale bioreactors. Their results showed that the highest yield of shikonin i.e. 71 mg L−1 with cell biomass which was treated with 16 Gy as against 17.7 mg L−1 with non-irradiated cultures. Whereas, shikonin yields were only 42.5 mg L−1 and 31.4 mg L−1 with 2 and 32 Gy treatments. Thus, a 4-fold increment in shikonin accumulation with 16 Gy treated cells compared to that of control cultures. Further, Chung et al. (Citation2006) also showed an increment in p-hydroxybenzoic acid geranyl transferase enzyme activity with 16 Gy treated cells which has led to the accumulation of shikonin in γ-rays treated cells. El-Beltagi et al. (Citation2011) carried out γ-irradiation (0, 5, 10, 15, and 20 Gy) of callus cultures of rosemary (Rosmarinus officinalis) and displayed an increase in induction of reactive oxygen species (ROS) such as superoxide radicle (O2-), hydrogen peroxide (H2O2) and hydroxyl radical (HO•) with γ-irradiation and concomitant increment in various antioxidant defense enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase. The increment in ROS was responsible for the accumulation of total phenolics and total flavonoids in γ-irradiated rosemary callus cultures and El-Beltagi et al. (Citation2011) realized the highest activity prooxidants and antioxidants with the callus cultures treated with 20 Gy of γ-rays. Mariadoss et al. (Citation2020) exposed the callus cultures of Rubia cordifolia with γ-irradiation at 2, 4, 6, 8, 10, 12, 14, and 16 Gy and callus cultures were maintained for four successive subcultures. They reported that the callus cultures that were irradiated at 8 Gy accumulated a maximum alizarin of 26.86 mg g−1 DW and a purpurin of 44.85 mg g−1 DW after the fourth sub-cultures, which was 6-fold and 11-fold higher than those of the nonirradiated callus cultures. Subsequently, they selected the callus line which was irradiated at 8 Gy, and established cell suspension cultures in bioreactors with a helical ribbon and Rushton turbine impellers. The cell suspension cultures in a bioreactor using a helical ribbon impellor accumulated 63.58% more anthraquinones than those in the bioreactors equipped with Rushton turbine impellers.
Table 1. Improvement of secondary metabolites in vitro using gamma radiation.
Increased production of plumbagin was recorded in adventitious root cultures of Plumbago indica by Jaisi et al. (Citation2013) with γ-irradiation. They studied the effect of 0.5, 10, 15, 20, and 25 Gy and selected the most appropriate dose of γ-rays i.e. 20 Gy. Subsequently, they irradiated 0, 5, 10, 15, and 20 days-old cultures with γ-irradiation and they observed an increase of plumbagin content with all ages of cultures. However, they recorded maximum production of plumbagin with 10-day-old cultures and such cultures contained 2.94-fold higher plumbagin compared to control cultures. These results demonstrate that irradiation dose and age of cultures are critical for the accumulation of SMs in cell and organ cultures. Smoke tree (Cotinus coggygria) and strawberry (Fragaria × ananassa) callus cultures were experimented by Ciocan et al. (Citation2023) for the production of anthocyanins and they treated callus cultures with 15, 20, 25, 30, 35, and 40 Gy of γ-irradiation and they illustrated that all the treatments were responsible for the increase in anthocyanin accumulation. However, 20 Gy-treated smoke-tree callus cultures and 25 Gy-treated strawberry callus cultures accumulated highest i.e. 2.8 mg g−1 DW and 1.65 mg g−1 DW anthocyanin content.
Sage (Salvia officinalis) in vitro-regenerated plants were irradiated with γ-rays at 10, 15, and 20 Gy by Radomir et al. (Citation2023), and assessed phenolic content, biological activity, and cellular ultrastructure in in vitro-grown plants as well as in plants after acclimatization. They recorded an 80% increase in rosmarinic acid in in vitro-regenerated plants which were irradiated at 20 Gy. They recorded an increase in carnosic acid, and salvianolic acid F with the increase in radiation dose. Radomir et al. (Citation2023) also noticed an increase in carnosic acid in the acclimatized sage plants, which have been regenerated after irradiation at 10 Gy treatment. Radomir et al. (Citation2023) have also evaluated the antioxidant capabilities and antiproliferative properties of the extracts of irradiated plants on the human epidermoid carcinoma cells and extracts from irradiated plants depicted excellent antioxidant and antiproliferation activities. They also recorded that the use of gamma irradiation on sage plantlets led to chloroplast dedifferentiation and also a destructive effect on chloroplast and mitochondria. Rosmarinic acid and carnosic acid are reported to possess excellent antioxidant and antimicrobial properties and are used in health foods and cosmetic industries. Whereas, salvianolic acid F has several therapeutic potentials and is utilized in hepatic, and neural protection and cancer treatments (Ho and Hong Citation2011). Therefore, low-dose γ-irradiation treatment of in vitro-grown sage plants is quite useful in enhancing bioactive compounds in plantlets. In another contrasting study, El-Garhy et al. (Citation2016) assessed the effect of γ-irradiation in Silybum marianum plants on the expression of chalcone synthase (CHS) genes. They exposed the seeds of Silybum marianum to 100, 200, 400, 600, 800, and 1000 Gy γ-irradiation doses and then germinated seeds with a mixture of sand and peat moss (1:1 v: v). Then after one month, they assessed the expression of CHS genes and showed that plants treated with γ-irradiation CHS2 were more expressed when compared to the CHS1 gene. They recorded the highest expression level for the CHS2 gene (13.8-fold increase) under the effect of 200 Gy, whereas the CHS3 gene was up-regulated under all treatments, with the highest expression (11.9-fold increase) under the effect of 200 Gy. Based on such results we predict that exposure of in vitro cultures or in vivo plants to γ-irradiation is responsible for initial oxidant stress and accumulation of pro-oxidants which might be responsible for triggering the expression genes that are involved in the specialized metabolite biosynthetic pathways.
