ABSTRACT
Parasitoid wasps of the genus Trichogramma are used worldwide as biological control agents against lepidopteran pests. Trichogramma wasps develop inside eggs of a wide range of host species, most of them moths. They are generally considered as diurnal insects. Here, we investigated whether Trichogramma wasps can also successfully parasitise host eggs at night under controlled laboratory conditions. Eggs of the moth Ephestia kuehniella were offered under dark conditions (scotophase) to females of Trichogramma brassicae and Trichogramma evanescens either from 9:00 PM to 9:00 AM or from 11:00 AM to 5:00 PM at four different temperatures (5°C, 10°C, 15°C and 20°C). Both species are known to parasitise E. kuehniella eggs in the photophase during daytime. The results show that T. brassicae did not parasitise eggs in the scotophase at night and only very few in the artificially induced scotophase during daytime from 10°C to 20°C. In contrast, T. evanescens parasitised more eggs in the dark both at night and artificially induced scotophase during daytime. Parasitism in the scotophase already started at 5°C, with more eggs being parasitised and more offspring being produced at higher temperatures. T. evanescens displayed higher parasitism activity in the induced scotophase during daytime than in the scotophase at night. The present study suggests that Trichogramma are capable of successfully parasitising host eggs at night, even at low temperatures, but that nocturnal activity with respect to parasitism varies between wasp species.
Introduction
Egg parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae) are used worldwide as important biological agents to control agricultural pests. Trichogramma wasps are known to attack eggs of more than 400 species, mainly of the order Lepidoptera (Li, Citation1994; Polaszek, Citation2010; Smith, Citation1996; Stinner, Citation1977). Trichogramma wasps are generally considered as polyphagous, although the level of polyphagy is likely to vary between and even within species in nature (Pinto & Stouthamer, Citation1994).
Trichogramma wasps display different strategies to find their host eggs. A wide range of mainly laboratory studies have shown that they can exploit a variety of chemical cues including host products (e.g. scales and host pheromones) and plant cues (e.g. those induced by host egg deposition) (for a review, see Fatouros, Dicke, Mumm, Meiners, & Hilker, Citation2008). Although the wasps may find lepidopteran host eggs mainly by walking and flying short distances within a host habitat, some species may also hitch-hike with adult butterflies and moths to disperse over longer distances and/or to obtain immediate access to freshly laid host eggs (Fatouros & Huigens, Citation2012; Fatouros, Huigens, van Loon, Dicke, & Hilker, Citation2005; Huigens & Fatouros, Citation2013; Huigens et al., Citation2009, Citation2010). We expect that this phoretic behaviour may also occur at low temperatures at night. During a field survey in The Netherlands, Trichogramma brassicae Bezdenko (Hymenoptera: Trichogrammatidae) was found on a female moth of Xestia c-nigrum L. (Lepidoptera: Noctuidae) at night at a temperature below 10°C (Fatouros & Huigens, Citation2012; Woelke, Citation2008). It is, however, still unknown what occurs in between transport on an adult moth and actual parasitism. More specifically, can female Trichogramma wasps parasitise moth eggs at low temperatures at night?
To the best of our knowledge, nocturnal parasitism by Trichogramma wasps has not been extensively investigated. These wasps are generally considered as diurnal insects. Studies on the circadian rhythm of locomotor activity of T. brassicae have shown that these wasps are day-active and are not active during the scotophase (Allemand, Pompanon, Fleury, Fouillet, & Boulétreau, Citation1994; Pompanon, Fouillet, & Bouletréau, Citation1995, Citation1999). It has been shown that both sexes of T. brassicae start to become active approximately one hour before lights were turned on, while activity increases quickly and wasps remain active until the lights were turned off (Pompanon, Fouillet, & Bouletréau, Citation1999). Males are most active in the morning when they are searching for females to mate with and females are active in the morning until the end of the afternoon when they are searching for suitable hosts (recorded activity at a constant temperature of 22°C) (Pompanon et al., Citation1999). Adult female T. brassicae wasps start emerging from a host egg two hours before sunrise, though the majority of individuals emerge later in the morning, while male eclosion precedes that of the females by approximately half an hour (Pompanon et al., Citation1995). This emergence pattern is similar in T. bourarachae Pintureau & Babault, T. minutum Riley and T. evanescens Westwood (all Hymenoptera: Trichogrammatidae) (Forsse, Smith, & Bourchier, Citation1992; Pompanon et al., Citation1995).
