?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Trichogramma spp. have been used or have been proposed for use in biological control programmes of lepidopteran pests, including the European corn borer, Ostrinia nubilalis, the cotton bollworm, Helicoverpa armigera, and the spruce budworm, Choristoneura fumiferana. Releases are typically made by placing cards with parasitised eggs at set points in the field. However, this method can be cost-prohibitive due to its laborious nature. As a result, labour-saving mechanised release programmes have been developed, including distribution by spray equipment. However, few have investigated applying Trichogramma with a standard pesticide application (i.e. ‘tank mix’). As a first step to ascertain the feasibility of such a system, we observed the effect of immersion of T. ostriniae pupae in field-relevant concentrations of eight herbicides (bentazon, clethodim, fomesafen, quizalofop, Glacial acetic acid, glyphosate, imazamox, imazethapyr), three fungicides (Copper Hydroxide, flutriafol, and penthiopyrad), one insecticide (K+ salt of fatty acid), and five adjuvants (Ammonium Sulfate, Crop Oil Concentrate, Methylated seed oil, Non-ionic surfactant, and Urea-Ammonium Nitrate) on the emergence of T. ostriniae adults. The herbicides GAA and clethodim; the fungicides copper hydroxide, flutriafol, and penthiopyrad; and the adjuvants COC, MSO, and NIS all reduced T. ostriniae emergence compared to a water control. No emergence was observed with exposure to quizalofop, GAA, or K+ salt of fatty acid treatments. Other treatments did not affect emergence compared to water. Thus, deploying Trichogramma with a standard pesticide application may be a feasible labour-saving distribution method that warrants further investigation.
Introduction
Trichogramma is a genus of gregarious endoparasitoid wasp widely used to control lepidopterous pests (Pinto & Stouthamer, Citation1994; Querino et al., Citation2010; Smith, Citation1994, Citation1996; Zang et al., Citation2021). At the turn of the millennium, Trichogramma spp. were released on over 32 million hectares of forest and cropland each year (VanLenteren, Citation2000). Although, this figure may be an overestimate as some of the hectarage were double or tipple counted in the former USSR (VanLenteren, Citation2000).
Trichogramma spp. have several attributes that make them ideal candidates for biological control. Benefits include ease of rearing and the destruction of the host prior to its damaging stage (Davies et al., Citation2011; Hassan, Citation1994; Hegazi et al., Citation2012; Oliveira et al., Citation2014; Pak, Citation1988; Sarwar & Salman, Citation2015; VanLenteren, Citation2000; Wang et al., Citation2014). Ease of rearing is facilitated by the use of factitious hosts such as Corcyra cephalonica, Ephestia kuehniella, and Sitotroga cerealella (Bigler et al., Citation1987; Dysart, Citation1973; El-Wakeil, Citation2007; Gowda et al., Citation2021; Hassan, Citation1993; Wang et al., Citation2014; Zang et al., Citation2021).Inundative releases of Trichogramma spp. have been made in various environments, including forestland, tree fruit, and field and vegetable crops (Hassan, Citation1993; Martel et al., Citation2021; Wang et al., Citation2014; Zang et al., Citation2021). Trichogramma minutum has been trialled for control of the spruce budworm, Choristoneura fumiferana, in North American forests (Martel et al., Citation2021; Smith et al., Citation1990). Trichogramma maidis has been used to control the European corn borer, Ostriniae nubilalis, in corn in Switzerland (Bigler, Citation1986). Trichogramma chilonis has been used to control Helicoverpa armigera (Masood et al., Citation2011). Trichogramma ostriniae has been trialled for control of the western bean cutworm Striacosta albicosta (Seaman, Citation2017). A more comprehensive list of Trichogramma uses is reported by Smith (Citation1994) and Cluever (Citation2023).
Usually, Trichogramma are released during their pupal stage inside the host egg. Host eggs are glued to a substrate such as cardboard, sometimes called a ‘Trichocard,’ which is then fastened to the plant (Dionisio & Calvo, Citation2022; Gagnon et al., Citation2017; Knutson, Citation1998; Sarwar & Salman, Citation2015; Schmidt et al., Citation2003; Smith, Citation1994; Zhan et al., Citation2021). Smith (Citation1996) stated that at least six release points should be made per hectare. However, the minimum number of release points and the number released at each point varies (See Basso et al., Citation2020; Bueno et al., Citation2011; Chapman et al., Citation2009; Geremias & Parra, Citation2014; Hommay et al., Citation2002; Martel et al., Citation2021 for examples). Releases are often more effective if release points are spaced in such a way as to give a minimal distance between each point (Askar et al., Citation2018; Basso et al., Citation2020; Bueno et al., Citation2011; Tohamy & Kassem, Citation2007; Zachrisson & Parra, Citation1998). This spacing allows uniform population dispersion to be attained quickly (Geetha & Balakrishnan, Citation2010; Geremias & Parra, Citation2014; Gusev & Lebedev, Citation1988; Mahrughan et al., Citation2015; Smith, Citation1994). While this approach is technically simple to implement, it can be costly in terms of labour and may be difficult to achieve (Ables et al., Citation1979; Basso et al., Citation2020; Ji et al., Citation2022; Kienzle et al., Citation2012; Martel et al., Citation2021; Parra, Citation2010; Smith, Citation1996; Zang et al., Citation2021; Zhan et al., Citation2021).
Bigler (Citation1986) stated that deploying Trichogramma on cards would require thirty minutes of labour per hectare in corn. So, this would cost the grower USD 3.63 ha−1 if one assumes the U.S. 2022 federal minimum wage or CAD 7.78 ha−1 if one assumes the Canadian 2022 federal minimum wage (Anonymous, Citation2021, Citation2022). However, others have estimated that it would take more time. Anonymous (Citation1974) stated that it would take one person an entire day to apply Trichogramma across ten hectares (as cited by Jones et al., Citation1977). Birthal et al. (Citation2000) stated that two Trichogramma releases would take 4.23 person-days ha−1 in cotton. Jones et al. (Citation1977) states that the lack of mechanised release methods is the main economic factor limiting the use of Trichogramma. Additionally, Stinner (Citation1977) and Van Lenteren and Bueno (Citation2003) posit that the primary limitation to inundative biological control is economic.
Releasing Trichogramma in capsules from crewed or uncrewed aircraft may save on labour during releases; however, this may still prove uneconomical due to the time and expense required to prepare the capsules (Ables et al., Citation1979; Zhan et al., Citation2021). Additionally, broadcast releases may offer greater uniformity and efficacy (Gardner & Giles, Citation1997; Mikhal’tsov & Purshkarev, Citation1981; Pas’ko et al., Citation1982). Thus, broadcast releases of loose Trichogramma may be advantageous (Smith, Citation1994).
Broadcast releases of biological control agents are often made with carriers to allow for a more precise dispersal. Carriers include sawdust, tobacco seed, water, bran, vermiculite, and hydrocolloid mixtures (Bouse et al., Citation1980; Gardner & Giles, Citation1997; Kienzle et al., Citation2012; Martel et al., Citation2021; Pas’ko et al., Citation1982; Reeves, Citation1975). Gardner and Giles (Citation1997) proposed applying T. pretiosum through standard insecticide application equipment. Filippov (Citation1989) stated that 140–150 hectares could be treated in 8 h through these methods. According to Dionne et al. (Citation2018) application of T. ostriniae via boom sprayer may be 1.7 times faster than manual placement in sweet corn. Unfortunately, they note that spraying T. ostriniae costs 29.7% more than manual placement due to the need for additional T. ostriniae and adjuvants (Guar and Xanthan gum). Therefore, we propose using pre-existing herbicide or fungicide applications to distribute T. ostriniae.