The effect of gamma irradiation on the accumulation of terpenoid compounds in cell and organ cultures
The medical herb ginseng (Panax ginseng) is one of the most popular medicines and health foods in the world. Ginsenosides, which are triterpenoid saponins, are ginseng’s main active ingredients (Ratan et al. Citation2021). While ginseng contains a variety of ginsenosides, Rg3 is one of the least abundant. Despite this, Rg3 offers a wide range of therapeutic benefits, including antioxidant, antiaging, anti-inflammatory, and anticancer effects (Lee et al. Citation2020). Thus, an increased production of ginsenoside Rg3 is highly beneficial. The application of γ-irradiation is useful in structural alterations of chemical compounds and the accumulation of useful phytochemicals (Hamdani et al. Citation2017; Ognyanov et al. Citation2022).
Varied researchers have used γ-irradiation for the induction of mutant adventitious root lines in ginseng (). For example, Kim et al. (Citation2009) treated ginseng embryogenic callus with γ-rays of 0, 10, 30, 50, 70, and 100 Gy and induced adventitious root lines. They recorded the highest frequency of adventitious root formation and proliferation at γ-irradiation of 30 Gy and induced 152 different adventitious root lines. They selected five mutant adventitious root lines and designated them as MAR3 lines (MAR3-9, MAR3-10, MAR3-13, MAR3-26, and MAR3-109), and among them, the MAR3-26 line had 2.5-fold higher total ginsenoside content (Rb1, Rb2, Rc, Rd, Re, Rf, and Rg1) compared to naturally cultivated ginseng roots. In another study, Kim et al. (Citation2013) induced two different mutant adventitious root lines namely MAR 5-2 and MAR 5-9 by treating the embryogenic callus of ginseng with 50 Gy γ-irradiation. They studied the expression of genes related to ginsenoside biosynthesis in mutant lines and reported the increased expression of squalene epoxidase and dammarenediol synthase genes in the MAR-2 line and the overexpression of the phytosterol synthase gene in MAR5-9 line. They recorded a 0.8-fold increase in total ginsenoside content in the MAR 5-9 line compared to native ginseng. Le et al. (Citation2019) utilized different callus lines (CS1 and CS2) and one-year-old (AR1) and 20-year-old (AR2) adventitious root lines and treated callus lines with 0, 20, 30, 50, 75, and 100 Gy and adventitious roots with 0, 20, 40, 60, 80 and 100 Gy γ-rays respectively. Of the varied γ-irradiation treatments, 20 Gy-dose induced promising mutant root lines from AR1 and they selected the best-performing lines in terms of growth and proliferation and designated them as IG-20-12, IG-20-16, IG-20-19, and IG-20-20. The newly induced mutant lines were differentially accumulating varied ginsenosides such as Rb1, Rb2, Rb3, Rc, Rd, Rg2 Rh2, and CK. The total ginsenoside content in IG-20-12, IG-20-16, IG-20-19, and IG-20-20 mutant root lines were 3.0, 2.3, 4.2, and 3.0-fold higher than control, respectively.