Beside light and darkness regimes (Allemand et al., Citation1994; Pompanon et al., Citation1995, Citation1999; Reznik, Voinovich, & Karpova, Citation2009), temperature is also an important factor that influences locomotion in Trichogramma wasps. Temperature influences not only walking speed and walking activity (Suverkropp, Bigler, & van Lenteren, Citation2001), and flight activity (Forsse et al., Citation1992), but also parasitism (Maceda, Hohmann, & dos Santos, Citation2003; Pak & van Heiningen, Citation1985). In general, activity increases with higher temperatures until a certain optimal temperature is reached, after which activity decreases.
The aim of this study was to investigate whether female Trichogramma wasps can parasitise moth eggs in the dark at low temperatures, and not to compare nocturnal with diurnal parasitism of host eggs. Trichogramma wasps are well known to parasitise eggs under light conditions during daytime. We, however, also expect occasional parasitism of eggs of nocturnal host species in the dark during relatively warm summer nights, for example, shortly after sunset or just before sunrise. Such could, for example, occur after a hitch-hiking experience with a nocturnal female moth (Fatouros & Huigens, Citation2012; Huigens & Fatouros, Citation2013). To investigate whether the ability of parasitism in the dark is wasp species-specific, we investigated different strains of T. brassicae and the closely related T. evanescens. T. brassicae is known to parasitise eggs of butterflies, day-active moths and nocturnal moths (Polaszek, Citation2010). This also applies to T. evanescens, but this species is known to parasitise eggs of much more nocturnal moth species than T. brassicae (Polaszek, Citation2010). We thus expect T. evanescens to parasitise more eggs in the dark than T. brassicae.
Materials and methods
Insects
Females without previous oviposition experience of two iso-female strains were tested per wasp species, that is, T. brassicae strains Y175 and J007 and T. evanescens strains GD011 and GD025. These strains have previously been discriminated by a molecular key based on the Internal Transcribed Spacer 2 gene region (Stouthamer, Hu, van Kan, Platner, & Pinto, Citation1999, sequences are available in an extensive electronic database with ITS-2 sequences of Trichogramma wasps managed by Prof. Richard Stouthamer at the University of California, Riverside, USA) and were used in previous diurnal studies (Fatouros, Bukovinszkine‘Kiss, Dicke, & Hilker, Citation2007; Fatouros, Broekgaarden, et al., Citation2008; Fatouros, Bukovinszkine‘Kiss, et al., Citation2005, Fatouros, Huigens, et al., Citation2005, Fatouros et al., Citation2012; Huigens, de Swart, & Mumm, Citation2011; Huigens et al., Citation2009, Citation2010). Iso-female strain Y175 originated from a vegetable garden in Lienden, The Netherlands, and was collected from a paper card with eggs of the cabbage moth Mamestra brassicae L. (Lepidoptera: Noctuidae) attached to a cabbage plant in the summer of 1999. Iso-female strain J007 was collected from an adult female X. c-nigrum moth at 7.2°C during a moth catch late in the evening (when it was dark) in the summer of 2007 in Ede, The Netherlands (Fatouros & Huigens, Citation2012; Woelke, Citation2008). Iso-female strains GD011 and GD025 were both collected from eggs of the butterfly Pieris rapae L. (Lepidoptera: Pieridae) present on Brassica oleracea L. plants (Brussels sprouts; Brassicaceae) in the summer of 2006 in Wageningen, The Netherlands.