Previous studies have assessed the toxicity of pesticides to Trichogramma (Bastos et al., Citation2006; Khan & Ruberson, Citation2017; Nusillard et al., Citation2023) and other egg parasitoids (Carmo et al., Citation2010; Turchen et al., Citation2016). For instance, Nusillard et al. (Citation2023) assessed the trophic effects of copper sulfate on T. cordubensis reared on Lobesia botrana. Bueno et al. (Citation2008) assessed the effect of 14 pesticides on the egg, larval, and pupal stages of T. pretiosum by immersion in solution for five seconds. Hassan et al. (Citation1998) assessed the effect of 21 pesticides on T. cacoeciae by residue exposure and to pupae by spaying the host. They also assessed the effect of duration of toxicity on leaves. Bastos et al. (Citation2006) assessed the effect of 20 pesticide formulations on T. pretiosum survival by immersing pupae in solution for five seconds. Khan and Ruberson (Citation2017) assessed the effect of eleven pesticides on T. pretiosum by immersion of eggs, larvae, and pupae in solution for ten seconds. Turchen et al. (Citation2016) assessed the effects of three insecticides on Telonmus podisi. Carmo et al. (Citation2010) observed the effect of exposure to 28 pesticide residues on T. remus parasitism and emergence. Sublethal effects on egg parasitoids include a reduction in fecundity (Fontes et al., Citation2018; González et al., Citation2013; Turchen et al., Citation2016; Wang et al., Citation2012), longevity (Ribeiro et al., Citation2021; Wang et al., Citation2012), response to host volatiles (Bayram et al., Citation2010), and wing deformities (Tai et al., Citation2022; Xie et al., Citation2022). For a more detailed review of pesticide toxicity to Trichogramma see Cluever (Citation2023).
However, these previous studies aimed not to evaluate the potential of a parasitoid and pesticide tank mix, but to assess the effect of pesticide exposure to Trichogramma already in the field. Thus, the authors did not expose Trichogramma to solutions for sufficient time to be relevant in a field-applied mixture.
The timing of standard pesticide applications may often align with the time when Trichogramma spp. can be used. For example, in western Nebraska, the pre-closure herbicide application in dry bean occurs in early July (NCL pers. observation). This application often aligns with the start of the western bean cutworm, S. albicosta flight (Cluever et al., Citation2021; Hanson et al. Citation2015). Additionally, corn fungicide applications may align with this timing (Jackson-Ziems, Citation2020).
Trichogramma ostriniae Pang et Chen is native to central and northern China (Li, Citation1994; Pang & Chen, Citation1974; Zong et al., Citation1988). This species has been used for inundative biological control of the Asian corn borer, Ostrinia furnacalis, and was imported to the United States in the 1990s (Lobdell et al., Citation2005; Wang et al., Citation2014). Since then, it has been used to control the European corn borer, O. nubilalis, in bell pepper, potato, sweet corn, and field corn in eastern North America (Chapman et al., Citation2009; Gagnon et al., Citation2017; Gardner et al., Citation2012; Kuhar et al., Citation2004; Wright et al., Citation2001).
Releasing T. ostrinae through a planned pesticide application could be an effective, low-cost, time-saving novel application process. As a first step in testing this novel distribution method, we assessed how immersion of T. ostriniae pupae in pesticide solutions affects emergence rates. We posit that most of the herbicides and fungicides will not affect T. ostriniae emergence as they are generally less harmful than insecticides and are protected by their host’s egg (Rakes et al., Citation2021; Smith, Citation1996). However oil-based pesticides may have less trouble penetrating the waxy host egg (Cônsoli et al., Citation1999) as noted by Khan and Ruberson (Citation2017) for S-metolachlor.
Materials and methods
Source of Trichogramma ostriniae
Trichogramma ostriniae pupae were obtained from a colony maintained at the Panhandle Research, Extension, and Education Center (PREEC) in Scottsbluff, Nebraska. The colony was maintained on sterilised Ephestia kuehniella Zeller eggs (Beneficial Insectary, Redding, CA) in a growth chamber with an 18:6 (L:D) h photoperiod at ≈25°C. The T. ostriniae used to intimate the PREEC colony were procured from the Cornell University Insectary in 2017. The Cornell University Insectary colony was started from T. ostriniae procured from the United States Department of Agriculture Animal and Plant Health Inspection Service (USDA APHIS) Mission Biological control center in 1993. The USDA obtained T. ostriniae from northern China (VanLenteren, Citation2000). Many other studies have used this isolate (Chapman et al., Citation2009; Gardner et al., Citation2012; Kuhar et al., Citation2002, Citation2004; Wright et al., Citation2001). All T. ostriniae used in this experiment were pupae inside loose E. kuehniella eggs.
Preparation of treatments
Commercial formulations of herbicides, fungicides, and one insecticide () were mixed with filtered (reverse osmosis) water at field-relevant concentrations. Pesticides and adjuvants were chosen based upon the level of their usage in the United States especially in dry edible bean and corn. The concentrations were chosen based upon the minimum and maximum that one could reasonably expect to see in the field (Knezevic, Citation2018; NCL personal Observation). The mixtures were placed in 20 ml glass scintillation vials. Then 0.1 g of T. ostriniae pupae inside an E. kuehniella host were added to each vial. Vials were placed in a fume hood arranged in an RCBD design for the duration of exposure. Each treatment was replicated four times with two separate T. ostriniae cohorts for a total of eight replications.
Table 1. Treatments (products and concentrations).
An aliquot (∼1–2 ml) of T. ostriniae pupae was pipetted from vials at 1.5, 3, 6.5, 9, 12, and 22.5 h of treatment exposure and placed onto a bleached white coffee filter cut into 12.5 cm2 triangles (Walmart, Bentonville, AR). These exposure durations were chosen to include the range of times a chemical may be held in suspension between mixing and application for a commercial field application. Most of the moisture was removed from the filter paper using a vacuum breaker. Then the coffee filter was allowed to dry before placing it in a 47 mm Petri dish sealed with parafilm.
Evaluation of mortality
Trichogramma ostriniae pupae were incubated on the lab bench at room temperature (≈21 °C) for ten days and then placed in the freezer (−20 °C) to kill any emerged wasps. Twenty E. kuehniella eggs in each dish were chosen haphazardly and examined for emergence holes under a dissecting microscope.
Statistical analysis
The effect of exposure time on emergence was analysed with base R software and the drc: Analysis of Dose-Response Curves Package in R version 4.2.1 (R Core Team, Citation2022; Reitz et al., Citation2015). The responses from the clethodim, glacial acetic acid, imazamox, and non-ionic surfactant were fitted to a Type II Weibull model (Seber & Wild, Citation1989):
where f(x) is the proportion of T. ostriniae alive at hour x, c is the lower limit, d is the upper limit, e is the number of hours at which 50% response between the upper and lower limits occurs, the inflexion point (i.e. LT50), and b is the slope of the line at the inflexion point.
The responses from the quizalofop and copper hydroxide treatments were fitted to a two and three-parameter exponential decay model, respectively (OCED, Citation2006):
where f(x) is the proportion of T. ostriniae alive at hour x, c is the lower limit, d is the upper limit, e is the number of hours at which 50% response between the upper and lower limits occurs, the inflexion point (i.e. LT50).