The effect of gamma irradiation on the accumulation of nitrogen-containing compounds in cell and organ cultures
A large number of plant secondary metabolites have nitrogen as part of their structure. They are synthesized from amino acids and are classified into alkaloids, cyanogenic glycosides, and nonprotein amino acids. Alkaloids offer a wide range of therapeutic uses in medicine, including analgesic, asthmatic, anticancer, antihypertensive, antipyretic, and antihyperglycemic properties (Aryal et al. Citation2022). Several studies have been carried out on the effect of γ-irradiation on the callus, embryogenic tissues, and plantlet cultures for the production of alkaloids (). Mujib et al. (Citation2022) irradiated embryogenic tissues of Catharanthus roseus with 20, 40, 60, 80, and 100 Gy γ-rays and cultured them on the nutrient medium and they observed increment callus biomass growth (1.65 g) with 20 Gy exposed tissues and a significant decrease in callus growth with 100 Gy treatment (0.33 g). They recorded an increment in percentage embryogenesis (81.31% in γ-rays treated vs. 73% in control), number of torpedo embryos/culture (18.12% in γ-rays treated vs. 9% in control), and number of cotyledonary embryos/culture (12.21% γ-rays treated vs. 6.25% in control) with the treatment of 20 Gy γ-rays. They recorded the highest amount of vinblastine in the leaves of somatic embryo-regenerated plants (15.13 µg g−1 DW) with 40 Gy irradiation treatment compared to the control (13.30 µg g−1 DW) and an increase of 13.75% was reported. They also reported an increment in vinblastine content in the stem of regenerated plants, and the embryos at initiation, proliferation, and maturation stages and in germinating embryos. Almukhtar et al. (Citation2019) tested the effect of γ-irradiation on the production of cardiac glycosides in the plants of Digitalis lanata. They irradiated D. lanata seeds at 0, 25, and 50 Gy γ-rays, germinated them on the nutrient medium, and recorded an increment in seed germination (100%) with 50 Gy γ-rays treatment compared to the control group (30%). Again, they documented a significant increment in the accumulation of digoxin (45.54 µg g−1 DW in γ-rays treated vs. 6.40 µg g−1 DW in control), digitoxin (91.87 µg g−1 DW in γ-rays treated vs. 20.17 µg g−1 DW in control) and gitoxin glycosides (68.70 µg g−1 DW in γ-rays treated vs. 13.09 µg g−1 DW in control) with 50 Gy γ-rays treated plants. In another study, Fulzele et al. (Citation2015) experimented influence of γ-irradiation on callus cultures of Nothapodytes foetida on the production of the anticancer compound camptothecin. They exposed callus cultures of N. foetida which were cultured on a nutrient medium at doses ranging from 5, 10, 15, 20, 25, and 30 Gy γ-rays and sub-cultured the callus on the same nutrient medium for seven cycles. They described γ-irradiation significantly boosted the production levels and increased the total camptothecin and 9-methoxy-camptothecin yields by 20-fold at 20 Gy and by only 2 and 9 folds at 10 Gy, respectively. The above reports substantiate that γ-irradiation is quite useful in triggering nitrogen-containing compounds in plant in vitro cultures.
Mechanism of action of gamma irradiation in eliciting secondary metabolism and induction of mutation in cell and organ cultures
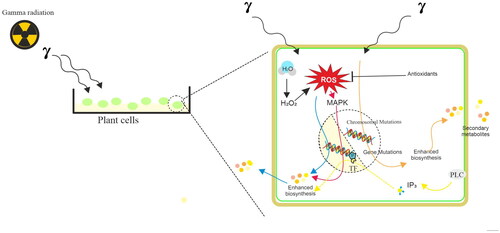
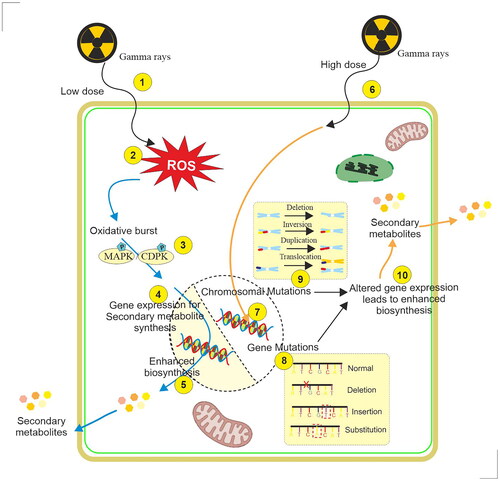
Gamma rays are electromagnetic radiations of shorter wavelengths (λ, < 0.001 nm) arising from the radioactive decay of atomic nuclei. The characteristic features of γ-rays are energy ranging from 5–6000 keV, linear energy transfer > 0.2 keV/µm, maximum travel in the air > 15000 cm, and relative biological effectiveness of 1 (Gudkov et al. Citation2019). Plants as sessile organisms have evolved in such a way that they withstand doses of ionizing radiations including γ-rays as high as 50 Gy by biochemical and physiological adjustments (Gudkov et al. Citation2019). Therefore, exposure to plants with lower doses of γ-radiation (1–20 Gy) is reported to be stimulatory in terms of enhancing physiological processes such as seed germination, seedling growth, plant growth, and development. Whereas, higher doses (more than 100 Gy) are mutagenic and lethal, however, the response of plants to γ-radiation depends upon species, variety, cultivar considered, the developmental stage at the time of irradiation, and even between the individuals (Jan et al. Citation2012). Gamma radiation is reported to be initially responsible for the radiolysis of water molecules and the production of reactive oxygen species (ROS) (). Superoxide anion (O2-•), hydroxyl radical (OH•), singlet oxygen (1O2) species, and hydrogen peroxide (H2O2) are formed with exposure of γ-radiation by water molecules of cells which is responsible for oxidative stress in the cells (Esnault et al. Citation2010). Further, it was proposed that ROS generation might be triggered in the cells because activation of specialized enzymes, such as NADPH-oxidase of the plasma membrane (Qi et al. Citation2015; Vanhoudt et al. Citation2014).