All strains were reared for many generations on Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs under standardised laboratory procedures and conditions at the Laboratory of Entomology of Wageningen University & Research (22 ± 1°C; 60% relative humidity and a 16:8h light:darkness (L:D) period with a photophase from 6 AM to 10 PM). E. kuehniella eggs were obtained from Koppert Biological Systems B.V. During the rearing procedures, a superfluous amount of honey is always provided as food source for the wasps. A female wasp only lays a single egg per oviposition event inside an E. kuehniella egg (M. E. Huigens, personal observations). One wasp thus normally emerges from one E. kuehniella egg. Only under limited host conditions (which is not the case in our study) superparasitism may occasionally occur: two wasp eggs allocated in one E. kuehniella egg may then result in two emerging wasps (M. E. Huigens, personal observations). Wasps of all strains are known to parasitise eggs in the photophase during daytime (Woelke & Huigens, personal observations; Fatouros, Broekgaarden, et al., Citation2008; Fatouros, Bukovinszkine‘Kiss, et al., Citation2005, Fatouros, Huigens, et al., Citation2005; Huigens et al., Citation2009, Citation2010).
Darkness and temperature regimes
Cohorts of both wasp species were exposed to two different darkness regimes in combination with four different temperatures (5°C, 10°C, 15°C or 20°C). In the presence of host eggs, wasps were either transferred to a dark climate cabinet at a time that they normally experience daytime conditions, that is, from 11 AM to 5 PM, or wasps were exposed to darkness for 12 hours from 9 PM till 9 AM the next day at a time when they would normally experience their night time. A temperature range between 5°C and 20°C is typical for summer and autumn nights in The Netherlands (Royal Netherlands Meteorological Institute, De Bilt, The Netherlands).
Experimental set-up
Forty, mated female wasps without previous oviposition experience (two-days-old and honey-fed) of both species and of each strain were individually placed in glass vials closed with cotton wool. These female wasps were individually provided with a small, single drop of honey to make sure that they had a sufficient amount of food during the darkness and temperature regimes. Just before transfer into a dark climate cabinet, each wasp was offered 140 ± 10 E. kuehniella eggs that were present on a small round egg card (radius 5 mm). After a darkness and temperature treatment, wasps were removed from the vials with E. kuehniella eggs and the eggs were placed in a climate room (22 ± 1°C, 60% relative humidity, 16:8 L:D). Parasitised eggs, which can be recognised by their dark (grey to black) colour were counted 7 days after treatment (see Results section on ‘parasitism response levels of female wasps’). After 12 days, emerged wasps were counted (see Results section on ‘number of host eggs parasitised by T. evanescens females; number of emerging progeny’) and sexed.
Data analysis
We compared the parasitism response levels of females between and within T. brassicae and T. evanescens species and strains. Females were scored for whether they parasitised eggs (scored 1) or not (scored 0), which was then treated as the response variable. Parasitism was compared by using a Generlised Linear Model (GLIM) for binary data, with logit link function and a binomial distribution for errors. As the two species showed highly significant differences in their responses, we fit the same model to each species separately, with temperature and light regimes and the two strains as fixed factors in the models. Subsequent differences between the four temperature regimes were examined by requesting linear contrasts.
To compare the offspring production by T. evanescens between treatment groups, we counted (i) the number of eggs parasitised and (ii) the number of emerged offspring in a vial. As parasitism was absent or sporadic at temperatures of 5°C and 10°C, only wasps of strain GD025 at 10°C yielded enough observations for data analysis.
We first tested the effects of fixed factors (strain, temperature and light) and their interactions on the levels of parasitism and the number of emerged offspring, using a three-way General Linear Model (GLM), using a model that included temperature regimes (15°C and 20°C) that were fully replicated in both strains and light regimes.
In a separate ANOVA test, we subsequently compared the remaining treatment group (10°C, 6 hours, strain GD025) to the rest of the groups in that treatment level (light, strain). Both dependent variables showed homogeneous variances across treatment groups (Levene’s test) and the residuals of the fitted model were normally distributed.