For the remainder of the treatments, there was no effect of time on survival, and regression analysis was not used in the analysis. Thus, the effect of treatment on survival at 22.5-hour exposure was analysed using Proc Glimmix (SAS Institute Inc., Citation2019), using a beta distribution with cohort as a nested effect (Shahaba, Citation2012; Stroup, Citation2013). Following a significant treatment effect, a post hoc Dunnett-Hsu test (significance at P ≤ 0.05) was used to compare herbicide, fungicide, and adjuvant treatments with awater control.
Results
Effect of time
For most treatments, the duration of exposure did not affect T. ostriniae emergence. However, GAA (low concentration), clethodim, copper hydroxide (low concentration), imazamox (low concentration), NIS, and quizalofop did show a significant effect of duration of exposure on T. ostriniae survival (). The LT50 was between 0.8 and 37.4 h for all treatments with a significant time effect. The 22.5-h exposure will be used to assess the effect of exposure on emergence as it is the ‘worst-case’ scenario (i.e.it is unlikely that an applicator would leave a mixed solution unsprayed for longer).
Table 2. Parameter estimates and S.E.s for the effect of exposure time on the mortality of T. ostriniae exposed to various adjuvants, herbicides, and fungicides.
Effect of compounds
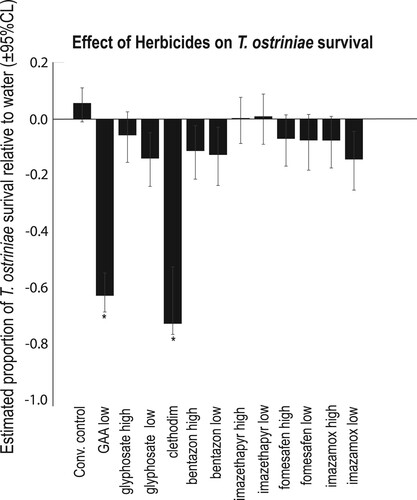
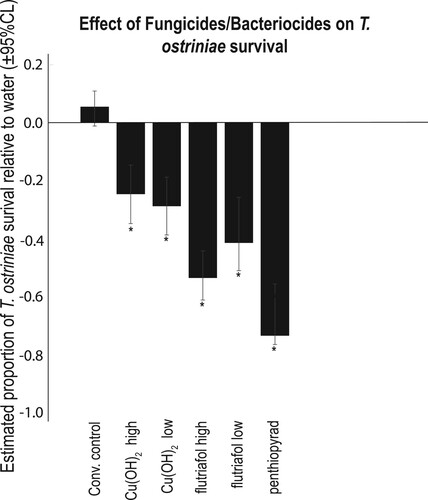
Chemical treatment had a significant effect on T. ostriniae emergence () (F = 21.49; df = 26, 165; P < 0.0001) at 22.5 h of exposure. According to a post hoc Dunnett’s test, the air treatment was not significantly different from water (). No T. ostriniae emerged in the quizalofop, GAA (High concentration), or K+ salt of fatty acid treatments at 22.5 h of exposure. Only two other herbicides negatively affected the emergence of T. ostriniae compared to water (). The fungicide/bactericide treatments significantly reduced emergence by 25–73% depending on treatment (). All adjuvants significantly reduced emergence except for Ammonium sulfate and both concentrations of Urea-Ammonium nitrate ().
Discussion
Our finding that emergence is not affected by immersion in water is similar to Gardner and Giles (Citation1997), who found that emergence of T. pretiosum from eggs immersed in water ranged from about eighty to ninety per cent of an open-air control. Also similar to our findings, Khan and Ruberson (Citation2017) found that the emulsifiable concentrate S-metolachlor negatively impacted the emergence rate of T. pretiosum when exposed in the pupal stage. However, the non-emulsifiable concentrates glufosinate-ammonium and nicosulfuron did not. Bastos et al. (Citation2006) found moderately-reduced T. pretiosum emergence with exposure to the herbicides clomazone and a mixture of paraquat and diuron compared to water. However, Oliveira et al. (Citation2014) did not find a decrease in the emergence of T. galloi with exposure to clomazone. In our study, all of the fungicides negatively affected T. ostriniae emergence. These findings contrast Khan and Ruberson (Citation2017), who found that myclobutanil, pyraclostrobin, and a mixture of trifloxystrobin and tebuconazole had little effect on the emergence of T. pretiosum. However, the studies mentioned here only exposed Trichogramma pupae for 5–10 s (Bastos et al., Citation2006; Khan & Ruberson, Citation2017; Oliveira et al., Citation2014).
Other factors impacting parasitoid deployment by co-mixing with agrichemicals might include product formulation, parasitoid host species, and application method. All treatments tested in this study were formulated products; therefore, negative effects on emergence could be due to inert rather than active ingredients. For example, Giolo et al. (Citation2005) found that glyphosate formulations containing isopropylamine salts were more harmful to T. pretiosum adults than potassium or ammonium salts. Additionally, despite being smaller, S. cerealella eggs have a mucus layer on the surface of the egg that E. kuehniella lacks. Also, the thickness of the chorion and the vitelline membrane is thicker in S. cerealella compared with E. kuehniella (Bastos et al., Citation2006; Cônsoli et al., Citation1999). Pesticides affect pupae developing in S. cerealella less than those developing in E. kuehniella (Bastos et al., Citation2006). The length of E. kuehniella and S. cerealella eggs are 520.7 and 584.7 µm, respectively (Cônsoli et al., Citation1999); therefore, filter mesh size would need to accommodate the host egg size. Preliminary studies (unpublished data) indicate that a 16-mesh size would allow for the passage of E. kuehniella eggs. Lastly, Knutson (Citation1996) found that passage through spray equipment reduced the emergence of an unspecified species of Trichogramma by about twenty per cent. However, Gardner and Giles (Citation1997) and Gauthier and Khelifi (Citation2016) did not find reduced emergence after the pupae were sprayed. In a study by Khelifi et al. (Citation2019), an important source of mortality is a non-pneumatic pressure regulator. Therefore, all applicator system aspects should be investigated before deployment in the field. Though not examined in this study, sublethal effects of pesticides may also be a factor in biological control success. Sublethal effects include reduction in fecundity (Tai et al., Citation2022), longevity (Li et al., Citation2015; Thubru et al., Citation2018), perception of host cues (Bayram et al., Citation2010), and an increase in wing malformations (Tai et al., Citation2022). For instance, azadiracthin has been shown to reduce the fecundity of Telenomus podisi (Turchen et al., Citation2016), abacemtin of Trichogramma achaeae (Fontes et al., Citation2018), and essential oil of Origanum vulgarae of Trissolcus basalis (González et al., Citation2013). Wang et al. (Citation2012) saw reduced longevity and fecundity of Trichogramma chilonis when exposed to fipronil and avermectins. Ribeiro et al. (Citation2021) also saw reduced longevity and fecundity of Trichogramma chilonis when exposed to clothianidin. Bayram et al. (Citation2010) found exposure to sublethal levels of cyfluthrin reduced Telonomus busseolae response to Sesamia nonagrioides pheromone. Tai et al. (Citation2022) found increased rates of wing deformity among Trichogramma ostriniae individuals exposed to atrazine, difenoconazole, and propiconazole.