Figure 3. Radiolysis of water molecules and singling pathways caused by low-dose gamma ray irradiation of plant cells. 1. γ-irradiation, 2. Release of oxidants (superoxide anion (O2-•), hydroxyl radical (OH•), and hydrogen peroxide (H2O2) by splitting of water molecule), 3. Accumulation of reactive oxygen species (ROS), 4. Opening of calcium channels and increased concentration of Ca2+, 5. Activation of NADPH-oxidase and release of hydrogen peroxide (H2O2), 6. Activation of phospholipase-c and phosphatidylinositol 4,5-bisphosphate (PIP2) and release of diacylglycerol (DAG) and inositol triphosphate (IP3), 7. Release of antioxidants [superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), peroxiredoxins (PRX), glutathione peroxidase (GPX)] counterbalances the effect of ROS, 8. Activation of MAPKs, protein phosphorylation, and accumulation of singling molecules, 9. Activation of CDPKs, protein phosphorylation, and accumulation of singling molecules.
![Figure 3. Radiolysis of water molecules and singling pathways caused by low-dose gamma ray irradiation of plant cells. 1. γ-irradiation, 2. Release of oxidants (superoxide anion (O2-•), hydroxyl radical (OH•), and hydrogen peroxide (H2O2) by splitting of water molecule), 3. Accumulation of reactive oxygen species (ROS), 4. Opening of calcium channels and increased concentration of Ca2+, 5. Activation of NADPH-oxidase and release of hydrogen peroxide (H2O2), 6. Activation of phospholipase-c and phosphatidylinositol 4,5-bisphosphate (PIP2) and release of diacylglycerol (DAG) and inositol triphosphate (IP3), 7. Release of antioxidants [superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), peroxiredoxins (PRX), glutathione peroxidase (GPX)] counterbalances the effect of ROS, 8. Activation of MAPKs, protein phosphorylation, and accumulation of singling molecules, 9. Activation of CDPKs, protein phosphorylation, and accumulation of singling molecules.](/cms/asset/a5297333-9253-4d16-a123-bb2f7bab90ba/irab_a_2324469_f0003_c.jpg)
ROS are strong oxidants and play an important role in cell signaling, differentiation, cell survival, and death (Mittler Citation2017; Waszczak et al. Citation2018). However, in order to mitigate the effects of oxidative stress plants activate both enzymatic and non-enzymatic oxidant defense machinery to scavenge excess ROS (Sewelam et al. Citation2016). Varied antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), peroxiredoxins (PRX), glutathione peroxidase (GPX), and glutathione reductase (GR) production were described to be accumulated in plants upon exposure to γ-radiation (Jan et al. Citation2012). It has been shown in different plants that increased in content of antioxidant enzymes upon exposure to γ-radiation. For example, irradiation of rosemary calluses (5–20 Gy) activates SOD, CAT, CAT, ascorbate peroxidase and (GR) (El-Beltagi et al. Citation2011). The balance between ROS and antioxidants may be triggering a further cascade of events during γ-radiation mediated singling events. It has been demonstrated that the ROS singling system is functionally coupled with other signaling systems in plants such as calcium and hormonal singling (Mittler Citation2017; Gilroy et al. Citation2016). Varied shreds of evidence have also supported the view that putative γ-radiation singling cascades may also include phospholipase C/D, phosphoinositide-dependent kinase (PKD), Ca2+-binding protein (calmodulin; calcium and calmodulin complex stimulates calcium-dependent kinases (CDPK)), mitogen-activated protein-activated protein kinase (MAPK). However, conclusive molecular, genome, and proteomic information are not elucidated in this direction. Nevertheless, the role of certain transcription factors such as HSF, ZAT, WRKY, and Myb families in relation to γ-radiation mediated singling cascade has been predicted by Kim et al. (Citation2011) and Goh et al. (Citation2014) ().
The high doses of γ-radiation are responsible for both partially due to the absorption of energy by DNA molecules as well as ROS which leads to DNA damage. It was estimated that a significant part (about 70–80%) of the γ-radiation-related DNA damage is caused by ROS formed during the radiolysis of water and around 20–30% of the damage is due to the direct absorption of high energy by the target DNA (Gudkov et al. Citation2019). DNA damage in response to γ-radiation is of two kinds, namely single-strand breaks (SSBs) or double-strand breaks (DSBs). Both point mutation substitution of nucleotides, and chromosomal mutations are reported in plants when they are exposed to ionizing radiations (Esnault et al. Citation2010) (). DNA repair mechanisms do exist in plants as in the case human system, however, the role of repair mechanisms involved in plants has not been understood very well. Homologous recombination (HR) and non-homologous system (NHR) repair systems are reported to be present in plants (Hefner et al. Citation2003). However, repair errors may lead to chromosomal aberrations as observed during mitosis or meiosis (Misra et al. Citation1991; Zaka et al. Citation2002). It has been shown that ionizing radiation may result in the formation of an altered progeny that exhibits specific adaptive abilities depending on the species considered (Hernández-Muñoz et al. Citation2019; Lal et al. Citation2020; Riviello-Flores et al. Citation2022).