To investigate the effects of wasp strain, light and temperature regimes on the sex ratios of the produced offspring, a GLIM for proportions was used. For each vial, the fraction of females within each vial was modelled as logits: logit(p) = log(p/(1 – p), assuming a binomial distribution for errors. Parasitoid strain (G011, G025), light (6 or 12 hours) and temperature (15°C, 20°C) regimes were included as fixed factors. Overdispersion in the data was corrected by allowing the variance functions of the binomial distribution to have a multiplicative overdispersion factor by dividing the square root of the deviance of the model by the degrees of freedom (McCullagh & Nelder, Citation1989). Analyses were carried out using SAS 9.2.
Results
Parasitism response levels of female wasps
When including all darkness and temperature regimes together, significantly much more female T. evanescens wasps parasitised E. kuehniella eggs than female T. brassicae wasps did (, P < .001). The frequency of females parasitising E. kuehniella eggs was similar between the two strains in the case of both T. brassicae and T. evanescens ( and ). In T. brassicae, there was no significant difference in the frequency of parasitising females between the temperature regimes (). Moreover, the overall frequency of parasitising T. brassicae wasps was very low (<5% of the females had parasitised eggs). In contrast, females of T. evanescens parasitised more eggs with increasing temperature (). When comparing the frequency of parasitising females during the two darkness regimes, more females parasitised eggs during the period of 6 hours than the period of 12 hours of darkness; these differences were significant for both T. brassicae and T. evanescens ().
Figure 1. Percentage of female T. brassicace (strain Y175 and J007) and T. evanescens (strain GD011 and GD025) that parasitised eggs of the moth E. kuehniella at different temperatures during (A) 6 hours of scotophase from 11 AM to 5 PM and (B) 12 hours of scotophase from 9 PM to 9 AM the next day. In total, 40 two-days-old, mated females per strain were tested for each temperature treatment.
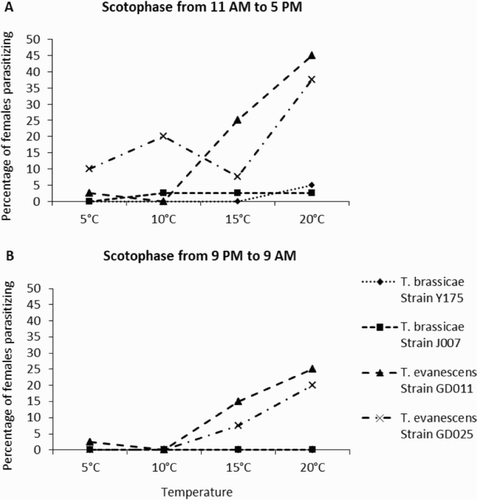
Table 1. Comparison of the parasitism response levels of females of T. brassicae (strain Y175 and J007) and T. evanescens (strain GD011 and GD025) within each species at different temperatures (5°C, 10°C, 15°C and 20°C) during 6 hours of scotophase (from 11 AM to 5 PM) and 12 hours of scotophase (from 9 PM to 9 AM the next day). In total, 40 two-days-old, mated females per strain were tested for each temperature treatment. The factor ‘Light’ includes the comparison between the two light regimes.
T. brassicae wasps parasitised eggs only during the 6 hours, and not during the 12 hours of scotophase period (). Females of strain J007 start parasitising eggs at 10°C and those of strain Y175 only at 20°C. In contrast, female T. evanescens wasps did parasitise eggs during the 6 and 12 hours of scotophase (). Females of both T. evanescens strains started parasitising eggs at 5°C during the 6 hours of darkness. During the 12 hours of darkness, strain GD011 started parasitising at 5°C, whereas strain GD025 started parasitising at 15°C.
Number of host eggs parasitised by T. evanescens females
We also compared the number of host eggs parasitised per parasitising T. evanescens female, as a measure of parasitism activity, at different temperatures and darkness regimes (). Here, T. brassicae was not included because there were very few females of this species that parasitised hosts ( and ).
Table 2. Mean number of parasitised eggs (±standard error) and mean offspring production (±standard error) per T. brassicae strain (Y175 and J007) and T. evanescens strain (GD011 and GD025) at different temperatures (5°C, 10°C, 15°C and 20°C) during 6 hours of scotophase (from 11 AM to 5 PM) and 12 hours of scotophase (from 9 PM to 9 AM the next day). In total, 40 two-days-old, mated females per strain were tested for each temperature treatment.