Even the products that reduce survival may be candidates for carriers if a broadcast release’s improved effectiveness and labour savings outweigh the loss due to toxicity. A 2022 search for Trichogramma costs resulted in costs between $20.00 and $28.80 USD 100 000−1 (JDC Personal observation). The U.S. minimum wage varies by state but averages $9.80 h−1 with a standard deviation of $2.46 (Anonymous, Citation2022). Although, farm workers can be paid below a state’s minimum wage. Furthermore, the agricultural sector is facing a labour shortage in many regions such Canada (Agriculture Canada, Citation2023; Komarnicki, Citation2012), the European Union (European Labour Authority, Citation2022; Mitaritonna & Ragot, Citation2020), India (Gunabhagya, Citation2017), Japan (Liu-Farrer et al., Citation2023), Mexico (Zahniser et al., Citation2018), United Kingdom (Byrne, Citation2018; Scott et al., Citation2008; Siudek & Zawojska, Citation2016), and United States (Simnitt & Martin, Citation2022; Zahniser et al., Citation2018). Thus, many growers may not have time or staff to make conventional Trichogramma releases even if they have the financial resources.
The time stated in the literature to deploy Trichocards manually can vary from 0.5 to 17 hr ha−1 (). Application by aircraft can be expensive furthermore, the high speed and altitude may cause many of the parasitoids to miss their target (Bzowska-Bakalarz et al., Citation2020; Zhan et al., Citation2021). Another option is unmanned aerial vehicles which depending on the model can treat 15 hectares in a twenty-minute flight (Zang et al., Citation2021). Parra and Coelho (Citation2022) state that the labour for drone application costs $2–3 ha−1 in Brazil However, agricultural drones can have a large upfront cost of $10,000 or more (Nixon, Citation2022) which may dissuade some growers (Michles et al., Citation2020). Furthermore, drone usage may require a special licence and flight is limited as to where the drone may be flown (FAA, Citation2023; Transport Canada, Citation2019). Indeed, Michles et al. (Citation2020) stated that legal barriers prevent some German growers from using drones. A further limitation of drones is limited flight duration (Martel et al., Citation2021). Costs associated with tractor application can be variable depending on the model and the current cost of fuel but are estimated to be $0.86 USD ha−1 for fuel, $2.79 ha−1 for repairs, and $8.60 ha−1 for depreciation (Klein & McClure, Citation2022). However, it should be noted that these would be incurred whether Trichogramma are released or not as this method takes advantage of pre-existing applications. See for a comparison of the costs of various release methods.
Table 3. Cost (USD 100 ha−1) comparisons of release methods with a labour cost of $9.80 hr−1 taking into account increased mortality in broadcast treatments.
In the U.S., approximately 75, 84, and 11 million acres of corn, soybean, and cotton are planted with herbicide-tolerant cultivars, respectively (Dodson, Citation2020; USDA-NASS, Citation2019). Much of this tolerance is to glyphosate. The low toxicity of glyphosate to Trichogramma may allow for broader adoption of spray application. For example, in cotton, glyphosate applications vary but, in some cultivars, may be made throughout much of the growing season (Beck, Citation2020; Wright & Munier, Citation2015). This may allow for control of damaging pests such as the the cotton bollworm, Heliothis zea, and the tobacco budworm, H. virescens (Bouse et al., Citation1980; Knutson, Citation1998; Stinner et al., Citation1974). In 2006, these pests caused over $10 million in collective damage in Georgia alone (Roberts & Ruberson, Citation2007). Furthermore, the relatively low toxicity of the low concentration of copper hydroxide may allow for Trichogramma release with late-season disease control. Applications of copper hydroxide in dry bean and corn may be made weekly up to harvest and thus may align with the ideal time for western bean cutworm, Striacosta albicosta, control (Kocide, Citation2015; Seymour et al., Citation2010). It may also allow for control of European corn borer, Ostrinia nubilalis, in corn and potato (Anonymous, Citation2015; Kocide, Citation2015; Pavlista, Citation2014).
Conclusion
This study represents the first step to proving the practicability of deploying Trichogramma with some commonly used pesticide formulations. Most herbicides did not affect T. ostriniae emergence, although most of the adjuvants and all of the fungicides tested did. Subsequent trials should assess (1) the sublethal effects of pesticides on T. ostriniae including possible effects on fecundity, longevity, host finding, and wing malformations (2) the effect of the spray equipment on mortality, (3) the ability of parasitised eggs to adhere to the foliage, and (4) the level of parasitism achieved.
Geolocation information
Nebraska, USA.
Statements and Declarations
JDC, NCL, and JDB contributed to the design of the study. JDC and CWB conducted to day to day activities associated with the experiment. JDC produced the first draft of this manuscript with contributions from JDB and NCL. All authors commented on and approved the manuscript.
Acknowledgements
The authors thank Kayla Mollet, Luis Ochoa, and Rick Patrick for assisting with conducting the experiment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, JDB, upon reasonable request.
Additional information
Funding
References
- Ables, J. R., Reeves, B. G., Morrison, R. K., Kinzer, R. E., Jones, S. L., Ridgway, R. L., & Bull, D. L. (1979). Methods for the field release of insect parasites and predators. Transactions of the ASAE, 22(1), 0059–0062. https://doi.org/10.13031/2013.34966
- Agriculture Canada. (2023, May 18). What We Heard Report—Agricultural Labour Strategy. Agriculture Canada. https://agriculture.canada.ca/en/department/transparency/public-opinion-research-consultations/what-we-heard-report-agricultural-labour-strategy
- Anonymous. (1974). Methodological directions on mass rearing and utilization of Trichogramma for the control of pests of farm crops. Proceedings 1972 All-union seminar of specialists of productive biolaboratories. All-union seminar of specialists of productive biolaboratories, Moscow.
- Anonymous. (2015, September 17). European corn borer (on potato). CropWatch. https://cropwatch.unl.edu/potato/euro_corn_borer
- Anonymous. (2021). Payroll Legislation. https://www.payworks.ca/payroll-legislation/MinimumWage.asp
- Anonymous. (2022). The economic Policy Institute: Minimum wage tracker (Vol. 2022, Issue 22 April). https://www.epi.org/minimum-wage-tracker/#/min_wage
- Askar, S., Tabikha, R., & El-Hussieni, M. (2018). Dispersal of Pteromalus puparum L. and Trichogramma evanescens, Parasitoids of Artogia rapae L. (Lepidoptera: Pieridae) in Cauliflower Fields, at El-Behera Governorate, Egypt. Egyptian Bulletin of Entomological Researrch, 24(2), 161–165.
- Basso, C., Chiaravalle, W., Maignet, P., Basso, C., Chiaravalle, W., & Maignet, P. (2020). Effectiveness of Trichogramma pretiosum in controlling lepidopterous pests of soybean crops. Agrociencia (Uruguay), 24(SPE2). https://doi.org/10.31285/agro.24.419
- Bastos, C. S., de Almeida, R. P., & Suinaga, F. A. (2006). Selectivity of pesticides used on cotton (Gossypium hirsutum) toTrichogramma pretiosumreared on two laboratory-reared hosts. Pest Management Science, 62(1), 91–98. https://doi.org/10.1002/ps.1140
- Bayram, A., Salerno, G., Onofri, A., & Conti, E. (2010). Sub-lethal effects of two pyrethroids on biological parameters and behavioral responses to host cues in the egg parasitoid Telenomus busseolae. Biological Control, 53(2), 153–160. https://doi.org/10.1016/j.biocontrol.2009.09.012
- Beck, L. (2020). Weed management in cotton. New Mexico State University, College of Agriculture, Consumer, and Environmental Sciences. https://pubs.nmsu.edu/_a/A239/#:~:text=Pre%2Dplant%20incorporated%20(PPI),to%20provide%20residual%20weed%20control.