Figure 4. Illustration of the mechanism of elicitation of specialized metabolites in plant cell and organ cultures by low and high doses of γ-irradiation. 1. Exposure of cells with low dose γ-irradiation, 2. Accumulation of reactive oxygen species (ROS), 3. Activation of MAPKs/CDPKs, protein phosphorylation, and accumulation of singling molecules, 5. Enhanced biosynthesis of specialized metabolites, 6. Exposure of cells with higher dose γ-irradiation, 7. Interaction of electrons with DNA, 8. Gene mutation, 9. Chromosomal mutation, 10. Altered gene expression leads to enhanced production of specialized metabolites.
Conclusion
It is clear from the available literature and evidence that gamma irradiation of in vitro cell and organ culture is a promising method for eliciting the biosynthetic process and accumulation of valuable SMs. Useful mutant lines were also induced with the application of gamma irradiation with in vitro cultures. Elicitation of in vitro cultures has demonstrated the induction and accumulation of oxidants in the plant cells, which are responsible for stimulating varied signaling cascade of events of hyperaccumulation of SMs. Stimulation of the antioxidant system to balance the detrimental effect of oxidants in plant cell and organ cultures is also evident. The elicitation effect of gamma irradiation was found to be tremendously influenced by the type of tissue used, species, cultivar, stage of growth and development, and other features such as dose and duration of exposure. However, finding the best conditions, doses, and combinations of treatments should be evaluated with particular plant species and specific types of cultures. There is a lack of information on the mechanism of elicitation in cell and organ cultures induced by gamma irradiation, future studies should focus on the elucidation of specific steps and key singling molecules, gene expression, and gene products that trigger secondary metabolism. Studies on genomic, proteomic, and metabolomic studies of specific systems and particular which are involved in secondary metabolic processes will elucidate these events. In the near future, attention should be paid to induction mutations using in vitro grown cells, study of the kind of mutation (point or chromosomal mutations), characterization, and stabilization of mutant lines.
Authors’ contributions
HNM: conceptualization; HNM: investigation, data curation, and formalization; SYP: resources and validation; HNM, KSJ, KYP, and SYP: writing, review, and editing. All the authors have read and agreed to the published version of this manuscript.
Acknowledgments
Hosakatte Niranjana Murthy is thankful for the “Brain Pool” (Grant No. 2022H1D3A2A02056665) of the National Research Foundation, Republic Korea, and Dr. Rajaramanna fellowship awarded by the Department of Atomic Energy, Government of India (Grant No. D.O. No. 1003/6/2023/R&D/II/DAE/6461).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Hosakatte Niranjana Murthy
Hosakatte Niranjana Murthy was a Professor at Department of Botany, Karnatak University, India, currently working as a Professor at Department of Biotechnology, KLE Technological University, Hubballi, India and Brain Pool Fellow at Department of Horticulture, Chungbuk National University, Cheongju, Republic of Korea. He is a researcher in the field of Plant Biotechnology and involved in teaching the graduate and master students.
Kadanthottu Sebastian Joseph
Kadanthottu Sebastian Joseph is an Assistant Professor at Department of Life Sciences, CHRIST (Deemed to be University), Bengaluru, India. He is involved in teaching the graduate and master students.
Kee Yoeup Paek
Kee Yoeup Paek is an Endowment Professor at Department of Horticulture, Chungbuk National University, Cheongju, Republic of Korea and CEO, Well Green Biotech Co., Chungbuk National University, Cheongju, Republic of Korea. He is a researcher in the field of Plant Biotechnology.
So Young Park
So Young Park is a Professor at Department of Horticulture, Chungbuk National University, Cheongju, Republic of Korea. She is a researcher in the field of Plant Biotechnology and involved in teaching the graduate and master students.