When comparing both T. evanescens strains, the number of parasitised eggs per female differed significantly (). Female wasps of strain GD025 parasitised more host eggs than those of strain GD011. The number of parasitised eggs was similar for the two light regimes and increased with temperature (). The number of parasitised eggs per female at 10°C was more than those observed at 15°C and 20°C within the same strain (G025) and light regimes (6 hours) (ANOVA, F2, 25 = 15.151, P < .001, Tukey Honestly Significant Difference (HSD) P < .001). None of all possible two- or three-way interaction had a significant effect (P > .05), which indicated that the effects of changing the light regime and temperature on parasitism were independent of each other and the strains of T. evanescens ().
Table 3. Tests of between-subjects effects with dependent variable offspring of T. evanescens strain (GD011 and GD025) at different temperatures during 6 hours of scotophase (from 11 AM to 5 PM) and 12 hours of scotophase (from 9 PM to 9 AM the next day), excluding treatment at 10°C for 6 hours of scotophase as this was not replicated in both strains. In total, 40 two-days-old, mated females per strain were tested for each temperature treatment. The factor ‘Light’ includes the comparison between the two light regimes.
The differences in the number of emerged offspring reflected those observed in the case of parasitised eggs. When comparing the offspring production between both T. evanescens strains (), we found a significant difference between the strains (GLM, F1, 64 = 13.061, P = .001). Strain GD025 produced more offspring than strain GD011 ( and ). There was no significant difference between the light regimes (GLM, F1, 64 = 0.313, P = .578) and between the temperature regimes 15°C and 20°C (GLM, F1, 64 = 2.39, P = .127). However, the number of emerged wasps at 10°C was significantly lower than those emerged at 15°C and 20°C within the same strain (GD025) and light regimes (6 hours) (ANOVA, F2, 25 = 22.11, P < .001, Tukey HSD P < .001, ), indicating higher offspring emergence at higher temperatures.
There was no significant difference between the offspring sex ratio of either T. evanescens strains (, P = .382) and no difference in offspring sex ratio between the light (
, P = .693) and temperature regimes (
, P = .227).
Number of emerging progeny
The differences observed in the frequency of females parasitising E. kuehniella eggs between species were also reflected in the number of emerging progeny (). More T. evanescens offspring emerged than that of T. brassicae (, P < .001). There were no differences in the number of emerged wasps between the two strains of T. brassicae () and the two strains of T. evanescens ().
Table 4. Comparison of emerging progeny within each species of T. brassicae (strain Y175 and J007) and T. evanescens (strain GD011 and GD025) at different temperatures (5, 10, 15 and 20°C) during 6 hours of scotophase (from 11 AM to 5 PM) and 12 hours of scotophase (from 9 PM to 9 AM the next day). In total 40 two-days-old, mated females per strain were tested for each temperature treatment. The factor ‘Light’ includes the comparison between the two light regimes.
The proportion of emerging T. brassicae wasps was not significantly different between the temperature regimes (). In contrast, proportionally more T. evanescens wasps were emerging with increasing temperature (). When comparing the proportion of emerged wasps between the two darkness regimes, significantly more wasps were emerging during the 6 hours of darkness than during the 12 hours of darkness period for both T. brassicae and T. evanescens ().
Discussion
Our results indicate that Trichogramma wasps can successfully parasitise moth eggs at night, even at temperatures below 10°C, and that such nocturnal activity varies between wasp species ( and ). Female T. brassicae wasps can successfully parasitise moth eggs in the dark at temperatures as low as 10°C, but only in an artificially induced scotophase during daytime according to their circadian rhythm (). Females of this species did not successfully parasitise any moth egg during the scotophase at night. This is in accordance with previous studies on the circadian rhythm of another strain of T. brassicae in which females were only active during daytime (completely light) and not at night (complete darkness) (Allemand et al., Citation1994; Pompanon et al., Citation1995, Citation1999). On the other hand, female T. evanescens wasps did parasitise moth eggs in the induced darkness during daytime and at night.