- Bigler, F. (1986). Mass production of Trichogramma maidis Pint, et Voeg. And its field application against Ostrinia nubilalis Hbn. In Switzerland1. Journal of Applied Entomology, 101(1-5), 23–29. https://doi.org/10.1111/j.1439-0418.1986.tb00829.x
- Bigler, F., Meyer, A., & Bosshart, S. (1987). Quality assessment in Trichogramma maidis Pintureau et Voegelé reared from eggs of the factitious hosts Ephestia kuehniella Zell. and Sitotroga cerealella (Olivier). Journal of Applied Entomology, 104(1-5), 340–353. https://doi.org/10.1111/j.1439-0418.1987.tb00535.x
- Birthal, P. S., Sharma, O. P., & Kumar, S. (2000). Economics of integrated pest management: Evidences and issues. Indian Journal of Agricultural Economics, 55(4), 644–659.
- Bouse, L. F., Carlton, J. B., Jones, S. L., Morrison, R. K., & Ables, J. R. (1980). Broadcast aerial release of an egg parasite for lepidopterous insect control. Transactions of the ASAE, 23(6), 1359–1363. https://doi.org/10.13031/2013.34779
- Bueno, A., de, F., Batistela, M. J., Bueno, R. C. O., de, F., França-Neto, J., de, B., Naime Nishikawa, M. A., & Filho, A. L. (2011). Effects of integrated pest management, biological control and prophylactic use of insecticides on the management and sustainability of soybean. Crop Protection, 30(7), 937–945. https://doi.org/10.1016/j.cropro.2011.02.021
- Bueno, A., de, F., Bueno, R. C. O., de, F., Parra, J. R. P., & Vieira, S. S. (2008). Effects of pesticides used in soybean crops to the egg parasitoid Trichogramma pretiosum. Ciência Rural, 38(6), 1495–1503. https://doi.org/10.1590/S0103-84782008000600001
- Byrne, R. (2018, June 8). The migrant labour shortage is already here, and agri-tech can’t yet fill the gap. LSE Brexit, 1–2.
- Bzowska-Bakalarz, M., Bulak, P., Bereś, P. K., Czarnigowska, A., Czarnigowski, J., Karamon, B., Pniak, M., & Bieganowski, A. (2020). Using gyroplane for application of Trichogramma spp. Against the European corn borer in maize. Pest Management Science, 76(6), 2243–2250. https://doi.org/10.1002/ps.5762
- Carmo, E. L., Bueno, A. F., & Bueno, R. C. O. F. (2010). Pesticide selectivity for the insect egg parasitoid Telenomus remus. BioControl, 55(4), 455–464. https://doi.org/10.1007/s10526-010-9269-y
- Chapman, A. V., Kuhar, T. P., Schultz, P. B., & Brewster, C. C. (2009). Dispersal of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) in Potato Fields. Environmental Entomology, 38(3), 677–685. https://doi.org/10.1603/022.038.0319
- Cluever, J., Peterson, J., Wright, R., & Bradshaw, J. (2021). Degree-days for prediction of western bean cutworm flight. University of Nebraska-Lincoln, Institute of Agriculture and Natural Resources. https://cropwatch.unl.edu/2021/degree-days-prediction-western-bean-cutworm-flight#:~:text=Twenty%2Dfive%20percent%20of%20WBC,are%20reached%20(Table%201)
- Cluever, J. D. (2023). Management of western bean cutworm (Striacosta albicosta) in western Nebraska. University of Nebraska-Lincoln.
- Cônsoli, F. L., Kitajima, E. W., & Parra, J. R. P. (1999). Ultrastructure of the natural and factitious host eggs of Trichogramma galloi Zucchi and Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). International Journal of Insect Morphology and Embryology, 28b(3), 211–231. https://doi.org/10.1016/S0020-7322(99)00026-4
- Davies, A. P., Carr, C. M., Scholz, B. C. G., & Zalucki, M. P. (2011). Using Trichogramma Westwood (Hymenoptera: Trichogrammatidae) for insect pest biological control in cotton crops: An Australian perspective. Australian Journal of Entomology, 50(4), 424–440. https://doi.org/10.1111/j.1440-6055.2011.00827.x
- Dionisio, M. A., & Calvo, F. J. (2022). Integrated management of chrysodeixis chalcites esper (lepidoptera: Noctuidae) based on trichogramma achaeae releases in commercial banana crops in the canary islands. Agronomy, 12(12), Article 2982. https://doi.org/10.3390/agronomy12122982
- Dionne, A., Khelifi, M., Todorova, S., & Boivin, G. (2018). Design and testing of a boom sprayer prototype to release Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) in sweet corn for biocontrol of Ostrinia nubilalis (Hübner)(Lepidoptera: Crambidae). Transactions of the ASABE, 61(6), 1867–1879. https://doi.org/10.13031/trans.12922
- Dodson, L. (2020). Recent trends in G.E. adoption. United States Department of Agriculture- Economic Research Service. https://www.ers.ano.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us/recent-trends-in-ge-adoption.aspx
- Dysart, R. J.. (1973). The use of Trichogramma in the USSR. Proceedings of the Tall Timbers Conference on Ecological Animal Control by Habitat Management. 4:165–173
- El-Wakeil, N. E. (2007). Evaluation of efficiency of Trichogramma evanescens reared on different factitious hosts to control Helicoverpa armigera. Journal of Pest Science, 80(1), 29–34. https://doi.org/10.1007/s10340-006-0150-9
- European Labour Authority. (2022). EURES: Report on labour shortages and surpluses 2022 (p. 125).
- FAA (Federal Aviation Administration). (2023). Getting started: Congratulations on your new drone. https://www.faa.gov/uas/getting_started
- Filippov, N. A. (1989). The present state and future outlook of biological control in the USSR. Acta Entomologica Fennica, 53, 11–18.
- Fontes, J., Roja, I. S., Tavares, J., & Oliveira, L. (2018). Lethal and sublethal effects of various pesticides on trichogramma achaeae (hymenoptera: Trichogrammatidae). Journal of Economic Entomology, 111(3), 1219–1226. https://doi.org/10.1093/jee/toy064
- Gagnon, A.-È., Audette, C., Duval, B., & Boisclair, J. (2017). Can the Use of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) to Control Ostrinia nubilalis (Lepidoptera: Crambidae) Be Economically Sustainable for Processing Sweet Corn? Journal of Economic Entomology, 110(1), tow293–66. https://doi.org/10.1093/jee/tow293
- Gardner, J., & Giles, K. (1997). Mechanical distribution ofChrysoperla rufilabrisandTrichogramma pretiosum:Survival and uniformity of discharge after spray dispersal in an aqueous suspension. Biological Control, 8(2), 138–142. https://doi.org/10.1006/bcon.1996.0499
- Gardner, J., Wright, M. G., Kuhar, T. P., Pitcher, S. A., & Hoffmann, M. P. (2012). Dispersal of Trichogramma ostriniae in field corn. Biocontrol Science and Technology, 22(10), 1221–1233. https://doi.org/10.1080/09583157.2012.723676
- Gauthier, P., & Khelifi, M. (2016). Trichogramma pupae spraying technique development for biocontrol of the European corn borer in sweet corn crops. Csbe/SCGAB 2016 annual conference, Halifax.
- Geetha, N., & Balakrishnan, R. (2010). Dispersal pattern of Trichogramma chilonis Ishii in sugarcane field. Biological Control, 24, 1–7.