References
- Almukhtar SA, Alrubaye MA, Elkaaby EA, Kadhim ZK, Alkilabi CK. 2019. Effect of irradiation by gamma rays and the use of benzyl adenine to increase the production of cardiac glycoside compounds form Digitalis lanata in vitro. IOP Conf Ser: Earth Environ Sci. 388(1):012068. doi:10.1088/1755-1315/388/1/012068
- Aryal B, Raut BK, Bhattarai S, Bhandari S, Tandan P, Gyawali K, Sharma K, Ranabhat D, Thapa R, Aryal D, et al. 2022. Potential therapeutic applications of plant-derived alkaloids against inflammatory and neurodegenerative diseases. Evid Based Complement Alternat Med. 2022:7299778. doi:10.1155/2022/7299778
- Azeez H, Ibrahim K, Pop R, Pamfil D, Hârţa M, Bobiș O. 2017. Changes induced by gamma-ray irradiation on biomass production and secondary metabolites accumulation in Hypericum triquentrifolium Turra callus cultures. Ind Crops Prod. 108:183–189. doi:10.1016/j.indcrop.2017.06.040
- Chung BY, Lee YB, Baek MH, Kim JH, Wi SG, Kim JS. 2006. Effects of low-dose gamma-irradiation on production of shikonin derivatives in callus cultures of Lithospermum erythrorhizon S. Radiat Phys Chem. 75(9):1018–1023. doi:10.1016/j.radphyschem.2005.11.001
- Ciocan A-G, Maximilian C, Mitoi EM, Moldovan R-C, Neguț D, Iuga C-A, Helepciuc FE, Holobiuc I, Radu M, Vassu Dimov T, et al. 2023. The impact of acute low-dose gamma irradiation on biomass accumulation and secondary metabolites production in Cotinus coggygria Scop. And Fragaria × ananassa Duch. red callus cultures. Metabolites. 13(8):894. doi:10.3390/metabo13080894
- El-Beltagi HS, Ahmed OK, El-Desouky W. 2011. Effect of low doses γ-irradiation on oxidative stress and secondary metabolites production of rosemary (Rosmarinus officinalis L.) callus cultures. Radiat Phys Chem. 80(9):968–976. doi:10.1016/j.radphyschem.2011.05.002
- El-Garhy HAS, Khattab S, Moustafa MMA, Ali RA, Azeiz AZA, Elhalwagi A, Sherif FE. 2016. Silybin content and overexpression of chalcone synthase genes in Silybum marianum L. plants under abiotic elicitation. Plant Physiol Biochem. 108:191–202. doi:10.1016/j.plaphy.2016.07.011
- Esnault MA, Legue F, Chenal C. 2010. Ionizing radiation: Advances in plant response. Environ Exp Bot. 68(3):231–237. doi:10.1016/j.envexpbot.2010.01.007
- Fulzele DP, Satdive R, Kamble S, Singh S, Singh S. 2015. Improvement of anticancer drug camptothecin production by gamma irradiation on callus cultures of Nothapodytes foetida. Int J Pharm Res Allied Sci. 4:19–27.
- Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R. 2016. ROS, calcium, and electric signals: key mediators of rapid systematic signaling in plants. Plant Physiol. 171(3):1606–1615. doi:10.1104/pp.16.00434
- Goh EJ, Kim JB, Kim WJ, Ha BK, Kim SH, Kang SY, Seo YW, Kim DS. 2014. Physiological changes and anti-oxidative responses of Arabidopsis plants after acute and chronic gamma irradiation. Radiat Environ Biophys. 53(4):677–693. doi:10.1007/s00411-014-0562-5
- Gudkov SV, Grinberg MA, Sukhov V, Vodeneev V. 2019. Effect of ionizing radiation on physiological and molecular processes in plants. J Environ Radioact. 202:8–24. doi:10.1016/j.jenvrad.2019.02.001
- Hamdani AM, Wani IA, Gani A, Bhat NA, Masoodi FA. 2017. Effect of gamma irradiation on physicochemical, structural and rheological properties of plant exudate gums. Innov Food Sci Emerg Technol. 44:74–82. doi:10.1016/j.ifset.2017.07.014
- Hefner E, Preuss SB, Britt AB. 2003. Arabidopsis mutants sensitive to gamma radiation include homologue of the reman repair gene ERCC1. J Exp Bot. 54(383):669–680. doi:10.1093/jxb/erg069
- Hernández-Muñoz S, Pedraza-Santos ME, Antonio López P, Gómez-Sanabria JM, Morales-García JL. 2019. Mutagenesis in the improvement of ornamental plants. rchsh. 25(3):151–167. doi:10.5154/r.rchsh.2018.12.022
- Ho JHC, Hong CY. 2011. Salvianolic acids: small compounds with multiple mechanisms for cardiovascular protection. J Biomed Sci. 18(1):30. doi:10.1186/1423-0127-18-30
- Huang XQ, Dudareva N. 2023. Plant specialized metabolism. Curr Biol. 33(11):R473–R478. doi:10.1016/j.cub.2023.01.057
- Jan, Sumira, Parween, Talat, Siddiqi, T.O., Mahmooduzzafar,. 2012. Effect of gamma radiation on morphological, biochemical and physiological aspects of plants and plant products.Environ. Rev., 1. 20: 17–39. doi:10.1139/a11-021
- Jaisi A, Sakunphueak A, Panichayupakaranant P. 2013. Increased production of plumbagin in Plumbao indica root cultures by gamma ray irradiation. Pharm Biol. 51(8):1047–1051. doi:10.3109/13880209.2013.775163