The difference in parasitism behaviour during the scotophase between the two species may be explained by their known host range, which, with respect to nocturnal moths, seems wider for T. evanescens than for T. brassicae (Polaszek, Citation2010). Interspecific differences in day/night parasitism between closely related parasitoids have previously been shown in weevil parasitoids that parasitise hosts that differ in day/night activity. For example, the wasp Microctonus aethiopoides Loan (Hymenoptera: Braconidae) oviposits into its diurnal host Sitona discoideus Gyllenhal (Coleoptera: Curculionidae) primarily during the photophase, whereas M. hyperodae Loan (Hymenoptera: Braconidae) mainly parasitises its nocturnal host Listronotus bonariensis Kuschel (Coleoptera: Curculionidae) during the scotophase (Armstrong, Barratt, & Evans, Citation1996).
Our data demonstrate that T. evanescens females are capable of parasitising host eggs at low temperatures during the scotophase. The aim of the present study was not to compare nocturnal parasitism with diurnal parasitism. The vast majority of host eggs is, however, likely to be parasitised in the photophase during daytime. Trichogramma wasps also generally have more than 24 hours time to parasitise nocturnally laid host eggs until the head of the host’s caterpillar has sclerotised. Diurnal activity has been shown for many Trichogramma species, and also for the strains used in this study (Woelke & Huigens, personal observations and, for example, Fatouros, Broekgaarden, et al., Citation2008; Fatouros, Bukovinszkine‘Kiss et al., Citation2005; Fatouros et al., Citation2007; Fatouros, Huigens, et al., Citation2005; Fatouros, et al., Citation2012; Huigens et al., Citation2009, Citation2010, Citation2011; Woelke, Citation2008). Reznik et al. (Citation2009) found for T. principium Sug. et Sor. (Hymenoptera: Trichogrammatidae) that female wasps that had been parasitising eggs during daytime stopped parasitising Sitotroga cerealella Olivier (Lepidoptera: Gelechiidae) eggs at the onset of the scotophase. However, when the first contact with the host occurred during darkness, T. principium females did start parasitising host eggs. Parasitism by females during darkness was reduced compared to parasitism by females during daytime. Maybe this is also the case in T. evanescens, but further experiments are needed to investigate this.
Temperature strongly affected the number of parasitising T. evanecens females and their offspring production. Biever (Citation1972), Boldt (Citation1974) and Suverkropp et al. (Citation2001) found a similar effect of temperature on the walking activity of T. evanescens, T. brassicae, T. minutum and T. semifumatum Perkins (Hymenoptera: Trichogrammatidae). Pak and van Heiningen (Citation1985) compared the oviposition rates of T. maidis Pint. et Voeg., T. pretiosum Riley and T. semblidis Auriv. (all Hymenoptera: Trichogrammatidae) and showed that the number of parasitised eggs per female increased with increasing temperatures until 25°C, and thereafter decreased. In summary, these studies suggest that the ‘activity’ of Trichogramma wasps from temperate zones increases sharply between 10°C and 15°C, as in our study (), flattens around 25°C and thereafter declines.
In conclusion, our study shows (a) that some species of Trichogramma wasps can parasitise moth eggs during the scotophase, (b) that such behaviour is affected by temperature and (c) that parasitism during the scotophase differs between wasp species. More knowledge on the intra- and interspecific variation in parasitism activity of Trichogramma females at different light and temperature regimes should help to select the most suitable species and strains for the biological control of a given pest species.
Acknowledgements
We thank Foteini Pashalidou for assistance with the experiments; Jeroen Spitzen for arranging the climate cabinets at the Laboratory of Entomology, Wageningen University & Research; and Rieta Gols for comments on an earlier version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Allemand, R., Pompanon, F., Fleury, F., Fouillet, P., & Boulétreau, M. (1994). Behavioural circadian rhythms measured in real-time by automatic image analysis: Applications in parasitoid insects. Physiological Entomology, 19, 1–8. doi: 10.1111/j.1365-3032.1994.tb01067.x
- Armstrong, S. M., Barratt, B. I. P., & Evans, A. A. (1996). Circadian pattern of oviposition in the parasitoid Microctonus aethiopoides Loan and M. hyperodae Loan (Hymenoptera: Braconidae), in relation to host activity. Proceedings of the NZ Plant Protection Conference, 49, 280–284.