- Geremias, L. D., & Parra, J. R. P. (2014). Dispersal of Trichogramma galloi in corn for the control of Diatraea saccharalis. Biocontrol Science and Technology, 24(7), 751–762. https://doi.org/10.1080/09583157.2014.891723
- Giolo, F. P., Grützmacher, A. D., Procópio, S. O., Manzoni, C. G., Lima, C. A. B., & Nörnberg, S. D. (2005). Side-effects of glyphosate formulations on Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Planta Daninha, 23(3), 458–462. https://doi.org/10.1590/S0100-83582005000300009
- González, J. O. W., Laumann, R. A., da Silveira, S., Moraes, M. C. B., Borges, M., & Ferrero, A. A. (2013). Lethal and sublethal effects of four essential oils on the egg parasitoids Trissolcus basalis. Chemosphere, 92(5), 608–615. https://doi.org/10.1016/j.chemosphere.2013.03.066
- Gowda, G. B., Pandi, G. P. P., Ullah, F., Patil, N. B., Sahu, M., Adak, T., Pokhare, S., Yadav, M. K., Mahendiran, A., Mittapelly, P., Desneux, N., & Rath, P. C. (2021). Performance of Trichogramma japonicum under field conditions as a function of the factitious host species used for mass rearing. PLOS ONE, 16(8), e0256246. https://doi.org/10.1371/journal.pone.0256246
- Greenberg, S. M., Abashkin, A. S., Cherkasov, V. A., & Nikonov, P. V. (1990). The use of Trichoramma in the fight against a complex of pests of field crops. Agropromizdat.
- Gunabhagya, A. (2017). Agricultural labour shortage: An abysmal to agriculture in north eastern karnataka. Economic Affairs, 62(4), 589. https://doi.org/10.5958/0976-4666.2017.00071.7
- Gusev, G. V., & Lebedev, G. I. (1988). Present state of Trichogramma application and research. Les Colloques de l’INRA, 43, 477–481.
- Hanson, A. A., Moon, R. D., Wright, R. J., Hunt, T. E., & Hutchison, W. D. (2015). Degree-Day prediction models for the flight phenology of western bean cutworm (Lepidoptera: Noctuidae) assessed with the concordance correlation coefficient. Journal of Economic Entomology, 108(4), 1728–1738. https://doi.org/10.1093/jee/tov110
- Hassan, S. A. (1993). The mass rearing and utilization of Trichogramma to control lepidopterous pests: Achievements and outlook. Pesticide Science, 37(4), 387–391. https://doi.org/10.1002/ps.2780370412
- Hassan, S. A. (1994). Strategies to select Trichogramma species for use in biological control. In E. Wajnberg, & S. A. Hassan (Eds.), Biological Control with egg parasitoids (pp. 56–71). CAB International.
- Hassan, S. A., Hafes, B., Degrande, P. E., & Herai, K. (1998). The side-effects of pesticides on the egg parasitoid Trichogramma cacoeciae Marchal (Hym., Trichogrammatidae), acute dose-response and persistence tests. Journal of Applied Entomology, 122(1-5), 569–573. https://doi.org/10.1111/j.1439-0418.1998.tb01547.x
- Hegazi, E., Khafagi, W., Herz, A., Konstantopoulou, M., Hassan, S., Agamy, E., Atwa, A., & Shweil, S. (2012). Dispersal and field progeny production of Trichogramma species released in an olive orchard in Egypt. BioControl, 57(4), 481–492. https://doi.org/10.1007/s10526-011-9420-4
- Hommay, G., Gertz, C., Kienlen, J. C., Pizzol, J., & Chavigny, P. (2002). Comparison between the control efficacy of trichogramma evanescens westwood (hymenoptera: Trichogrammatidae) and Two trichogramma cacoeciae marchal strains against grapevine moth ( lobesia botrana Den. & schiff.), depending on their release density. Biocontrol Science and Technology, 12(5), 569–581. https://doi.org/10.1080/0958315021000016234
- Jackson-Ziems, T. (2020). Considerations for Foliar Fungicide Use in Corn. CropWatch. https://cropwatch.unl.edu/2020/considerations-foliar-fungicide-use-corn
- Ji, S., Gong, J., Cui, K., Zhang, Y., & Mostafa, K. (2022). Performance test and parameter optimization of trichogramma delivery system. Micromachines, 13(11), Article 11. https://doi.org/10.3390/mi13111996
- Jones, S.L., Morrison, R. K., Ables, J.R & Bull D. (1977). A new and improved technique for the field release of Trichogramma pretiosum. Southwestern Entomologist. 2: 210–215
- Khan, M. A., & Ruberson, J. R. (2017). Lethal effects of selected novel pesticides on immature stages of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Pest Management Science, 73(12), 2465–2472. https://doi.org/10.1002/ps.4639
- Khelifi, M., Gauthier, P., Todorova, S., & St-onge, M. (2019). New formulation for spraying biopesticides and spraying apparatus to control pest (United States Patent US20190208761A1). https://patents.google.com/patent/US20190208761A1/en
- Kienzle, J., Zimmermann, O., Wührer, B., Triloff, P., Morhard, J., Landsgesell, E., & Zebitz, C. P. W. (2012). New species and new methods of application—A new chance for Trichogramma in codling moth control? Pcoceedings of the Ecofruit. 15th International Conference on Organic Fruit-Growing, 20–22.
- Klein, R., & McClure, G. (2022). 2023 unl crop budgets. Center for Agricultural Profitability. https://cap.unl.edu/cropbudgets
- Knezevic, S. (eds.). (2018). 2018 Guide for weed, disease, and insect management in Nebraska. University of Nebraska-Lincoln.
- Knutson, A. (1996). Evaluation of the biosprayer for the application of Trichogramma to cotton. Beltwide Cotton Conferences.
- Knutson, A. (1998). The Trichogramma manual. Texas Agricultural Extension Service.
- Kocide. (2015). Kocide 3000. https://www3.epa.gov/pesticides/chem_search/ppls/091411-00002-20150507.pdf
- Komarnicki, E. (2012). Labour and skills shortages in Canada: Addressing current and future challanges (p. 119). House of Commons Standing committee on Human Resources, Skills and Social Development and the Status of Persons with Disabilities.
- Kuhar, T. P., Barlow, V. M., Hoffmann, M. P., Fleischer, S. J., Groden, E., Gardner, J., Hazzard, R., Wright, M. G., Pitcher, S. A., Speese, J., & Westgate, P. (2004). Potential of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) for biological control of European corn borer (Lepidoptera: Crambidae) in solanaceous crops. Journal of Economic Entomology, 97(4), 1209–1216. https://doi.org/10.1093/jee/97.4.1209
- Kuhar, T. P., Wright, M. G., Hoffmann, M. P., & Chenus, S. A. (2002). Life Table Studies of European Corn Borer (Lepidoptera: Crambidae) with and without Inoculative Releases of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae). Environmental Entomology, 31(3), 482–489. https://doi.org/10.1603/0046-225X-31.3.482
- Li, L.-Y. (1994). Worldwide use of Trichogramma for biological control on different crops: A survey. In E. Wajnberg, & S. A. Hassan (Eds.), Biological control with egg parasitoids (pp. 37–53). CAB International.
- Li, W., Zhang, P., Zhang, J., Lin, W., Lu, Y., & Gao, Y. (2015). Acute and sublethal effects of neonicotinoids and pymetrozine on an important egg parasitoid, Trichogramma ostriniae (Hymenoptera: Trichogrammatidae). Biocontrol Science and Technology, 25(2), 121–131. https://doi.org/10.1080/09583157.2014.957163
- Liu-Farrer, G., Green, A. E., Ozgen, C., & Cole, M. A. (2023). Immigration and labor shortages: Learning from Japan and the United Kingdom. Asian and Pacific Migration Journal, 0(0). https://doi.org/10.1177/01171968231188532
- Lobdell, C. E., Yong, T.-H., & Hoffmann, M. P. (2005). Host color preferences and short-range searching behavior of the egg parasitoid Trichogramma ostriniae. Entomologia Experimentalis et Applicata, 116(2), 127–134. https://doi.org/10.1111/j.1570-7458.2005.00306.x
- Mahrughan, A., Shirazi, J., Amir Maafi, M., & Dadpour, H. (2015). Dispersal of Trichogramma brassicae in tomato field. Journal of Crop Protection, 4(2), 173–180.