- Katiyar P, Pandey N, Keshavkant S. 2022. Gamma radiation: A potential tool for abiotic stress mitigation and management of agroecosystem. Plant Stress. 5:100089. doi:10.1016/j.stress.2022.100089
- Khalifa AM, Abd-Elshafy E, Abu-Khudir R, Gaafar RM. 2022. Influence of gamma radiation and phenylalanine on secondary metabolites in callus cultures of milk thistle (Silybum marianum L.). J Genet Eng Biotechnol. 20(1):166. doi:10.1186/s43141-022-00424-2
- Kim DS, Song M, Kim SH, Jang DS, Kim JB, Ha BK, Kim SH, Lee KJ, Kang SY, Jeong IY. 2013. The improvement of ginsenoside accumulation in Panax ginseng as a result of γ-irradiation. J Ginseng Res. 37(3):332–340. doi:10.5142/jgr.2013.37.332
- Kim DS, Kim JB, Goh EJ, Kim WJ, Kim SH, Seo YW, Jang CS, Kang SY. 2011. Antioxidant response to Arabidopsis plant to gamma irradiation: genome-wide expression profiling of the ROS scavenging and signal transduction pathways. J Plant Physiol. 168(16):1960–1971. doi:10.1016/j.jplph.2011.05.008
- Kim DS, Kim SY, Jeong IY, Kim JB, Lee GJ, Kang SY, Kim W. 2009. Improvement of ginsenoside production by Panax ginseng adventitious roots induced by γ-irradiation. Biologia Plant. 53(3):408–414. doi:10.1007/s10535-009-0079-y
- Kim JH, Lee MH, Moon YR, Kim JS, Wi SG, Kim TH, Chung BY. 2009. Characterization of metabolic disturbances closely linked to the delayed senescence of Arabidopsis leaves after gamma-irradiation. Environ Exp Bot. 67(2):363–371. doi:10.1016/j.envexpbot.2009.07.001
- Lal RK, Chanotiya CS, Gupta P. 2020. Induced mutation breeding for qualitative and quantitative traits and varietal development in medicinal and aromatic crops at CSIR-CIMAP, Lucknow (India): Past and recent accomplishment. Int J Radiat Biol. 96(12):1513–1527. doi:10.1080/09553002.2020.1834161
- Le KC, Ho TT, Paek KY, Park SY. 2019. Low-dose gamma radiation increases the biomass and ginsenoside content of callus and adventitious root cultures of wild ginseng (Panax ginseng Mayer). Ind Crops Prod. 130:16–24. doi:10.1016/j.indcrop.2018.12.056
- Lee H, Kong G, Tran Q, Kim C, Park J, Park J. 2020. Relationship between ginsenoside Rg3 and metabolic syndrome. Front Pharmacol. 11:130. doi:10.3389/fphar.2020.00130
- Mariadoss A, Satdive R, Fulzele DP, Ramamoorthy S, Doss GPC, Zayed H, Younes S, Rajasekaran C. 2020. Enhanced production of anthraquinones by gamma-irradiated cell cultures of Rubia cordifolia in a bioreactor. Ind Crops Prod. 145:111987. doi:10.1016/j.indcrop.2019.111987
- Misra HO, Sharma JR, Lal RK. 1991. Radiation induced cytomorphological changes in Hyocyamus muticus L. cytologia. 56(4):527–531. doi:10.1508/cytologia.56.527
- Mittler R. 2017. ROS Are good. Trends Plant Sci. 22(1):11–19. doi:10.1016/j.tplants.2016.08.002
- Moghaddam SS, Jaafar H, Ibrahim R, Rahmat A, Aziz MA, Philip E. 2011. Effects of acute gamma irradiation on physiological traits and flavonoid accumulation of Centella asiatica. Molecules. 16(6):4994–5007. doi:10.3390/molecules16064994
- Mohamed AA. 2009. Effect of low dose gamma irradiation on some phytochemicals and scavenger ability of in vitro Culantro (Eryngium foetidum L.) plantlets. Med Aromat Plant Sci Biotechnol. 3:32–36.
- Mujib A, Fatima S, Malik MQ. 2022. Gamma ray-induced tissue responses and improved secondary metabolites accumulation in Catharanthus roseus. Appl Microbiol Biotechnol. 106(18):6109–6123. doi:10.1007/s00253-022-12122-7
- Murthy HN, Joseph KS, Paek KY, Park SY. 2023a. Bioreactor systems for micropropagation of plants: present scenario and future prospects. Front Plant Sci. 14:1159588. doi:10.3389/fpls.2023.1159588
- Murthy HN, Joseph KS, Paek KY, Park SY. 2023b. Production of anthraquinones from cell and organ cultures of Morinda species. Appl Microbiol Biotechnol. 107(7-8):2061–2071. doi:10.1007/s00253-023-12440-4
- Murthy HN, Joseph KS, Paek KY, Park SY. 2023c. Bioreactor configurations for adventitious root culture: recent advances toward the commercial production of specialized metabolites. Crit Rev Biotechnol. 27:1–23. doi:10.1080/07388551.2023.2233690
- Murthy HN, Joseph KS, Paek KY, Park SY. 2023d. Nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites in cell and organ cultures: current status and future outlooks. Plant Growth Regul. doi:10.1007/s10725-023-01086-x