- Biever, K. D. (1972). Effect of temperatures on the rate of search by Trichogramma and its potential application in field releases. Environmental Entomology, 1(2), 194–197. doi: 10.1093/ee/1.2.194
- Boldt, P. E. (1974). Temperature, humidity, and host: Effect on rate of search of Trichogramma evanescens and T. minutum Auctt. (not Riley, 1871). Annals of the Entomological Society of America, 67, 706–708. doi: 10.1093/aesa/67.4.706
- Fatouros, N. E., Broekgaarden, C., Bukovinszkine‘Kiss, G., van Loon, J. J. A., Mumm, R., Huigens, M. E., … Hilker, M. (2008). Male-derived butterfly anti-aphrodisiac mediates induced indirect plant defense. Proceedings of the National Academy of Sciences of the United States of America, 105(29), 10033–10038. doi: 10.1073/pnas.0707809105
- Fatouros, N. E., Bukovinszkine‘Kiss, G., Dicke, M., & Hilker, M. (2007). The response specificity of Trichogramma egg parasitoids towards infochemicals during host location. Journal of Insect Behavior, 20(1), 53–65. doi: 10.1007/s10905-006-9062-z
- Fatouros, N. E., Bukovinszkine‘Kiss, G., Kalkers, L. A., Soler Gamborena, R., Dicke, M., & Hilker, M. (2005). Oviposition-induced plant cues: Do they arrest Trichogramma wasps during host location? Entomologia Experimentalis et Applicata, 115, 207–215. doi: 10.1111/j.1570-7458.2005.00245.x
- Fatouros, N. E., Dicke, M., Mumm, R., Meiners, T., & Hilker, M. (2008). Foraging behavior of egg parasitoids exploiting chemical information. Behavioral Ecology, 19(3), 677–689. doi: 10.1093/beheco/arn011
- Fatouros, N. E., & Huigens, M. E. (2012). Phoresy in the field: Natural occurrence of Trichogramma egg parasitoids on butterflies and moths. BioControl, 57(4), 493–502. doi: 10.1007/s10526-011-9427-x
- Fatouros, N. E., Huigens, M. E., van Loon, J. J. A., Dicke, M., & Hilker, M. (2005). Chemical communication: Butterfly anti-aphrodisiac lures parasitic wasps. Nature, 433, 704. doi: 10.1038/433704a
- Fatouros, N. E., Lucas-Barbosa, D., Weldegergis, B. T., Pashalidou, F. G., van Loon, J. J. A., Dicke, M., … Huigens, M. E. (2012). Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. Plos One, 7(8), doi: 10.1371/journal.pone.0043607
- Forsse, E., Smith, S. M., & Bourchier, R. S. (1992). Flight initiation in the egg parasitoid Trichogramma minutum: Effects of ambient temperature, mates, food, and host eggs. Entomologia Experimentalis et Applicata, 62, 147–154. doi: 10.1111/j.1570-7458.1992.tb00654.x
- Huigens, M. E., de Swart, E., & Mumm, R. (2011). Risk of egg parasitoid attraction depends on anti-aphrodisiac titre in the large cabbage white butterfly Pieris brassicae. Journal of Chemical Ecology, 37(4), 364–367. doi: 10.1007/s10886-011-9935-2
- Huigens, M. E., & Fatouros, N. E. (2013). A hitch-hiker’s guide to parasitism: The chemical ecology of phoretic insect parasitoids. In J. Wajnberg & S. Colazza (Eds.), Chemical ecology of insect parasitoids (pp. 86–111). Hoboken: John Wiley and Sons.