- Martel, V., Johns, R. C., Jochems-Tanguay, L., Jean, F., Maltais, A., Trudeau, S., St-Onge, M., Cormier, D., Smith, S. M., & Boisclair, J. (2021). The use of UAS to release the egg parasitoid Trichogramma spp. (Hymenoptera: Trichogrammatidae) against an agricultural and a forest pest in Canada. Journal of Economic Entomology, 114(5), 1867–1881. https://doi.org/10.1093/jee/toaa325
- Masood, A., Arif, M. J., Hamed, M., & Talpur, M. A. (2011). Field performance of Trichogramma chilonis aganist cotton bollworms infestation in different cotton varieties as a sustainable IPM aprroach. Pakistan Journal of Agriculture, Agricultural Engineering, and Veterinary Sciences, 27, 176–184.
- Michles, M., Von Hobe, C.-F., & Musshoff, O. (2020). A trans-theoretical model for the adoption of drones by large-scale German farmers. Journal of Rural Studies, 75, 80–88. https://doi.org/10.1016/j.jrurstud.2020.01.005
- Mikhal’tsov, V. P., & Purshkarev, B. V. (1981). Water as a means of Trichogramma release. Zashchita Rastenii, 9, 27–28.
- Mitaritonna, C., & Ragot, L. (2020). After COVID-19, will seasonal migrant agricultural workers in Europe be replaced by robots. CEPII Policy Brief, 33, 1–10.
- Nixon, A. (2022, January 25). Best drones For agriculture 2022: The ultimate buyer’s guide. Best Drone for the Job. https://bestdroneforthejob.com/drone-buying-guides/agriculture-drone-buyers-guide/
- Nusillard, W., Garinie, T., Lelièvre, Y., Moreau, J., Thiéry, D., Groussier, G., Frandon, J., & Louâpre, P. (2023). Heavy metals used as fungicide may positively affect Trichogramma species used as biocontrol agents in IPM programs. Journal of Pest Science. https://doi.org/10.1007/s10340-023-01624-6
- OCED (Organisation for Economic Co-operation and Development. (2006). Current approaches in the statistical Analysis of ecotoxicity data: A guidance to application—Annexes. OCED.
- Oliveira, H. N., Antigo, M. R., Carvalho, G. A., & Glaeser, D. F. (2014). Effect of selectivity of herbicides and plant growth regulators used in sugarcane crops on immature stages of Trichogramma galloi (Hymenoptera: Trichogrammatidae). Planta Daninha, 32(1), 125–131. https://doi.org/10.1590/S0100-83582014000100014
- Pak, G. A. (1988). Selection of Trichogramma for inundative biological control: A study of behavioural variations among strains and species of an egg-parasite genus [PhD]. Wageningen University.
- Pang, H. F., & Chen, T. L. (1974). Trichogramma of China (hymenoptera: Trichogrammatidae). Acta Entomologia Sinica, 17(4), 441–454.
- Parra, J. R. P. (2010). Egg parasitoids commercialization in the New world. In J. R. P. Consoli, & R. A. Zucchi (Eds.), Egg parasitoids in agroecosystems with emphasis on trichogramma (pp. 373–388). Springer.
- Parra, J. R. P., & Coelho, A. (2022). Insect rearing techniques for biological control programs, a component of sustainable agriculture in Brazil. Insects, 13(1), 105. https://doi.org/10.3390/insects13010105
- Pas’ko, A. K., Barabash, A. V., Grinberg, A. M., Vorotyntseva, A. F., voubetryn, I. N., & pynzar, B. V. (1982). Aerial distribution of Trichogramma. Zashchita Rastenii, 7, 17.
- Pavlista, A. D. (2014). European corn borer in potato. NebGuide. https://extensionpublications.unl.edu/assets/pdf/g2229.pdf.
- Pinto, J. D., & Stouthamer, R. (1994). Systematics of the Trichogrammatidae with emphasis on Trichogramma. In E. Wajnberg, & S. A. Hassan (Eds.), Biological control with egg parasitoids (pp. 1–28). Wallingford (UK): CAB International.
- Querino, R., Zucchi, R. A., & Pinto, J. D. (2010). Chapter 7: Systematics of the Trichogrammatidae (Hymenoptera: Chalcidoidea) with a focus on the genera attacking Lepidoptera. In F. L. Consoli, J. R. P. Parra, & R. A. Zucchi (Eds.), Egg parasitoids in agroecosystems with emphasis on trichogramma (pp. 191–218). Springer.
- Rakes, M., Pasini, R. A., Morais, M. C., Araújo, M. B., de Bastos Pazini, J., Seidel, E. J., Bernardi, D., & Grützmacher, A. D. (2021). Pesticide selectivity to the parasitoid Trichogramma pretiosum: A pattern 10-year database and its implications for Integrated Pest Management. Ecotoxicology and Environmental Safety, 208, 111504. https://doi.org/10.1016/j.ecoenv.2020.111504
- R Core Team. (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
- Reeves, B. G. (1975). Design and evaluation of facilities and equipment for mass production and field release of an insect parasite and an insect predator [Doctoral dissertation]. Texas A&M University.
- Reitz, C., Baty, F., Streibig, J. C., & Gerhard, D. (2015). drc: Dose-response Analysis using R. PLos One, 10(12), e0146021.
- Ribeiro, A. V., Holle, S. G., Hutchison, W. D., & Koch, R. L. (2021). Lethal and sublethal effects of conventional and organic insecticides on the parasitoid trissolcus japonicus, a biological control agent for halyomorpha halys. Frontiers in Insect Science, 1. https://doi.org/10.3389/finsc.2021.685755
- Roberts, P., & Ruberson, J. (2007). Cotton insects. In P. Guillebeau, N. Hinkle, & P. Roberts (Eds.), Summary of losses from insect damage and cost of control in Georgia 2006 (Vol. 1–Book, Section, pp. 2–4). University of Georgia.
- Sarwar, M., & Salman, M. (2015). Biological insecticide Trichogramma spp. (Hymenoptera: Trichogrammatidae) strikes for caterpillar control. International Journal of Entomology Research, 1(1), 31–36.
- SAS Institute Inc.(2019). SAS version 9.4, Cary, North Carolina.
- Schmidt, V. B., Linker, H. M., Orr, D. B., & Kennedy, G. G. (2003). Variation in biological parameters of Trichogramma spp. Purchased from commercial suppliers in the United States. BioControl, 48(5), 487–502. https://doi.org/10.1023/A:1025751428043
- Scott, S., McCormick, A., & Zaloznik, M. (2008). Staff shortages and immigration in agriculture. Migration Advisory Committee.
- Seaman, A. (2017). Final Report for ONE16-271—SARE Grant Management System (Grant Report No. ONE16-271). Sustainable Agriculture Research and Education. https://projects.sare.org/project-reports/one16-271/
- Seber, G. A., & Wild, C. J. (1989). Nonlinear regression. Wiley & Sons.