- Murthy HN, Joseph KS, Hahn JE, Lee HS, Paek KY, Park SY. 2023e. Suspension culture of somatic embryos for the production of high-value secondary metabolites. Physiol Mol Biol Plants. 29(8):1153–1177. doi:10.1007/s12298-023-01365-x
- Murthy HN, Joseph KS, Paek KY, Park SY. 2022. Anthraquinone production from cell and organ cultures of Rubia species: an overview. Metabolites. 13(1):39. doi:10.3390/metabo13010039
- Murthy HN, Dandin VS, Paek KY. 2016. Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem Rev. 15(1):129–145. doi:10.1007/s11101-014-9391-z
- Murthy HN, Lee EJ, Paek KY. 2014. Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss Organ Cult. 118(1):1–16. doi:10.1007/s11240-014-0467-7
- Narayani M, Srivastava S. 2017. Elicitation: a stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem Rev. 16(6):1227–1252. doi:10.1007/s11101-017-9534-0
- Ognyanov M, Denev P, Teneva D, Georgiev Y, Taneva S, Totseva I, Kamenova-Nacheva M, Nikolova Y, Momchilova S. 2022. Influence of gamma irradiation on different phytochemical constituents of dried rose hip (Rosa canina L.) fruits. Molecules. 27(6):1765. doi:10.3390/molecules27061765
- Qi W, Zhang L, Feng W, Xu H, Wang L, Jiao Z. 2015. ROS and ABA signaling are involved in the growth stimulation induced by low-dose gamma radiation in Arabidopsis seedling. Appl Biochem Biotechnol. 175(3):1490–1506. doi:10.1007/s12010-014-1372-6
- Radomir A-M, Temelie M, Moldovan R-C, Stoica R, Petrache A-M, Helepciuc F-E, Savu DI, Iuga C-A, Moroșanu A-M, Neguț CD, et al. 2023. Effect of gamma irradiation on phenolic content, biological activity, and cellular ultrastructure of Salvia officinalis L. cultured in vitro. Plant Cell Tiss Organ Cult. 154(1):141–160. doi:10.1007/s11240-023-02522-6
- Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J. 2016. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules. 21(2):182. doi:10.3390/molecules21020182
- Ratan ZA, Haidere MF, Hong YH, Park SH, Lee JO, Lee J, Cho JY. 2021. Pharmacological potential of ginseng and its major component ginsenosides. J Ginseng Res. 45(2):199–210. doi:10.1016/j.jgr.2020.02.004
- Reshi ZA, Ahmad W, Lukatkin AS, Javed SB. 2023. From nature to lab: A review of secondary metabolite biosynthetic pathways, environmental influences, and in vitro approaches. Metabolites. 13(8):895. doi:10.3390/metabo13080895
- Riviello-Flores MdlL, Cadena-Iñiguez J, Ruiz-Posadas LDM, Arévalo-Galarza MdL, Castillo-Juárez I, Soto Hernández M, Castillo-Martínez CR. 2022. Use of gamma radiation for the genetic improvement of underutilized plant varieties. Plants (Basel). 11(9):1161. doi:10.3390/plants11091161
- Sewelam N, Kazan K, Schenk PM. 2016. Global plant stress signaling: reactive oxygen species at the cross-road. Front Plant Sci. 7:187. doi:10.3389/fpls.2016.00187
- Taiz L, Zeiger E, Moller IM, Murphy A. 2015. Plant physiology and development. Sinauer Associates, Inc. Sunderland, MA, USA.
- Vanhoudt N, Horemans N, Wannijn J, Nauts R, Hees MV, Vandenhove H. 2014. Primary stress responses in Arabidopsis thaliana exposed to gamma radiation. J Environ Radioact. 129:1–6. doi:10.1016/j.jenvrad.2013.11.011
- Vardhan PV, Shukla LI. 2017. Gamma irradiation of medicinally important plant and the enactment of secondary metabolite production. Int J Radiat Biol. 93(9):967–979. doi:10.1080/09553002.2017.1344788
- Waszczak C, Carmody M, Kangasjärvi J. 2018. Reactive oxygen species in plant singling. Annu Rev Plant Biol. 69(1):209–236. doi:10.1146/annurev-arplant-042817-040322
- Wi SG, Chung BY, Kim JH, Baek MH, Yang DH, Lee JW, Kim JS. 2005. Ultrastructural changes of cell organelles in Arabidopsis stem after gamma irradiation. J Plant Biol. 48(2):195–200. doi:10.1007/BF03030408
- Zaka R, Chenal C, Misset MT. 2002. Study of external low irradiation does effects on induction of chromosome aberrations in Psium sativum root tip meristem. Mutat Res. 517(1-2):87–99. doi:10.1016/S1383-5718(02)00056-6
- Zhang JY, Bae TW, Boo KH, Sun HJ, Song IJ, Pham CH, Ganesan M, Yang DH, Kang HG, Ko SM, et al. 2011. Ginsenoside production and morphological characterization of wild ginseng (Panax ginseng Meyer) mutant lines induced by γ-irradiation (60Co) of adventitious roots. J Ginseng Res. 35(3):283–293. doi:10.5142/jgr.2011.35.3.283
- Zhao J, Davis LC, Verpoorte R. 2005. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 23(4):283–333. doi:10.1016/j.biotechadv.2005.01.003