- Huigens, M. E., Pashalidou, F. G., Qlan, M., Bukovinszky, T., Smid, H. M., van Loon, J. J. A., … Fatouros, N. E. (2009). Hitch-hiking parasitic wasp learns to exploit butterfly antiaphrodisiac. Proceedings of the National Academy of Sciences of the United States of America, 106(3), 820–825. doi: 10.1073/pnas.0812277106
- Huigens, M. E., Woelke, J. B., Pashalidou, F. G., Bukovinszky, T., Smid, H. M., & Fatouros, N. E. (2010). Chemical espionage on species-specific butterfly anti-aphrodisiacs by hitchhiking Trichogramma wasps. Behavioral Ecology, 21(3), 470–478. doi: 10.1093/beheco/arq007
- Li, L. Y. (1994). Worldwide use of Trichogramma for biological control on different crops: A survey. In J. Wajnberg & S. A. Hassan (Eds.), Biological control with egg parasitoids (pp. 37–53). Wallingford: CAB International.
- Maceda, A., Hohmann, C. L., & dos Santos, H. R. (2003). Temperature effects on Trichogramma pretiosum Riley and Trichogramma annulata De Santis. Brazilian Archives of Biology and Technology, 46(1), 27–32. doi: 10.1590/S1516-89132003000100005
- McCullagh, P., & Nelder, J. A. (1989). Generalized linear models. London: Chapman and Hall.
- Pak, G. A., & van Heiningen, T. G. (1985). Behavioural variations among strains of Trichogramma spp.: Adaptability to field-temperature conditions. Entomologia Experimentalis et Applicata, 38, 3–13. doi: 10.1111/j.1570-7458.1985.tb03491.x
- Pinto, J. D., & Stouthamer, R. (1994). Systematics of Trichogrammatidae with emphasis on Trichogramma. In J. Wajnberg & S. A. Hassan (Eds.), Biological control with egg parasitoids (pp. 1–36). Wallingford: CAB International.
- Polaszek, A. (2010). Species diversity and host associations of Trichogramma in Eurasia. In F. L. Consoli, J. R. P. Parra, & R. Zucchi (Eds.), Egg parasitoids in agroecosystems with emphasis on Trichogramma (pp. 237–266). Dordrecht: Springer.
- Pompanon, F., Fouillet, P., & Bouletréau, M. (1995). Emergence rhythms and protandry in relation to daily patterns of locomotor activity in Trichogramma species. Evolutionary Ecology, 9(5), 467–477. doi: 10.1007/BF01237829
- Pompanon, F., Fouillet, P., & Bouletréau, M. (1999). Physiological and genetic factors as sources of variation in locomotion and activity rhythm in a parasitoid wasp (Trichogramma brassicae). Physiological Entomology, 24(4), 346–357. doi: 10.1046/j.1365-3032.1999.00150.x
- Reznik, S. Y., Voinovich, N. D., & Karpova, S. G. (2009). Daily rhythms in parasitization of the angoumois grain moth Sitotroga cerealella Oliv. (Lepidoptera, Gelechiidae) eggs by the egg parasitoid Trichogramma principium Sug. et Sor. (Hymenoptera, Trichogrammatidae) females. Entomological Review, 89(1), 5–15. doi: 10.1134/S0013873809010023
- Smith, S. M. (1996). Biological control with Trichogramma: Advances, successes, and potential of their use. Annual Review of Entomology, 41, 375–406. doi: 10.1146/annurev.en.41.010196.002111
- Stinner, R. E. (1977). Efficacy of inundative releases. Annual Review of Entomology, 22, 515–531. doi: 10.1146/annurev.en.22.010177.002503
- Stouthamer, R., Hu, J., van Kan, F. J. P. M., Platner, G. R., & Pinto, J. (1999). The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma. BioControl, 43, 421–440. doi: 10.1023/A:1009937108715
- Suverkropp, B. P., Bigler, F., & van Lenteren, J. C. (2001). Temperature influences walking speed and walking activity of Trichogramma brassicae (Hym., Trichogrammatidae). Journal of Applied Entomology, 125(6), 303–307. doi: 10.1046/j.1439-0418.2001.00546.x
- Woelke, J. B. (2008). Hitch-hiking behavior of Trichogramma wasps on cabbage white butterflies and moths (MSc thesis). Laboratory of Entomology, Wageningen University, pp. 1–49.