- Seymour, R. C., Hein, G. L., Wright, R. J., & Campbell, J. B. (2010). Western bean cutworm in corn and dry beans. University of Nebraska-Lincoln, NebGuide.
- Shahaba, B. (2012). Bayesian analysis. In: Biostatistics with R. Use R!. Springer.
- Simnitt, S., & Martin, P. (2022). U.S. Fruit and vegetable industries try to cope with rising labor costs. USDA-ERS. https://www.ers.usda.gov/amber-waves/2022/december/u-s-fruit-and-vegetable-industries-try-to-cope-with-rising-labor-costs/
- Siudek, T., & Zawojska, A. (Eds.). (2016). Foreign labour in agricultural sectors of some EU countries. https://doi.org/10.22004/ag.econ.249797
- Smith, S. M. (1994). Methods and timing of releases of Trichogramma to control lepidopterous pests. In E. Wajnberg, & S. A. Hassan (Eds.), Biological control with Egg parasitoid (pp. 113–144). Wallingford (UK): CAB International.
- Smith, S. M. (1996). Biological control with Trichogramma: Advances, successes, and potential of their use. Annual Review of Entomology, 41(1), 375–406. https://doi.org/10.1146/annurev.en.41.010196.002111
- Smith, S. M., Wallace, D. R., Howse, G., & Meating, J. (1990). 3.5 Suppression of spruce budworm populations by Trichogramma minutum Riley, 1982–1986. Memoirs of the Entomological Society of Canada, 122(S153)(S153), 56–81. https://doi.org/10.4039/entm122153056-1
- Stinner, R. E. (1977). Efficacy of inundative releases. Annual Review of Entomology, 22(1), 515–531. https://doi.org/10.1146/annurev.en.22.010177.002503
- Stinner, R. E., Ridgway, R. L., Coppedge, J. R., Morrison, R. K., & Dickerson Jr, W. A. (1974). Parasitism of Heliothis eggs after field releases of Trichogramma pretiosum in cotton. Environmental Entomology, 3(3), 497–500. https://doi.org/10.1093/ee/3.3.497
- Stroup, W. W. (2013). Generalized linear mixed models: Modern concepts, methods and applications. CRC Press.
- Tai, H., Zhang, F., Xiao, C., Tang, R., Liu, Z., Bai, S., & Wang, Z. (2022). Toxicity of chemical pesticides commonly used in maize to Trichogramma ostriniae (Hymenoptera: Trichogrammatidae), an egg parasitoid of Asian corn borer. Ecotoxicology and Environmental Safety, 241, 113802. https://doi.org/10.1016/j.ecoenv.2022.113802
- Thubru, D. P., Firake, D. M., & Behere, G. T. (2018). Assessing risks of pesticides targeting lepidopteran pests in cruciferous ecosystems to eggs parasitoid, Trichogramma brassicae (Bezdenko). Saudi Journal of Biological Sciences, 25(4), 680–688. https://doi.org/10.1016/j.sjbs.2016.04.007
- Tohamy, T. H., & Kassem, M. M. A. (2007). Comparative efficacy of Trichogramma evanescens west. and the biocide (agerin) with recommended insecticides program for controlling cotton bollworms and its effect on cotton productivity in middle Egypt region. Journal of Plant Protection and Pathology, 32(7), 5663–5677. https://doi.org/10.21608/jppp.2007.219702
- Transport Canada. (2019, June 17). Knowledge Requirements for Pilots of Remotely Piloted Aircraft Systems 250 g up to and including 25 kg, Operating within Visual Line-of-Sight (VLOS)—TP 15263. AARV 12871687; AARV. https://tc.canada.ca/en/aviation/publications/knowledge-requirements-pilots-remotely-piloted-aircraft-systems-250-g-including-25-kg-operating-within-visual-line-sight-vlos-tp-15263
- Turchen, L. M., Golin, V., Butnariu, A. R., Guedes, R. N. C., & Pereira, J. B. (2016). Lethal and sublethal effects of insecticides on the egg parasitoid Telenomus podisi (Hymenoptera: Platygastridae). Journal of Economic Entomology, 109(1), 84–92. https://doi.org/10.1093/jee/tov273
- USDA-NASS. (2019). 2017 Census of Agriculture United States Summary and State Data. USDA-NASS. https://www.nass.usda.gov/Publications/AgCensus/2017/index.php
- VanLenteren, J. C. (2000). Success in biological control of arthropods by augmentation of natural enemies. In G. Gurr, & S. Wratten (Eds.), Biological control: Measures of success (pp. 77–103). Springer.
- Van Lenteren, J. C., & Bueno, V. H. P. (2003). Augmentative biological control of arthropods in Latin America. Biocontrol, 48(2), 123–139. https://doi.org/10.1023/A:1022645210394
- Wang, D.-S., He, Y.-R., Guo, X.-L., & Luo, Y.-L. (2012). Acute toxicities and sublethal effects of some conventional insecticides on Trichogramma chilonis (Hymenoptera: Trichogrammatidae). Journal of Economic Entomology, 105(4), 1157–1163. https://doi.org/10.1603/EC12042
- Wang, Z.-Y., He, K.-L., Zhang, F., Lu, X., & Babendreier, D. (2014). Mass rearing and release of Trichogramma for biological control of insect pests of corn in China. Biological Control, 68, 136–144. https://doi.org/10.1016/j.biocontrol.2013.06.015
- Wright, M. G., Hoffmann, M. P., Chenus, S. A., & Gardner, J. (2001). Dispersal behavior of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) in sweet corn fields: Implications for augmentative releases against Ostrinia nubilalis (Lepidoptera: Crambidae). Biological Control, 22(1), 29–37. https://doi.org/10.1006/bcon.2001.0948
- Wright, S. D., & Munier, D. J. (2015). Cotton: Agricultural pest management: Herbicide treatment table (Vol. 2022, Issue 22 April). http://ipm.ucanr.edu/PMG/r114700311.html
- Xie, L.-C., Jin, L.-H., Lu, Y.-H., Xu, H.-X., Zang, L.-S., Tian, J.-C., & Lu, Z.-X. (2022). Resistance of Lepidopteran egg parasitoids, Trichogramma japonicum and Trichogramma chilonis, to insecticides used for control of rice planthoppers. Journal of Economic Entomology, 115(2), 446–454. https://doi.org/10.1093/jee/toab254
- Zachrisson, B., & Parra, J. R. P. (1998). Capacidade de dispersão de Trichogramma pretiosum Riley, 1879 para o controle de Anticarsia gemmatalis Hübner, 1818 em soja. Scientia Agricola. https://bit.ly/3jwDprP
- Zahniser, S., Taylor, J. E., Hertz, T., & Charlton, D. (2018). Farm labor markets in the United States and Mexico pose challenges for U.S. Agriculture (p. 46). USDA-ERS.
- Zang, L.-S., Wang, S., Zhang, F., & Desneux, N. (2021). Biological control with Trichogramma in China: History, present status, and perspectives. Annual Review of Entomology, 66, 463–484. https://doi.org/10.1146/annurev-ento-060120-091620
- Zhan, Y., Chen, S., Wang, G., Fu, J., & Lan, Y. (2021). Biological control technology and application based on agricultural unmanned aerial vehicle (UAV) intelligent delivery of insect natural enemies (Trichogramma) carrier. Pest Management Science, 77(7), 3259–3272. https://doi.org/10.1002/ps.6371
- Zong, L., Zhong, C., & Lei, C. (1988). Studies of Trichogramma (Hymenoptera: Trichogrammatidae): Classification and geographical distribution in Hubei province of China. Trichogramma and Other Egg Parasites, 43, 57–67.